Introduction
Immune thrombocytopenia (ITP) is an autoimmune bleeding disorder, characterized by abnormally low levels of platelets (below 100 × 109/L) [, ]. Bleeding diathesis is the most common clinical sign of ITP, leading to substantial morbidity and mortality. The annual incidence of ITP was estimated at 3.3 cases per 100,000 adults, seriously affecting people’s quality of life []. Effective treatment aiming at achieving an adequate platelet count is an important measure to improve outcomes in adult patients with ITP.
Current first-line therapies for adult ITP include corticosteroids, intravenous immunoglobulin, and anti-D []. However, relapse and adverse events (AEs) are common in patients with these drugs []. Recently, thrombopoietin receptor agonists (TPO-RAs) have been actively used as the second-line therapy to stimulate platelet production and reduce the risk of bleeding. At present, five kinds of TPO-RAs are approved for the treatment of adult ITP, including eltrombopag, romiplostim, avatrombopag, recombinant human thrombopoietin (rhTPO), and hetrombopag. Eltrombopag is the first oral, small-molecule, non-peptide TPO-RA and was approved by the US Food and Drug Administration (FDA) in 2008. This drug can increase platelet counts after 8 days of daily oral dosing, with levels returning to baseline 12 days after the last dose []. Romiplostim is a recombinant fusion polypeptide administered weekly via subcutaneous injection [] and has been approved by the US FDA since 2008. However, eltrombopag carries the black box of hepatotoxicity, and romiplostim is subcutaneously injected that limits its convenience and compliance. Hence, a novel TPO-RA is in urgent need. Avatrombopag is an oral small-molecule, non-peptide TPO-RA that mimics the biological effects of TPO on platelet production without hepatotoxicity [] and was approved by the US FDA in 2018. The rhTPO is a full-length and glycosylated TPO developed by Chinese hamster ovary cells []. Hetrombopag is a novel, oral small-molecule, non-peptide TPO-RA produced by the structural modification of eltrombopag to enhance potency and minimize toxicity []. rhTPO and hetrombopag were approved by NMPA as a second-line therapy for ITP. Many randomized placebo-controlled trials have demonstrated that these TPO-RAs had highly effectiveness in increasing platelet count with a low rate of adverse events. However, the best approach for the treatment of adult ITP remains unclear because it is virtually impossible to perform head-to-head comparisons of all therapies in randomized controlled trials (RCTs).
Network meta-analysis (NMA) is a recognized method that extends a conventional pairwise meta-analysis to allow integrated analysis of three or more treatments []. To date, several studies have assessed the efficacy and safety of several options in adults with thrombocytopenia via NMA [-]. However, none of these studies included hetrombopag, and these studies were not focused on ITP or TPO-RA. In addition, JZ Deng analyzed the combined therapy of rhTPO with rituximab rather than monotherapy [], this would lead to overestimating the efficacy of rhTPO. Moreover, in terms of safety, all of these NMAs only compared the incidence of adverse events (AEs) of these treatment options, not treatment-related adverse events (TRAEs). However, incidence of AEs is generally affected by the external environment, patients’ personal characteristics, length of follow-up, etc. [, ]. TRAEs that have causal relationships with the drug can more accurately and objectively reflect the safety of drugs.
Therefore, in this study, we assessed the comparative efficacy and safety of different TPO-RAs (eltrombopag, romiplostim, avatrombopag, rhTPO, and hetrombopag) for adult ITP as the second-line therapy. We ranked the efficacy on increasing the platelet count, and the safety about TRAEs of each treatment to help establish evidence-based hierarchies.
Materials and Methods
We conducted a literature search to identify all published RCTs based on the search strategies suggested in the Cochrane Handbook for Systematic Reviews of Interventions. This review complied with preferred reporting items for systematic review and meta-analysis (PRISMA) []. A systematic literature search was to establish a clinically relevant ranking of the efficacy and safety of TPO-RAs for adults (≥18 years old) with ITP. The literature searching strategy and searching process was performed by two authors independently, and articles with different opinions were discussed to decide. The study was registered with INPLASY (No.: INPLASY2021120054).
Search Strategy
The electronic databases PubMed, Web of Science, and Embase were searched for publications listed between each database’s inception date and June 1, 2022. The search terms and MeSH used were mainly “avatrombopag,” “eltrombopag,” “romiplostim,” “hetrombopag,” “rhTPO,” “TPO-RA,” “Thrombopoiesis,” “thrombopoie,” “thrombocytopenia,” “TPO,” and “thrombopoietin receptor agonists.” More detailed terms were listed in online supplementary 1 (for all online suppl. material, see http://www.karger.com/doi/10.1159/000528642).
