INTRODUCTION
Cirrhosis is a frequent liver disease caused by various factors such as obesity, alcohol consumption, viral infections, and autoimmune diseases. The disease progresses from an asymptomatic phase to a symptomatic phase, leading to complications that can result in hospitalization, impaired quality of life, and high mortality.[]
Kidney dysfunction is a significant complication of liver cirrhosis that can lead to poor outcomes. The incidence of acute kidney injury (AKI) ranges from 20% to 50% in cirrhotic patients hospitalized due to acute decompensation.[] Moreover, AKI in liver disease patients often progresses to chronic kidney disease (CKD), and the prevalence of CKD in patients with cirrhosis has increased, reaching up to 50% in recent reports.[] Progression of CKD is higher in patients with other risk factors, including diabetes, obesity, and hypertension. The prevalence of CKD among liver transplantation candidates has increased more than 2-fold in the last decade, likely a result of an aging population coupled with increased frequency of comorbidities and an allocation system dependent on kidney dysfunction. The increased prevalence of CKD in cirrhotic patients is one of the factors determining the rise in simultaneous liver-kidney transplant (SLKT) utilization by nearly 300% in the past decade.[] Thus, management of AKI in cirrhotic patients is of most importance to avoid or delay CKD progression.
Hepatorenal syndrome (HRS) is renal disfunction caused by hemodynamic changes in patients with ascites and portal hypertension. The primary mechanism involves reduced renal perfusion due to increased sympathetic, renin-angiotensin-aldosterone, and vasopressin activities, as well as decreased cardiac output in cirrhosis-associated cardiomyopathy. Systemic inflammation and impaired renal autoregulation also contribute to renal injury, leading to structural kidney damage following severe or repeated episodes.[]
However, distinguishing between renal dysfunction caused by HRS and acute tubular necrosis (ATN) or persistent AKI can be challenging [Figure 1]. To further enhance the understanding of renal dysfunction in cirrhotic patients, exploring biomarkers, such as those associated with fibrosis, would be beneficial to differentiate between acute and potentially reversible HRS versus non-reversible conditions (ATN).[]
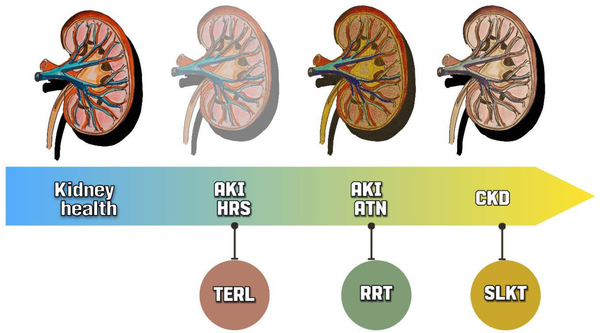
Figure 1.
Differential diagnoses of acute kidney injury in cirrhotic patients and potential therapeutics. AKI in cirrhotic patients can be challenging, as it requires distinguishing between HRS and ATN. Both conditions can progress to CKD. Treatment options also differ, with TERL being indicated for HRS and RRT being a better option for AKI-ATN. In cases of CKD, a liver transplant alone may not be sufficient, and a SLKT may be a more suitable choice. AKI, acute kidney injury; HRS, hepatorenal syndrome; ATN, acute tubular necrosis; CKD, chronic kidney disease; TERL, terlipressin, RRT, renal replacement therapy; SLKT, simultaneous liver-kidney transplant.
DIAGNOSTIC OF AKI
The latest consensus on the diagnosis of AKI was published in 2012 by the Kidney Disease: Improving Global Outcomes (KDIGO).[] The International Club of Ascites (ICA) performed a new recommendation in 2012 for the diagnosis of AKI, updated in 2015.[] Compared to the KDIGO, the ICA proposed criteria do not recommend the urine output criterion, as oliguria can be present in patients with preserved kidney function.[] ICA further divides KDIGO stage 1 into two subgroups based on the level of serum creatinine (sCr): stage 1A (sCr < 1.5 mg/dL) and stage 1B (sCr ≥ 1.5 mg/dL), and this subclassification is supported by differential outcomes.[]
Despite the improvement of the criteria, the diagnosis of AKI in cirrhotic patients remains challenging. SCr limitations are more remarkable in this population, with higher malnutrition rates, muscle mass loss, and fluid accumulation that may lead to underestimating sCr values [Figure 2].[]
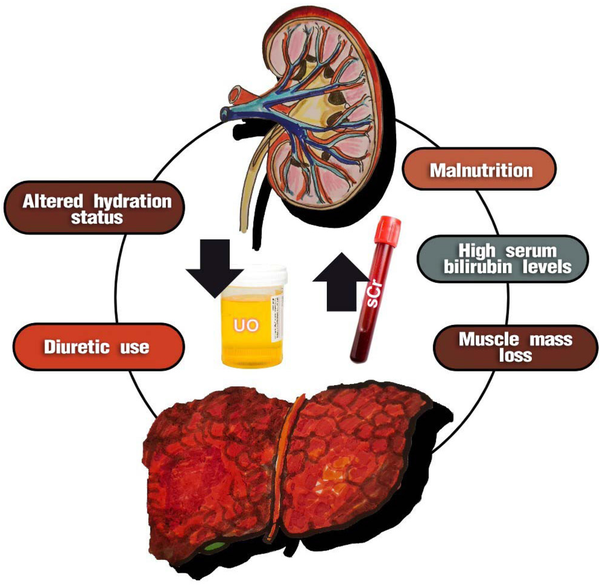
Figure 2.
Factors that can influence the standard diagnosis of AKI in cirrhotic patients. The standard diagnosis for assessing renal function in cirrhotic patients, based on increased sCr and reduced UO, has limitations. Some factors, such as malnutrition, muscle mass loss, and hypervolemia, can decrease serum creatinine levels. On the other hand, factors such as hypovolemia and high bilirubin levels can increase sCr levels, especially when the Jaffé method is used for evaluation. It is important to note that urine output may be preserved even in the presence of oliguria, and the use of diuretics is common in these patients. AKI, acute kidney injury; sCr, serum creatinine; UO, urine output.
The colorimetric Jaffé method is still commonly used to measure creatinine levels. In this method, creatinine reacts with an alkaline picrate solution to form a complex that absorbs light at a specific wavelength. The intensity of the color produced is proportional to the concentration of creatinine in the sample. However, in patients with hepatic cirrhosis, high serum bilirubin levels can also react with the alkaline picrate solution, producing a color similar to that of creatinine.[] This can result in overestimating creatinine levels, particularly when bilirubin levels exceed 25 mg/dL, leading to errors in evaluating kidney function.[]
Furthermore, acute changes in glomerular filtration rate (GFR) are not accompanied by a concomitant increase in sCr. In steady state, the production and elimination of sCr are in balance, and when GFR declines it may take 24-48 hours to detect an elevation in after an insult.[] It’s also worth noting that delayed diagnosis of AKI can significantly increase the in-hospital mortality rate by 2.7-fold for every 12-hour delay.[] Therefore, there is a critical need for earlier and more sensitive and specific biomarkers to improve AKI diagnosis in this population.