Study Selection
Studies that met all of the following criteria were included: (1) The studies were published RCTs, comprising any of the following interventions: avatrombopag, eltrombopag, romiplostim, hetrombopag, and rhTPO; (2) patients were adults (≥18 years old) with ITP; and (3) studies revealed at least one of the following two outcomes: the number of patients who achieved platelet response (platelet counts ≥50 × 109/L) as originally defined by each study, and TRAEs. Studies were excluded if (1) studies were reviews, meeting summaries, letters, etc.; (2) there was missing or incomplete information on the trial; and (3) patients received combined therapy for ITP, such as rhTPO with rituximab.
Data Extraction
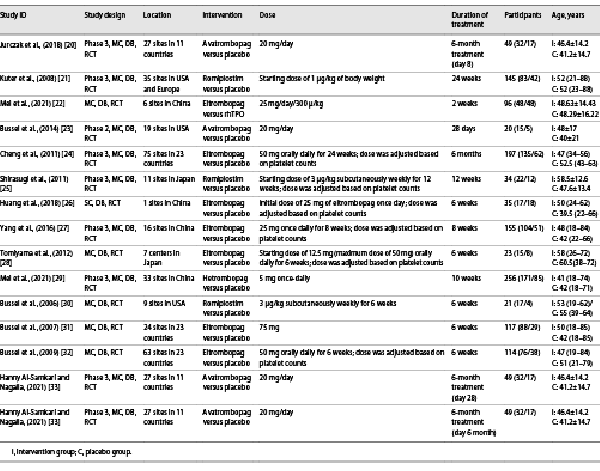
Two authors extracted data by screening the titles and abstracts; in case of disagreement, consensus was reached through discussion. We extracted the study design, location, interventions (dose, duration of treatment), age, baseline PLT, and outcome (platelet response rate, TRAEs rate). In one study, if several dose groups were set for the same therapy, only the dose group with the highest platelet response rate was included. We summarized all the results for interventions by different assessments in each eligible research.
Quality Assessment
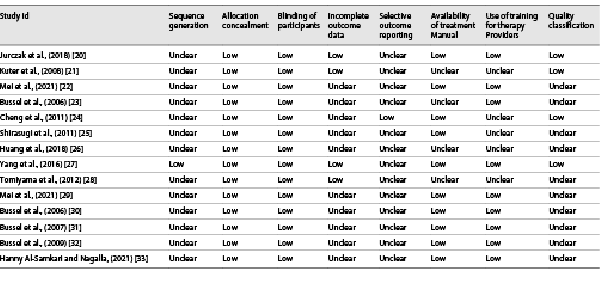
Study quality was assessed using adapted Cochrane risk-of-bias variables []. We evaluated the following aspects for quality assessment: sequence generation, allocation concealment, blinding (participants), incomplete outcome data, selective outcome reporting, availability of treatment manual, and use of training for therapy providers. Each item was scored as high (−1), unclear (0), or low (1) risk of bias. Overall, study quality was scored from 0 to 7, with a higher score indicating a lower risk of bias []. “Low risk of bias” means plausible bias unlikely to seriously alter the results (score: ≥4). “Unclear risk of bias” means plausible bias that raises some doubt about the results (score: −4 to 4). “High risk of bias” means plausible bias that seriously weakens confidence in the results (score: ≤−4). Contradiction in quality assessment was resolved through discussion.
Statistical Analysis
A network meta-analysis was done under the frequentist framework, obtaining pairwise odds ratios (ORs) and 95% confidence intervals (CIs) for platelet response rate and TRAEs rate. Heterogeneities were assessed by Wald χ2, with a p value > 0.05 considered as no heterogeneity, and the fixed-effects model was adopted; otherwise, a random-effects model with restricted maximum likelihood variance estimation was used. The funnel plot and Egger’s test of the intercept were employed to assess indications of publication bias. The surface under the cumulative ranking (SUCRA) was used to rank the included therapies for each outcome. Higher SUCRA scores correlate with better efficacy, whereas lower SUCRA scores correlate with better safety. We performed meta-analyses using Stata 13 software (StataCorp., College Station, TX, USA).
Results
Study Selection and Characteristics
Database searching produced a total of 5,193 citations, of which 3,876 remained after the duplicates were removed. A total of 123 articles were pulled for full-text articles, and 109 were excluded for different reasons (no relevant outcomes or non-RCT studies). See details in Figure 1.
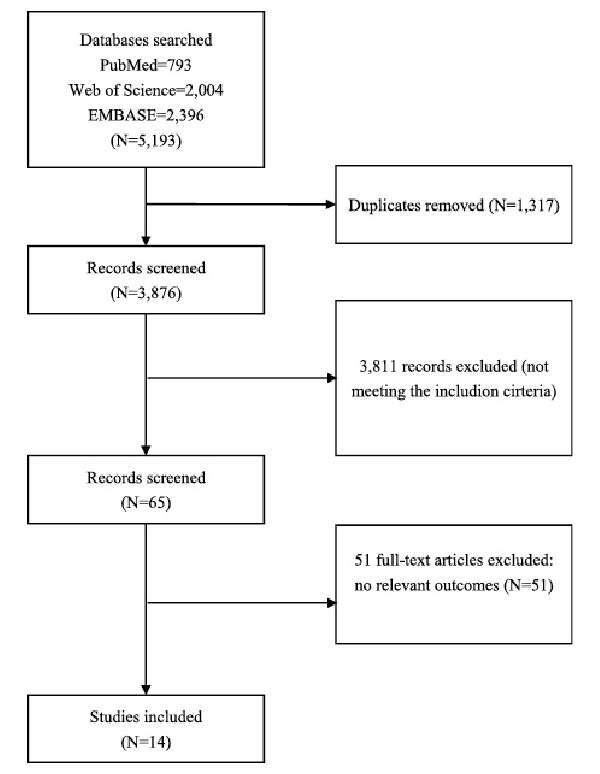
Fig. 1
Flowchart showing the search and study selection process for literature.
Ultimately, 1,360 participants reported in 14 unique publications [-] were available for the network meta-analysis (Table 1). As Table 1 showed, included studies were published between 2006 and 2021. Four interventional arms were included as follows: seven with eltrombopag, three with romiplostim, three studies with avatrombopag, and one with hetrombopag. Regarding the control arm, placebo was used in all RCTs except in one study that selected rhTPO as the control. Among the included literatures, the study with the shortest intervention duration was 2 weeks, and the longest intervention duration was 6 months. The dosage groups with the best effect were included in this study.
Quality Assessment of the Included Studies
Table 2 presented information on quality assessment. Sequence generation was described in detail for all RCTs, and the method of allocation concealment was described in seven RCTs. Most RCTs showed a low risk of bias because their protocols and outcomes were well described in each study. Most items were assessed as unclear sources of bias because of insufficient information. None of the studies met all quality criteria.
Outcomes
Platelet Response
Platelet response (platelet counts ≥50 × 109/L) during therapeutic or observational period was regarded as a dichotomous outcome. As many as six treatment arms were included in this analysis (Fig. 2a), and the analysis comprised four direct comparisons among five treatments. There was a significant inconsistency between the NMA (χ2 = 234.76, p < 0.001). For all relative treatment comparisons, avatrombopag (OR, 89.27; 95% CI: 22.56–353.28), romiplostim (OR, 51.10; 95% CI: 11.72–222.81), hetrombopag (28.85; 95% CI: 11.09–75.06), and eltrombopag (OR, 12.04; 95% CI: 7.56–19.18) showed a significantly better platelet response than the placebo. No significant differences were observed among avatrombopag and romiplostim, hetrombopag, rhTPO. Avatrombopag (OR, 7.42; 95% CI: 1.74–31.69) and rhTPO (OR, 3.86; 95% CI: 1.62–9.18) showed a better platelet response than eltrombopag with significance (Fig. 3a).