ETIOLOGY OF AKI IN CIRRHOTIC PATIENTS
The understanding of the etiology of AKI and mortality in cirrhotic patients is limited. AKI in cirrhotic patients has a multifactorial etiology, involving infections, volume depletion from gastrointestinal bleeding or fluid losses, glomerulonephritis associated with chronic hepatitis B or C infection, and nephrotoxicity.[] The main etiologies for AKI in cirrhosis are prerenal AKI and ATN. The two main causes of prerenal AKI are hypovolemia and HRS-AKI, while ATN is usually due to septic or hypovolemic shock and, less commonly, nephrotoxic drugs/agents. Bile cast nephropathy, glomerulonephritis, and postrenal obstruction are less-common causes of AKI that should be considered in the differential diagnosis. In large studies, AKI is attributed to hypovolemia (27%-50% of all cases), HRS-AKI (15%-43%), and ATN (14%-35%).[]
A study including 102 patients found that the main causes of AKI in cirrhotic patients were: infections (55.88%) and hypovolemia (31.37%). HRS occurred in 10.78% of patients, and parenchymal renal disease was the least common cause at 1.90%.[]
The mechanism of AKI in cirrhotic patients with infections is linked to impaired systemic arterial vasodilation. This is due to various bacterial products or cytokines (such as tumor necrosis factor α, interleukin-6 [IL-6]), nitric oxide, and other endogenous vasodilators synthesized in response to infection.[] Cirrhotic patients are at high risk of bacterial translocation, leading to increased circulating levels of lipopolysaccharide binding protein and enhanced production of tumor necrosis factor α, which worsens splanchnic vasodilation. Other significant immunologic factors triggered by hepatic injury include the release of damage-associated molecular pattern (DAMP) compounds, including high-mobility group box 1. These DAMP compounds interact through toll-like receptors 2 and 4, causing subsequent renal tubule injury.[]
The high incidence of AKI is due to the combination of an impaired effective arterial blood volume secondary to arterial vasodilation, with increased intrarenal vasoconstriction and impaired renal autoregulation that predisposes to renal dysfunction, and several precipitating factors related to cirrhosis, typically bacterial infections and gastrointestinal bleeding.[] The etiology of AKI is conceptually classified into three general categories: prerenal, intrarenal, and postrenal. Although this classification may be useful for establishing a differential diagnosis, AKI has mostly multifactorial, and pathophysiologic features that can be divided into different categories. Acute tubular necrosis, caused by either ischemia or nephrotoxicity, is common in the setting of AKI.[]
SCr is unable to provide information about the etiology of AKI, and the reliability of some parameters such as fractional excretion of sodium (FeNa), Feurea, in determining the type of AKI, ATN, HRS, or CKD in cirrhotic patients is not clear. In some AKI cases, kidney damage may develop, but clinical manifestations and kidney dysfunction may not be present, and it is called subclinical AKI.[] Biomarkers should be considered to better assess AKI in cirrhotic patients.
ROLE OF SCR IN MELD AND SLKT DIAGNOSIS AND SCORING
The Model End Stage Liver Disease (MELD) score assesses the waiting list mortality risk and determines deceased-donor liver allocation since February 2002. The MELD score is calculated using three routine laboratory test results: bilirubin, sCr, and international normalized ratio (INR).
SCr is a crucial component of the MELD score and significantly predicts complications such as AKI. Patients with renal dysfunction are commonly considered candidates for liver transplantation (LT).[] The weight of sCr serves as an independent predictor of survival after transplantation. The United Network for Organ Sharing (UNOS) has established a maximum 4 mg/dL value for sCr.[] Some studies have suggested replacing sCr with alternative biomarkers, such as cystatin C, in the MELD score.[] This change could improve the accuracy and fairness of the score in assessing liver disease severity and prioritizing patients for transplantation.
Another important score determining SLKT candidacy is the estimated glomerular filtration rate (eGFR). The eGFR considers age, gender, and sCr levels to calculate a value reflecting kidney function. However, it’s important to note that in advanced cirrhotic patients, who are often sarcopenic regardless of age, sex, and race, the correction of muscle mass by these factors may not accurately assess kidney function. This raises concerns about whether the correction could potentially benefit certain candidates over others.[] The study by Asrani et al. proposes a GFR model for liver disease in the presence of renal dysfunction—GRAIL. The results obtained with GRAIL have shown better accuracy in predicting the development of CKD and the need for kidney transplantation within five years after LT compared to other equations. However, it is worth noting that the model uses additional variables such as creatinine, blood urea nitrogen, age, gender, race, and albumin, which may not address the issue of sarcopenia.[]
Recently, studies have reported the non-use of correction for race; for example, Inker et al. results showed that the current equations using age, sex, and race overestimate the GFR in black individuals and, to a lesser extent, non-blacks. It is concluded that the new equations that incorporate creatinine and cystatin C but exclude race are more accurate and reduce the differences between racial groups.[]
These findings suggest that race adjustment in eGFR equations may impact SLKT candidacy for a subset of black patients listed for LT alone. The study highlights the need to develop novel algorithms for estimating GFRs free of race to promote equity in SLKT eligibility and outcomes.[]
Use of biomarkers to diagnostic AKI in cirrhosis patients
Due to the limitations of sCr, which are more pronounced in cirrhotic patients, and considering that AKI is a complex and heterogeneous process that involves the dynamics of pathology, it is unlikely that a single biomarker can answer all questions related to the diagnosis of AKI. However, other biomarkers have been studied to assist in the diagnosis of AKI in cirrhotic patients.
BIOMARKERS
Several biomarkers have been studied to overcome these limitations and to detect AKI earlier and with greater sensitivity than sCr.[] Some of these biomarkers include urinary cystatin C, urinary kidney injury molecule-1 (KIM-1), urinary interleukin-18 (IL-18), and urinary/plasma neutrophil gelatinase-associated lipocalin (NGAL).[] Although some biomarkers have shown promise in clinical practice, few have been widely adopted. NGAL has been particularly effective in early AKI diagnosis and predicting severity and the need for dialysis.[] Recently, biomarkers of cell cycle arrest, insulin-like growth factor-binding protein 7 (IGFBP7), and tissue inhibitor of metalloproteinases-2 (TIMP-2) have provided more insight into AKI physiopathology and increased the possibility of early diagnosis and intervention.[] While rapid assays for some of these markers are becoming available and ongoing trials may provide more guidance on early AKI management, their high cost and lack of robust evidence of improvement in patient outcomes continue to limit their use.[]
Interpreting the use of biomarkers in clinical practice
Despite the potential benefits of using biomarkers for diagnosing AKI in cirrhotic patients, several multicenter clinical studies have failed to demonstrate a performance level comparable to that of troponin in the myocardium.[] The difficulty in achieving good diagnostic performance for AKI using biomarkers can be explained by the time required for biomarker analysis, which must consider the dynamics of both “normal” and “disease” states in the population under consideration. Therefore, it is important to understand the cutoff value for both normality and AKI diagnosis in cirrhotic patients.