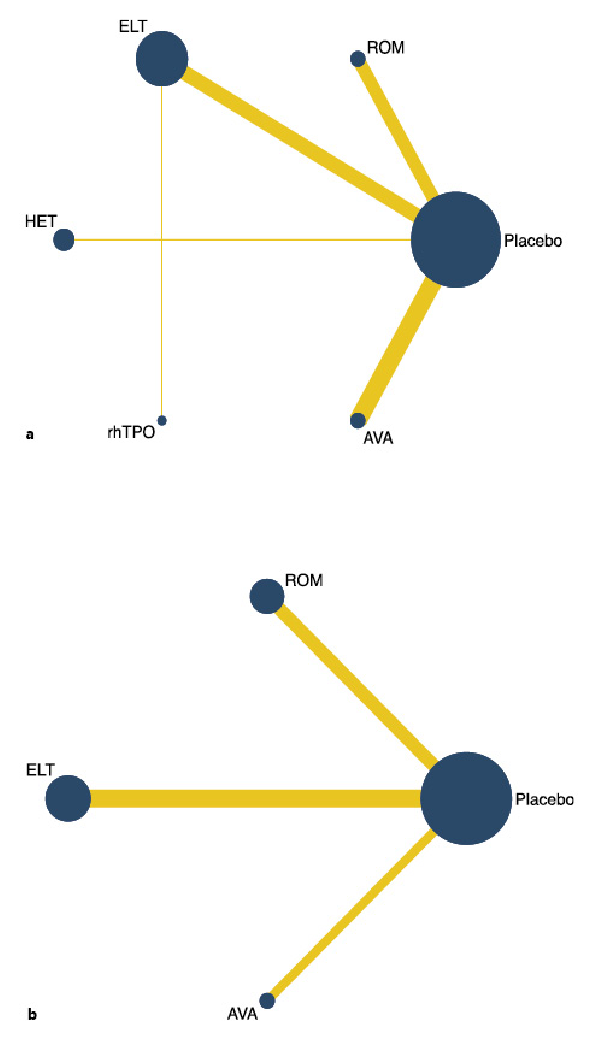
Fig. 2
Network map for all outcomes. a Platelet response. b Treatment-related adverse events. ELT, eltrombopag; ROM, romiplostim; AVA, avatrombopag; rhTPO, recombinant human thrombopoietin; HET, hetrombopag.
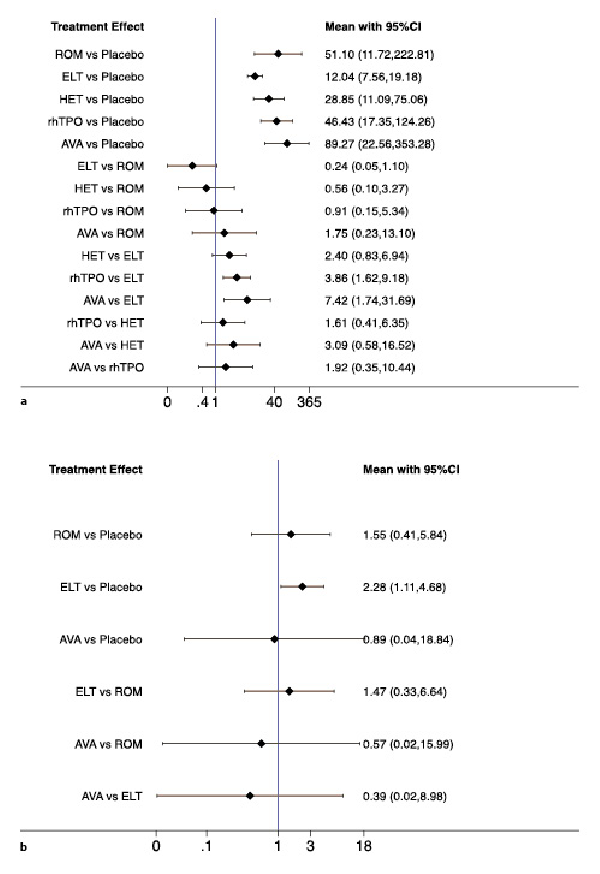
Fig. 3
Forest plot of the studies included. a Platelet response. b Treatment-related adverse events. ELT, eltrombopag; ROM, romiplostim; AVA, avatrombopag; rhTPO, recombinant human thrombopoietin; HET, hetrombopag.
Avatrombopag was ranked as the best treatment for platelet response according to its SUCRA value of 87.5, followed by romiplostim (71.1), rhTPO (68.8), hetrombopag (50.7), eltrombopag (21.9), and placebo (0.0) (Fig. 4a, 5a). These data indicate that the patients had the highest probability of achieving PR when treated with avatrombopag.