The analysis of kidney function in cirrhotic patients is challenging. However, preoperative studies in patients with advanced cirrhosis but without renal decompensation can help determine normality in this scenario. It is important to distinguish between HRS and CKD in situations involving liver transplantation or combined liver and kidney transplantation.[]
Comprehensive assessment of kidney function by biomarkers
The combined evaluation of a panel of biomarkers allows not only the stratification in the diagnosis of AKI but also the assessment of the site of injury (glomerular, proximal or distal tubular, and renal), as well as the type of injury—ischemic, nephrotoxic, and ATN. To comprehensively assess kidney function through biomarkers, we conducted a review of biomarkers that represent different dimensions of the Nephron, evaluating filtration (cystatin C and proenkephalin) and injury (NGAL, KIM-1, IL-18) in cirrhotic patients [Figure 3].
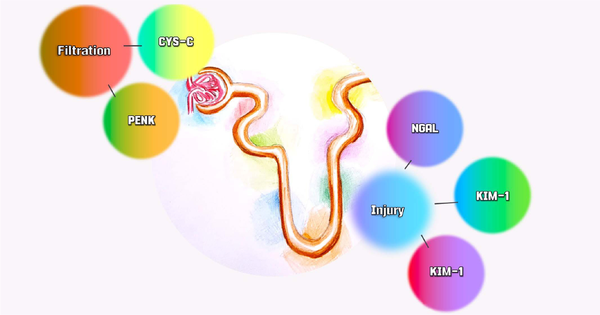
Figure 3.
Biomarkers of filtration and Nephron injury. Acute kidney injury is a complex and heterogeneous syndrome, and a single molecule is unlikely to address all the questions. Therefore, a panel involving multiple dimensions of the Nephron can be useful. This study addresses filtration biomarkers with PENK and CYS-C, and injury biomarkers with KIM-1, IL-18, and NGAL. PENK, proenkephalin; CYS-C, cystatin C; KIM-1, kidney injury molecule-1; IL-18, interleukin 18; NGAL, neutrophil gelatinase-associated lipocalin.
FILTRATION BIOMARKERS
Accurate measurement of kidney function is fundamental to the clinical care of patients with cirrhosis. It is important to distinguish between HRS and CKD in situations involving liver or combined liver and kidney transplantation.[] We have previously stated the limitations of using sCr in this population. One of the oldest renal filtration biomarkers is serum cystatin C, which has been studied over the years and has shown superiority over sCr. It is available in clinical practice in some hospitals. Meanwhile, the recent biomarker proenkephalin—PENK is still finding its place through clinical studies, with potential superiority over sCr and cystatin C.
Cystatin C
In 1961, Clausen discovered gamma-trace, a low-molecular-weight protein in cerebrospinal fluid (CSF) samples. The same year, Butler and Flyn described cystatin C as a gamma-electrophoretic profile protein in isolated urine samples from patients with various renal pathologies.[] However, it wasn’t until 1984 that Barret et al. identified the biological function of cystatin C as a cysteine inhibitor due to its structural and functional similarity with cystatin found in chicken eggs.[]
Cystatin C is a non-glycosylated protein of 13.36 kDa that is produced at a constant rate by all nucleated cells. It is freely filtered into the glomerulus, reabsorbed, and metabolized in the proximal renal tubule without renal or extrarenal secretion.[]
Unlike creatinine, serum levels of cystatin C are not significantly affected by sex, race, or muscle mass. However, concentrations of cystatin C increase with age.[] Glucocorticoids appear to alter the production of cystatin C, high levels increase its production, while medium and low levels have no effect.[]
Thyroid disorders, such as hypothyroidism, hyperthyroidism, and subclinical disorders, may alter the production of cystatin C. Patients with hypothyroidism have a reduction in serum cystatin C. In contrast, patients with hyperparathyroidism have higher serum cystatin C expression.[]
Is cystatin C a better biomarker than sCr in cirrhotic patients?
Estimating GFR based on cystatin C, based formulas shows a strong correlation with the gold-standard methods of GFR estimation, such as diethylenetriamine pentaacetate scan or iohexol-based clearance.[] Moreover, it is worth noting that cystatin C-based formulas provide a more accurate estimation of GFR than sCr-based formulas in cirrhotic patients, especially in hyperbilirubinemia.[] Some studies have even suggested that cystatin C may be superior to sCr in diagnosing AKI and replacing sCr in the MELD score (MELD-cystatin).[]
A systematic review and meta-analysis were performed to summarize different GFR estimating formulas, including CKD-EPI-creatinine, CKD-EPI-cystatin C, and CKD-EPI-creatinine-cystatin C equations in cirrhosis patients. The study found that the CKD-EPI-creatinine-cystatin C combined equation had the least bias, superior precision, highest P30 accuracy, good sensitivity, and best specificity among all GFR estimating formulas.[]
In a study by Souza et al., cystatin C-based equations were more precise in determining GFR than Crea-based equations in over 200 patients awaiting liver transplantation.[] This finding was also confirmed in various groups of patients with liver cirrhosis.[] Previous small studies have suggested that cystatin C can predict HRS and mortality.[]
In a study involving 112 cirrhotic patients with normal creatinine levels, it was found that cystatin C showed a stronger correlation with the GFR measured by 51Cr-Ethylenediaminetetraacetic acid (EDTA) compared to creatinine. In patients with normal sCr levels, cystatin C proved a valuable marker for predicting HRS and survival.[] These findings were corroborated by another study with eighty patients with liver cirrhosis, ascites, and normal sCr levels. The study revealed a significant association between cystatin C levels and HRS development and mortality.[]
In a recent study by Padia et al., the outcomes of 88 cirrhotic patients were evaluated, including the development of renal dysfunction (RD), acute-on-chronic liver failure (ACLF), and mortality. The study found that cystatin C was a useful predictor of RD development and ACLF, with odds ratio (OR) of 7.970 (2.700-23.530) and 5.486 (1.456-20.600), respectively.[] In a study by Wan et al., patients with liver disease were evaluated, and the cohort cystatin C value was 1.21 mg/dL at the diagnosis of AKI.[] Another study, which considered a larger cohort, found that a cutoff value of 1.50 mg/L predicted 90-day mortality based on cystatin C levels.[]
Serum cystatin C but not sCr was an independent predictor of new AKI on multivariate analysis. The study analyzed the association between serum cystatin C and pharmacokinetics, dosing, and clinical outcomes of medications in adults. Twenty-eight articles evaluating 16 different medications in 3455 participants were included. Cystatin C proved to be more predictive of medication levels and renal clearance than sCr, but further clarification is needed in a larger cohort.[]
The studies suggest that cystatin C is a promising alternative to using sCr as a biomarker for evaluating renal function in cirrhotic patients. Cystatin C-based formulas provide a more accurate estimation of GFR in cirrhotic patients. In addition, cystatin C has been associated with predicting renal complications, including the development of AKI and ACLF, as well as predicting mortality.
However, other studies in cirrhotic patients did not achieve positive results with cystatin C.[] This can be partially explained by immunosuppressants, such as glucocorticoids, which affect the production of cystatin C. In addition, the inflammatory state can also contribute to this lack of effectiveness.[] As well as the presence of hypothyroidism as a comorbidity, which reduces the production of cystatin C.[]
Proenkephalin (PENK)
PENK is filtrated by the glomerulus and is a newer biomarker of glomerular filtration.[] PENK is a stable surrogate marker for endogenous enkephalins. It is a monomeric peptide with a molecular weight of approximately 4.5 kDa. PENK is cleaved from the precursor peptide pre-proenkephalin A alongside enkephalins, which are endogenous opioids. Unlike cystatin C, PENK is not influenced by inflammation, making it a more reliable indicator of glomerular filtration injury.[]
Most studies involving PENK have focused on sepsis. In this regard, three notable studies were conducted. The first study, involving 101 septic patients, demonstrated an association between PENK levels and the diagnosis and severity of AKI based on the RIFLE criterion.[] The second study, which evaluated a cohort of 167 patients, observed that PENK levels increased with the severity of sepsis and the diagnosis of AKI.[] In the third study, which had a larger cohort of 978 patients, PENK was found to be a reliable determinant of AKI, the need for renal replacement therapy (RRT), and mortality, with OR of 4.0 (95% confidence interval [CI] 3.0-5.4) and 1.5 (95% CI 1.2-1.8), respectively.[]
Do we have studies on the use of PENK in cirrhotic patients?
Patients with cirrhosis can present varying levels of inflammation. Unlike cystatin C, PENK is not influenced by inflammation, making it a promising biomarker for diagnosing AKI. The combined use of functional and structural markers, as recommended by the acute dialysis quality initiative (ADQI), can be particularly beneficial in identifying cases of subclinical AKI characterized by structural loss without functional impairment. In these cases, sCr alone may not be sufficient for diagnosis, but using biomarkers could be helpful. In a study conducted by Lima et al. in liver transplant patients, PENK, cystatin C, and the sCr were evaluated during the same period.[] PENK determined Subclinical AKI preoperatively in 32 (56%) patients. SCr and cystatin C were not useful in predicting severe AKI.[] However, PENK had an area under curve (AUC) of 0.83 for predicting severe AKI in 48 hours after LT and was independently associated with severe AKI in pre-operative. with OR 4.40 and OR 44.64 in the post-operative.[]
Therefore, further studies should explore the use of PENK in cirrhotic patients, particularly to assess its superiority over cystatin C, which has been considered the gold standard in cirrhosis. These investigations can provide additional insights into PENK’s effectiveness and clinical relevance as a biomarker in hepatic cirrhosis.
INJURY BIOMARKERS
We have previously seen that biomarkers such as PENK have not yet been analyzed in the context of liver disease. However, injury biomarkers, including cirrhotic patients, are the most studied in evaluating kidney function in various scenarios.
NGAL
In 1993, Kjeldsen et al. discovered a lipocalin covalently bound to neutrophil gelatinase and named it NGAL.[] NGAL is a 25-kilodalton protein covalently bound to gelatinase in neutrophil-specific granules. Although NGAL is expressed at very low levels in various human tissues, including the kidneys, trachea, lungs, stomach, and colon (adult and fetal).[] Its anti-inflammatory function is verified by increased expression in proliferative epithelia, areas of inflammation, and intestinal malignancy.[]
In normal kidneys, NGAL is only expressed in the distal tubules and the collecting ducts.[] While its physiological function in the kidneys is unknown, it is believed to play a role in renal morphogenesis.[] NGAL also has a significant role in regulating cell proliferation, repair processes, and tubular re-epithelization. This marker corresponds to an additional iron transport pathway, which increases the transcription of heme oxygenase. This enzyme protects and preserves proximal tubular cells with proliferative and anti-apoptotic effects.[]
However, some conditions can interfere with its performance, such as sepsis, chronic obstructive pulmonary disease, and cardiac dysfunction, which may act as confounding factors.[] The performance of NGAL seems to be influenced by age (higher prediction in children), sex, and baseline kidney function.[]
NGAL is the most useful biomarker in several scenarios; previous studies have reported the ability of NGAL to determine renal dysfunction in cirrhotic patients.[] A recent study by Gambino et al. suggests that urinary NGAL is an excellent biomarker for distinguishing between ATN-AKI and HRS-AKI. The differential diagnosis has important clinical implications due to using terlipressin and albumin in HRS-AKI.[] The best threshold for distinguishing ATN-AKI from other forms of AKI was 220 ng/mL, with a sensitivity of 89% and a specificity of 78%.[] However, it is important to note that the study did not compare NGAL with commonly used clinical markers such as FeNa and FeU, and the sample size was small (64 HRS-AKI and 27 ATN-AKI). Further validation through additional studies is necessary.
Previously, studies from our group have also demonstrated the ability of urinary and plasma NGAL in the diagnosis of severe AKI, the need for RRT, and mortality in LT.[]
Recent studies on the use of NGAL should focus on using this biomarker in combination with the standard sCr marker not only for the differential diagnosis of AKI but also for the diagnosis of subclinical AKI and its use in clinical practice as a guide for therapies, early RRT, and dual transplant.
Kidney injury of molecule-1 (KIM-1)
KIM-1, also known as T-cell immunoglobulin and mucin domain-1 (TIM-1), was originally identified as a hepatitis virus receptor (HAVCR-1), a type of transmembrane-1 protein, which is strongly expressed by renal insults of ischemic and/or toxic origin. It also plays several roles in T and B cells in cell biology.[]
Expression and structure data suggest that KIM-1 is an upregulated epithelial cell adhesion molecule in cells dedifferentiated in replication. KIM-1 has a role in restoring morphological integrity and function in the kidney after ischemia.[]
In ischemic AKI, apoptosis and loss of epithelial cells contribute to the obstruction of the tubule lumen. In this process, KIM-1 can recognize phagocyte dead cells, a receptor that recognizes phosphatidylserine in the apoptotic cells, directing them to the lysosomes. KIM-1 also serves as an oxidized lipoprotein receptor and is, therefore, suitable for cell recognition by apoptosis. KIM-1 is the only non-myeloid phosphatidylserine receptor that transforms epithelial cells into semiprofessional phagocytes.[]
In renal injury, KIM-1 is markedly upregulated in proximal renal tubule cells by stimuli that promote dedifferentiation, including ischemic or nephrotoxic injury. KIM-1 is also upregulated in cases of tubulointerstitial disease, polycystic kidney disease, and renal cell carcinoma, indicating its potential as a biomarker for these conditions.[]
What is the potential use of KIM-1 as a biomarker in cirrhotic patients?
KIM-1 has been studied as a possible biomarker in cirrhotic patients. It is believed that elevated levels of KIM-1 may indicate kidney damage associated with liver cirrhosis.[] Recent studies have found that as the eGFR levels decrease in cirrhosis patients, there is a significant increase in blood KIM-1 levels.[] Furthermore, the research shows that individuals with AKI caused by decompensated cirrhosis have notably higher levels of urinary KIM-1 compared to non-AKI and healthy control groups; the KIM-1 was also useful to the progression of AKI to stage 3.[] In the study by Humphreys et al. with mice, the expression of KIM-1 after renal injury has been reported to promote renal fibrosis and to provide a link between acute and recurrent lesions with progressive CKD.[]
IL-18
In 1989, IL-18 was first described as “inducing interferon-γ IFN-γ factor” and was isolated in the serum of mice after intraperitoneal injection of endotoxin. Years before, the mice had been pre-treated with Propionibacterium acnes, which stimulates the reticuloendothelial system, particularly the Kupffer cells of the liver. In 1995, the name was changed to IL-18.[]
IL-18 induces the synthesis and release of interferon-gamma and other inflammatory cytokines, such as IL-8, IL-4, and IL-13, and tumor necrosis factor. In this way, IL-18 modulates the action of several immunologically active cells: macrophages, monocytes, lymphocytes, and granulocytes. In addition, IL-18 can induce apoptosis. The precursor of IL-18 can be found in blood monocytes of healthy individuals and keratinocytes, epithelial cells throughout the gastrointestinal tract. Peritoneal and rat spleen macrophages also contain the precursor IL-18 in the absence of disease.[]
IL-18 is synthesized as a 23 kDa inactive precursor, expressed in the healthy kidney in the distal Nephron, specifically by the cells interspersed at the distal convoluted tubule, the connecting tubule, and the collecting duct. IL-18 is an early cascade component of inflammatory cytokines; its localization suggests that intercalated renal cells may contribute to the immediate immune response of the kidney. Ischemic injury and inflammatory conditions trigger the immature intracellular cleavage of IL-18 in its active form, which is then detected in the urine as an injury marker.[]
IL-18 has been extensively studied in different clinical settings: AKI, psoriasis, heart failure, inflammatory bowel disease, and diseases such as multiple sclerosis, acute respiratory distress syndrome, cardiac surgery, and metastatic melanoma.[]
Elevated levels of IL-18 have been observed in the serum of patients with primary biliary cholangitis, biliary atresia, and chronic liver disease of different etiologies. Additionally, these elevated levels of IL-18 are correlated with disease severity.[] IL-18 plays a significant role in the development of liver fibrosis by directly affecting the activation of hepatic stellate cells (HSCs); it can be identified as a potential target for treating liver fibrosis.[]
Despite not being a recent biomarker and having an important role in liver diseases, there are few studies on the scenario of cirrhosis and renal dysfunction. In the study conducted by Sirota et al.[] in 2013, IL-8 in plasma and IL-8, IL-18, and NGAL in the urine of 40 patients within 24 hours after liver transplantation were analyzed for the diagnosis of AKI. The result appeared favorable, with an AUC of 0.74 and P-value of 0.04 by IL-18.
CONCLUSION
In conclusion, the diagnosis of AKI in cirrhotic patients is challenging due to limitations such as altered volume status, malnutrition, and the use of diuretics that can affect sCr levels. Although several biomarkers have been studied to assist in the diagnosis of AKI in cirrhotic patients, few have been widely adopted. Cystatin C has shown superiority over sCr in determining GFR and is more precise in determining GFR in various groups of patients with liver cirrhosis. The combined evaluation of a panel of biomarkers allows the stratification in the diagnosis of AKI and the assessment of the site and type of injury. Accurate estimation of kidney function in cirrhosis is crucial for prognosis and decisions regarding dual-organ transplantation.
Author contributions
Lima C, Macedo E: Conceptualization, Investigation, Project administration, Validation, Visualization, Writing—Original draft, Writing—Review and Editing. All authors read and approved the final version of the manuscript.
Ethics approval
Not applicable.
Informed consent
Not applicable.
Source of funding
This work was carried out with financial support from the National Council for Scientific and Technological Development (CNPq), funding process 402189/2023-0.
Conflict of interest
The authors declare to have no competing interests.
Data availability statement
No additional data.
How to cite this article: Lima C, Macedo E. Biomarkers in acute kidney injury and cirrhosis. J Transl Crit Care Med. 2024;6:e23-00014. doi: 10.1097/JTCCM-D-23-00014
REFERENCES
1.
Ginès P, Krag A, Abraldes JG, Solà E, Fabrellas N, Kamath PS. Liver cirrhosis. Lancet. 2021;398(10308):1359-1376.2.
Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4(1):23.3.
Garcia-Tsao G, Parikh CR, Viola A. Acute kidney injury in cirrhosis. Hepatology. 2008;48(6):2064-2077.4.
de Carvalho JR, Villela-Nogueira CA, Luiz RR, et al. Acute kidney injury network criteria as a predictor of hospital mortality in cirrhotic patients with ascites. J Clin Gastroenterol. 2012;46(3):e21-e26.5.
Fagundes C, Barreto R, Guevara M, et al. A modified acute kidney injury classification for diagnosis and risk stratification of impairment of kidney function in cirrhosis. J Hepatol. 2013;59(3):474-481.6.
Warner NS, Cuthbert JA, Bhore R, Rockey DC. Acute kidney injury and chronic kidney disease in hospitalized patients with cirrhosis. J Investig Med. 2011;59(8):1244-1251.7.
Piano S, Rosi S, Maresio G, et al. Evaluation of the Acute Kidney Injury Network criteria in hospitalized patients with cirrhosis and ascites. J Hepatol. 2013;59(3):482-489.8.
Patidar KR, Naved MA, Grama A, et al. Acute kidney disease is common and associated with poor outcomes in patients with cirrhosis and acute kidney injury. J Hepatol. 2022;77(1):108-115.9.
Tonon M, Rosi S, Gambino CG, et al. Natural history of acute kidney disease in patients with cirrhosis. J Hepatol. 2021;74(3):578-583.10.
Bassegoda O, Huelin P, Ariza X, et al. Development of chronic kidney disease after acute kidney injury in patients with cirrhosis is common and impairs clinical outcomes. J Hepatol. 2020;72(6):1132-1139.11.
Formica RN, Aeder M, Boyle G, et al. Simultaneous liver-kidney allocation policy: a proposal to optimize appropriate utilization of scarce resources. Am J Transplant. 2016;16(3):758-766.12.
Chancharoenthana W, Leelahavanichkul A. Acute kidney injury spectrum in patients with chronic liver disease: where do we stand? World J Gastroenterol. 2019;25(28):3684-3703.13.
Khwaja A. KDIGO clinical practice guidelines for acute kidney injury. Nephron Clin Pract. 2012;120(4):c179-c184.14.
Angeli P, Gines P, Wong F, et al. Diagnosis and management of acute kidney injury in patients with cirrhosis: revised consensus recommendations of the International Club of Ascites. Gut. 2015;64(4):531-537.15.
Khatua CR, Sahu SK, Barik RK, et al. Validation of International Club of Ascites subclassification of stage 1 acute kidney injury in chronic liver disease. JGH Open. 2019;3(4):290-294.16.
Huelin P, Piano S, Solà E, et al. Validation of a staging system for acute kidney injury in patients with cirrhosis and association with acute-on-chronic liver failure. Clin Gastroenterol Hepatol. 2017;15(3):438-445.e5.17.
Nair S, Verma S, Thuluvath PJ. Pretransplant renal function predicts survival in patients undergoing orthotopic Liver Transplantation. Hepatology. 2002;35(5):1179-1185.18.
Yoshida S, Suda G, Ohara M, et al. Overestimated renal function in patients with liver cirrhosis predicts poor prognosis. Hepatol Res. 2022;52(7):603-613.19.
Cholongitas E, Marelli L, Kerry A, et al. Different methods of creatinine measurement significantly affect MELD scores. Liver Transpl. 2007;13(4):523-529.20.
Yong K, Dogra G, Boudville N, Pinder M, Lim W. Acute kidney injury: controversies revisited. Int J Nephrol. 2011; 2011:762634.21.
Nickolas TL, O’Rourke MJ, Yang J, et al. Sensitivity and specificity of a single emergency department measurement of urinary neutrophil gelatinase-associated lipocalin for diagnosing acute kidney injury. Ann Intern Med. 2008;148(11):810-819.22.
Maiwall R, Kumar A, Bhardwaj A, Kumar G, Bhadoria AS, Sarin SK. Cystatin C predicts acute kidney injury and mortality in cirrhotics: a prospective cohort study. Liver Int. 2018;38(4):654-664.23.
Slack A, Yeoman A, Wendon J. Renal dysfunction in chronic liver disease. Crit Care. 2010;14(2):214.24.
Ginès P, Solà E, Angeli P, Wong F, Nadim MK, Kamath PS. Hepatorenal syndrome. Nat Rev Dis Primers. 2018;4(1):23.25.
Musunuri B, Gopal S, Tantry BV, Shenoy S, Shetty AJ. Predictors of short-term mortality in patients of cirrhosis of liver presenting as acute kidney injury: an In-hospital prospective observational study. J Clin Exp Hepatol. 2023;13(6):989-996.26.
Bernardi M, Moreau R, Angeli P, Schnabl B, Arroyo V. Mechanisms of decompensation and organ failure in cirrhosis: from peripheral arterial vasodilation to systemic inflammation hypothesis. J Hepatol. 2015;63(5):1272-1284.27.
Biancofiore G, Critchley LA, Lee A, et al. Evaluation of a new software version of the FloTrac/Vigileo (version 3.02) and a comparison with previous data in cirrhotic patients undergoing Liver Transplant surgery. Anesth Analg. 2011;113(3):515-522.28.
Park SW, Kim M, Brown KM, D’Agati VD, Lee HT. Paneth cell-derived interleukin-17A causes multiorgan dysfunction after hepatic ischemia and reperfusion injury. Hepatology. 2011;53(5):1662-1675.29.
Nadim MK, Garcia-Tsao G. Acute kidney injury in patients with cirrhosis. N Engl J Med. 2023;388(8):733-745.30.
Gupta K, Bhurwal A, Law C, et al. Acute kidney injury and hepatorenal syndrome in cirrhosis. World J Gastroenterol. 2021;27(26):3984-4003.31.
Turgut F, Awad AS, Abdel-Rahman EM. Acute Kidney Injury: Medical Causes and Pathogenesis. J Clin Med. 2023;12(1):375.32.
Ronco C, Kellum JA, Haase M. Subclinical aki is still aki. Crit Care. 2012;16(3):313.33.
Kamath PS, Kim WR, Group ALDS. The model for end-stage liver disease (MELD). Hepatology. 2007;45(3):797-805.34.
UNOS. [homepage on the Internet]. Virginia: United Network for Organ Sharing. Accessed 23 June, 2023. http://www.unos.org35.
Inker LA, Eneanya ND, Coresh J, et al. New creatinine- and cystatin C-based equations to estimate GFR without race. N Engl J Med. 2021;385(19):1737-1749.36.
Asrani SK, Jennings LW, Trotter JF, et al. A model for glomerular filtration rate assessment in liver disease (GRAIL) in the presence of renal dysfunction. Hepatology. 2019;69(3):1219-1230.37.
Panchal S, Serper M, Bittermann T, Asrani SK, Goldberg DS, Mahmud N. Impact of race-adjusted glomerular filtration rate estimation on eligibility for simultaneous liver-kidney Transplantation. Liver Transpl. 2022;28(6):959-968.38.
Ostermann M, Zarbock A, Goldstein S, et al. Recommendations on acute kidney injury biomarkers from the acute disease quality initiative consensus conference: a consensus statement. JAMA Netw Open. 2020;3(10):e2019209.39.
Schneider AG, Bellomo R. Urinalysis and pre-renal acute kidney injury: time to move on. Crit Care. 2013;17(3):141.40.
Han WK, Bailly V, Abichandani R, Thadhani R, Bonventre JV. kidney injury molecule-1 (KIM-1):a novel biomarker for human renal proximal tubule injury. Kidney Int. 2002;62(1):237-244.41.
Parikh CR, Mishra J, Thiessen-Philbrook H, et al. Urinary IL-18 is an early predictive biomarker of acute kidney injury after cardiac surgery. Kidney Int. 2006;70(1):199-203.42.
Di Somma S, Magrini L, De Berardinis B, et al. Additive value of blood neutrophil gelatinase-associated lipocalin to clinical judgement in acute kidney injury diagnosis and mortality prediction in patients hospitalized from the emergency department. Crit Care. 2013;17(1):R29.43.
Bennett M, Dent CL, Ma Q, et al. Urine NGAL predicts severity of acute kidney injury after cardiac surgery: a prospective study. Clin J Am Soc Nephrol. 2008;3(3):665-673.44.
Lima C, de Paiva Haddad LB, de Melo PDV, et al. Early detection of acute kidney injury in the perioperative period of Liver Transplant with neutrophil gelatinase-associated lipocalin. BMC Nephrol. 2019;20(1):367.45.
Hall IE, Yarlagadda SG, Coca SG, et al. IL-18 and urinary NGAL predict dialysis and graft recovery after kidney Transplantation. J Am Soc Nephrol. 2010;21(1):189-197.46.
Jia HM, Huang LF, Zheng Y, Li WX. Diagnostic value of urinary tissue inhibitor of metalloproteinase-2 and insulin-like growth factor binding protein 7 for acute kidney injury: a meta-analysis. Crit Care. 2017;21(1):77.47.
Bargnoux AS, Piéroni L, Cristol JP. Analytical study of a new turbidimetric assay for urinary neutrophil gelatinase-associated lipocalin (NGAL) determination. Clin Chem Lab Med. 2013;51(12):e293-e296.48.
Westhoff JH, Tönshoff B, Waldherr S, et al. Urinary tissue inhibitor of metalloproteinase-2 (TIMP-2) • insulin-like growth factor-binding protein 7 (IGFBP7) predicts adverse outcome in pediatric acute kidney injury. PLoS One. 2015;10(11):e0143628.49.
Oh DJ. A long journey for acute kidney injury biomarkers. Ren Fail. 2020;42(1):154-165.50.
Bera C, Wong F. Management of hepatorenal syndrome in liver cirrhosis: a recent update. Therap Adv Gastroenterol. 2022;15:17562848221102679.51.
Singapura P, Ma TW, Sarmast N, et al. Estimating glomerular filtration rate in cirrhosis using creatinine-based and cystatin C-based equations: systematic review and meta-analysis. Liver Transpl. 2021;27(11):1538-1552.52.
Clausen J. Proteins in normal cerebrospinal fluid not found in serum. Proc Soc Exp Biol Med. 1961;107:170-172.53.
Butler EA, Flynn FV. The occurrence of post-gamma protein in urine: a new protein abnormality. J Clin Pathol. 1961;14(2):172-178.54.
Barrett AJ, Davies ME, Grubb A. The place of human gamma-trace (cystatin C) amongst the cysteine proteinase inhibitors. Biochem Biophys Res Commun. 1984;120(2):631-636.55.
Filler G, Bökenkamp A, Hofmann W, Le Bricon T, Martínez-Brú C, Grubb A. Cystatin C as a marker of GFR—history, indications, and future research. Clin Biochem. 2005;38(1):1-8.56.
Finney H, Newman DJ, Price CP. Adult reference ranges for serum cystatin C, creatinine and predicted creatinine clearance. Ann Clin Biochem. 2000;37 (Pt 1):49-59.57.
Risch L, Herklotz R, Blumberg A, Huber AR. Effects of glucocorticoid immunosuppression on serum cystatin C concentrations in renal transplant patients. Clin Chem. 2001;47(11):2055-2059.58.
Bökenkamp A, van Wijk JA, Lentze MJ, Stoffel-Wagner B. Effect of corticosteroid therapy on serum cystatin C and β2-microglobulin concentrations. Clin Chem. 2002;48(7):1123-1126.59.
Fricker M, Wiesli P, Brändle M, Schwegler B, Schmid C. Impact of thyroid dysfunction on serum cystatin C. Kidney Int. 2003;63(5):1944-1947.60.
den Hollander JG, Wulkan RW, Mantel MJ, Berghout A. Is cystatin C a marker of glomerular filtration rate in thyroid dysfunction? Clin Chem. 2003;49(9):1558-1559.61.
Manetti L, Pardini E, Genovesi M, et al. Thyroid function differently affects serum cystatin C and creatinine concentrations. J Endocrinol Invest. 2005;28(4):346-349.62.
Trimarchi H, Muryan A, Martino D, et al. Creatinine- vs. cystatin C-based equations compared with 99mTcDTPA scintigraphy to assess glomerular filtration rate in chronic kidney disease. J Nephrol. 2012;25(6):1003-1015.63.
Herget-Rosenthal S, Bökenkamp A, Hofmann W. How to estimate GFR-serum creatinine, serum cystatin C or equations? Clin Biochem. 2007;40(3/4):153-161.64.
Velayudham B, Thomas RG, Vasudevan C, Senthilkumar RP, Thirumalvalavan, Murugesan. Serum cystatin C unmasks renal dysfunction in cirrhosis and performs better in estimation of glomerular filtration rate. Saudi J Kidney Dis Transpl. 2020;31(6):1320-1330.65.
De Souza V, Hadj-Aissa A, Dolomanova O, et al. Creatinine- versus cystatine C-based equations in assessing the renal function of candidates for Liver Transplantation with cirrhosis. Hepatology. 2014;59(4):1522-1531.66.
Omar M, Abdel-Razek W, Abo-Raia G, Assem M, El-Azab G. Evaluation of serum cystatin C as a marker of early renal impairment in patients with liver cirrhosis. Int J Hepatol. 2015;2015:309042.67.
Rognant N, Lemoine S. Evaluation of renal function in patients with cirrhosis: where are we now? World J Gastroenterol. 2014;20(10):2533-2541.68.
Ahn HS, Kim YS, Kim SG, et al. Cystatin C is a good predictor of hepatorenal syndrome and survival in patients with cirrhosis who have normal serum creatinine levels. Hepato-gastroenterology. 2012;59(116):1168-1173.69.
Sharawey MA, Shawky EM, Ali LH, Mohammed AA, Hassan HA, Fouad YM. Cystatin C: a predictor of hepatorenal syndrome in patients with liver cirrhosis. Hepatol Int. 2011;5(4):927-933.70.
Padia G, Mahajan B, Kumar A, et al. Cystatin C and interleukin-6 for prognosticating patients with liver cirrhosis and acute kidney injury for renal replacement therapy: A prospective study. Indian J Crit Care Med. 2021;25(6):658-663.71.
Wan ZH, Wang JJ, You SL, et al. Cystatin C is a biomarker for predicting acute kidney injury in patients with acute-on-chronic liver failure. World J Gastroenterol. 2013;19(48):9432-9438.72.
Markwardt D, Holdt L, Steib C, et al. Plasma cystatin C is a predictor of renal dysfunction, acute-on-chronic liver failure, and mortality in patients with acutely decompensated liver cirrhosis. Hepatology. 2017;66(4):1232-1241.73.
Barreto EF, Rule AD, Murad MH, et al. Prediction of the renal elimination of drugs with cystatin C vs creatinine: a systematic review. Mayo Clin Proc. 2019;94(3):500-514.74.
Moghaddam SN, Ganji MR, Kochari MR, Tofangchiha S. Serum cystatin-C is not superior to serum creatinine in predicting glomerular filtration rate in cirrhotic patients. Middle East J Dig Dis. 2013;5(4):209-216.75.
Oddoze C, Morange S, Portugal H, Berland Y, Dussol B. Cystatin C is not more sensitive than creatinine for detecting early renal impairment in patients with diabetes. Am J Kidney Dis. 2001;38(2):310-316.76.
Schück O, Teplan V, Jabor A, Stollová M, Bláha V. Comparison of serum cystatin C and creatinine in patients with liver cirrhosis. Biomed Pap Med Fac Univ Palacky Olomouc Czech Repub. 2004;148(1):25-28.77.
Delanaye P, Nellessen E, Cavalier E, et al. Is cystatin C useful for the detection and the estimation of low glomerular filtration rate in heart transplant patients? Transplantation. 2007;83(5):641-644.78.
Cimerman N, Brguljan PM, Krasovec M, Suskovic S, Kos J. Serum cystatin C, a potent inhibitor of cysteine proteinases, is elevated in asthmatic patients. Clin Chim Acta. 2000;300(1/2):83-95.79.
Macedo E, Lima C. Comprehensive assessment of kidney health in acute kidney injury: can it be achieved? Nephron. 2019;143(3):188-192.80.
Denning GM, Ackermann LW, Barna TJ, et al. Proenkephalin expression and enkephalin release are widely observed in non-neuronal tissues. Peptides. 2008;29(1):83-92.81.
Slominski AT, Zmijewski MA, Zbytek B, et al. Regulated proenkephalin expression in human skin and cultured skin cells. J Invest Dermatol. 2011;131(3):613-622.82.
Marino R, Struck J, Hartmann O, et al. Diagnostic and short-term prognostic utility of plasma pro-enkephalin (pro-ENK) for acute kidney injury in patients admitted with sepsis in the emergency department. J Nephrol. 2015;28(6):717-724.83.
Kim H, Hur M, Lee S, et al. Proenkephalin, neutrophil gelatinase-associated lipocalin, and estimated glomerular filtration rates in patients with sepsis. Ann Lab Med. 2017;37(5):388-397.84.
Hollinger A, Wittebole X, François B, et al. Proenkephalin A 119-159 (penkid) is an early biomarker of septic acute kidney injury: the kidney in sepsis and septic shock (kid-SSS) study. Kidney Int Rep. 2018;3(6):1424-1433.85.
Lima C, Gorab DL, Fernandes CR, Macedo E. Role of proenkephalin in the diagnosis of severe and subclinical acute kidney injury during the perioperative period of liver transplantation. Pract Lab Med. 2022;31:e00278.86.
Kjeldsen L, Johnsen AH, Sengelov H, Borregaard N. Isolation and primary structure of NGAL, a novel protein associated with human neutrophil gelatinase. J Biol Chem. 1993;268(14):10425-10432.87.
Cowland JB, Borregaard N. Molecular characterization and pattern of tissue expression of the gene for neutrophil gelatinase-associated lipocalin from humans. Genomics. 1997;45(1):17-23.88.
Nielsen BS, Borregaard N, Bundgaard JR, Timshel S, Sehested M, Kjeldsen L. Induction of NGAL synthesis in epithelial cells of human colorectal neoplasia and inflammatory bowel diseases. Gut. 1996;38(3):414-420.89.
Schmidt-Ott KM, Chen X, Paragas N, Levinson RS, Mendelsohn CL, Barasch J. C-kit delineates a distinct domain of progenitors in the developing kidney. Dev Biol. 2006;299(1):238-249.90.
Mori K, Lee HT, Rapoport D, et al. Endocytic delivery of lipocalin-siderophore-iron complex rescues the kidney from ischemia-reperfusion injury. J Clin Invest. 2005;115(3):610-621.91.
Yang J, Goetz D, Li JY, et al. An iron delivery pathway mediated by a lipocalin. Mol Cell. 2002;10(5):1045-1056.92.
Marakala V. Neutrophil gelatinase-associated lipocalin (NGAL) in kidney injury-A systematic review. Clin Chim Acta. 2022;536:135-141.93.
Legrand M, Darmon M, Joannidis M. NGAL and AKI: the end of a myth? Intensive Care Med. 2013;39(10):1861-1863.94.
Huelin P, Solà E, Elia C, et al. Neutrophil gelatinase-associated lipocalin for assessment of acute kidney injury in cirrhosis: a prospective study. Hepatology. 2019;70(1):319-333.95.
Fagundes C, Pépin MN, Guevara M, et al. Urinary neutrophil gelatinase-associated lipocalin as biomarker in the differential diagnosis of impairment of kidney function in cirrhosis. J Hepatol. 2012;57(2):267-273.96.
Verna EC, Brown RS, Farrand E, et al. Urinary neutrophil gelatinase-associated lipocalin predicts mortality and identifies acute kidney injury in cirrhosis. Dig Dis Sci. 2012;57(9):2362-2370.97.
Belcher JM, Sanyal AJ, Peixoto AJ, et al. Kidney biomarkers and differential diagnosis of patients with cirrhosis and acute kidney injury. Hepatology. 2014;60(2):622-632.98.
Gambino C, Piano S, Stenico M, et al. Diagnostic and prognostic performance of urinary neutrophil gelatinase-associated lipocalin in patients with cirrhosis and acute kidney injury. Hepatology. 2023;77(5):1630-1638.99.
Rennert PD. Novel roles for TIM-1 in immunity and infection. Immunol Lett. 2011;141(1):28-35.100.
Mukherjea D, Whitworth CA, Nandish S, Dunaway GA, Rybak LP, Ramkumar V. Expression of the kidney injury molecule 1 in the rat cochlea and induction by cisplatin. Neuroscience. 2006;139(2):733-740.101.
Ichimura T, Bonventre JV, Bailly V, et al. Kidney injury molecule-1 (KIM-1), a putative epithelial cell adhesion molecule containing a novel immunoglobulin domain, is up-regulated in renal cells after injury. J Biol Chem. 1998;273(7):4135-4142.102.
Ichimura T, Asseldonk EJPV, Humphreys BD, Gunaratnam L, Duffield JS, Bonventre JV. Kidney injury molecule-1 is a phosphatidylserine receptor that confers a phagocytic phenotype on epithelial cells. J Clin Invest. 2008;118(5):1657-1668.103.
Savill J, Fadok V. Corpse clearance defines the meaning of cell death. Nature. 2000;407:784-788.104.
Zhang Z, Humphreys BD, Bonventre JV. Shedding of the urinary biomarker kidney injury molecule-1 (KIM-1) is regulated by MAP kinases and juxtamembrane region. J Am Soc Nephrol. 2007;18(10):2704-2714.105.
Karmakova TA, Sergeeva NS, Kanukoev KY, Alekseev BY, Kaprin AD. Kidney injury molecule-1 predicts mortality and renal replacement therapy in patients with cirrhosis and bacterial infections. J Hepatol. 2016;65(2):323-329.106.
Lei L, Li LP, Zeng Z, et al. Value of urinary KIM-1 and NGAL combined with serum Cys C for predicting acute kidney injury secondary to decompensated cirrhosis. Sci Rep. 2018;8(1):7962.107.
Humphreys BD, Xu F, Sabbisetti V, et al. Chronic epithelial kidney injury molecule-1 expression causes murine kidney fibrosis. J Clin Invest. 2013;123(9):4023-4035.108.
Okamura H, Nagata K, Komatsu T, et al. A novel costimulatory factor for gamma interferon induction found in the livers of mice causes endotoxic shock. Infect Immun. 1995;63(10):3966-3972.109.
Ix JH, Shlipak MG. The promise of tubule biomarkers in kidney disease: a review. Am J Kidney Dis. 2021;78(5):719-727.110.
Puren AJ, Fantuzzi G, Dinarello CA. Gene expression, synthesis, and secretion of interleukin 18 and interleukin 1beta are differentially regulated in human blood mononuclear cells and mouse spleen cells. Proc Natl Acad Sci USA. 1999;96(5):2256-2261.111.
Yazigi F, Romanelli MF, McCullough PA. Which marker should we use to assess acute renal injury? Cleve Clin J Med. 2011;78(4):237-245.112.
Gauer S, Sichler O, Obermüller N, et al. IL-18 is expressed in the intercalated cell of human kidney. Kidney Int. 2007;72(9):1081-1087.113.
Dinarello CA, Novick D, Kim S, Kaplanski G. Interleukin-18 and IL-18 binding protein. Front Immunol. 2013;4:289.114.
Parikh CR, Abraham E, Ancukiewicz M, Edelstein CL. Urine IL-18 is an early diagnostic marker for acute kidney injury and predicts mortality in the intensive care unit. J Am Soc Nephrol. 2005;16(10):3046-3052.115.
Haase M, Bellomo R, Story D, Davenport P, Haase-Fielitz A. Urinary interleukin-18 does not predict acute kidney injury after adult cardiac surgery: a prospective observational cohort study. Crit Care. 2008;12(4):R96.116.
Yamano T, Higashi T, Nouso K, et al. Serum interferon-gamma-inducing factor/IL-18 levels in primary biliary cirrhosis. Clin Exp Immunol. 2000;122(2):227-231.117.
Urushihara N, Iwagaki H, Yagi T, et al. Elevation of serum interleukin-18 levels and activation of Kupffer cells in biliary atresia. J Pediatr Surg. 2000;35(3):446-449.118.
Ludwiczek O, Kaser A, Novick D, et al. Plasma levels of interleukin-18 and interleukin-18 binding protein are elevated in patients with chronic liver disease. J Clin Immunol. 2002;22(6):331-337.119.
Knorr J, Kaufmann B, Inzaugarat ME, et al. Interleukin-18 signaling promotes activation of hepatic stellate cells in mouse liver fibrosis. Hepatology. 2023;77(6):1968-1982.120.
Sirota JC, Walcher A, Faubel S, et al. Urine IL-18, NGAL, IL-8 and serum IL-8 are biomarkers of acute kidney injury following liver transplantation. BMC Nephrol. 2013;14:17.