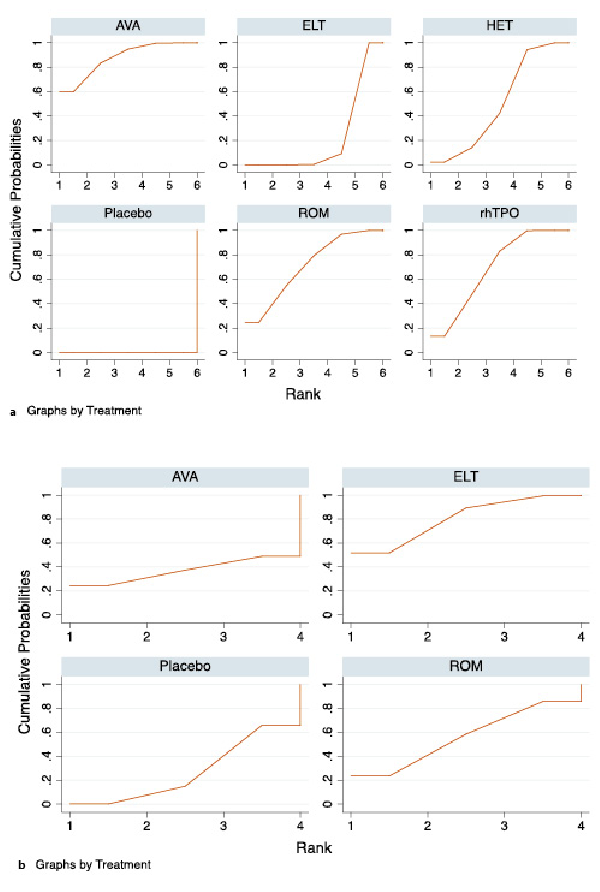
Fig. 4
The surface under the cumulative ranking curve (SUCRA) is shown for each treatment. a Platelet response. b Treatment-related adverse events. ELT, eltrombopag; ROM, romiplostim; AVA, avatrombopag; rhTPO, recombinant human thrombopoietin; HET, hetrombopag.
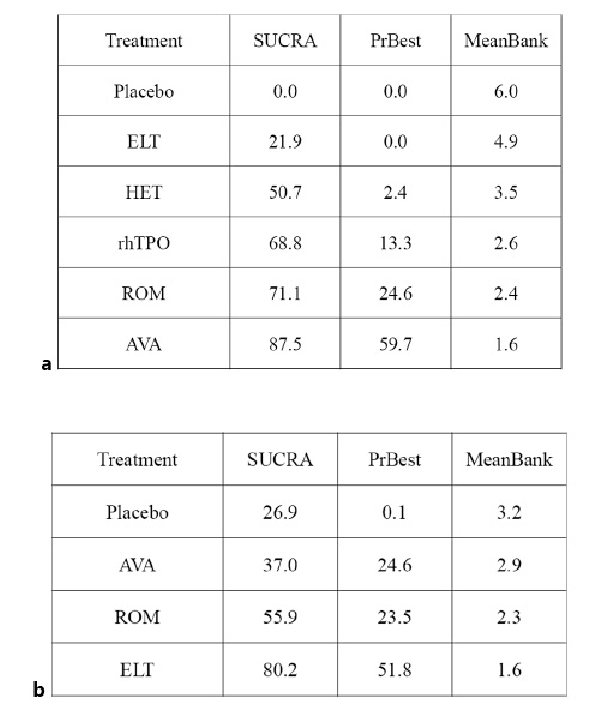
Fig. 5
SUCRA rank of each intervention. a Platelet response. b Treatment-related adverse events. ELT, eltrombopag; ROM, romiplostim; AVA, avatrombopag; rhTPO, recombinant human thrombopoietin; HET, hetrombopag.
Treatment-Related Adverse Events
In total, six studies included data regarding TRAEs. There was no evidence of inconsistencies (χ2 = 5.48, p < 0.140). These comprised three direct comparisons among the three treatments (Fig. 2b). All possible pairwise comparisons were made (Fig. 3b), and the pooled data showed no significant differences in TRAEs between patients receiving the three types of interventions. The SUCRA rankings revealed that avatrombopag carried the lowest TRAEs risk (37.0), whereas eltrombopag carried the highest risk (80.2). Additionally, romiplostim (55.9) was associated with a modest risk (Fig. 4b, 5b).
Discussion
This systematic review and network meta-analysis on the efficacy and safety of five TPO-RAs for ITP included 14 trials with 1,360 randomly assigned participants. Avatrombopag may be the better choice as a second-line treatment for adult ITP among the TPO-RAs, with the highest platelet response and lower risk of TRAEs. Eltrombopag appeared to be the treatment with the less platelet response and a higher risk of TRAEs.
Platelet response rate has been the most commonly used primary outcome of response to ITP treatment, which is objectively and easily compared [, ]. In this study, all TPO-RAs had a better platelet response than the placebo. Avatrombopag ranked as the most effective option, followed by romiplostim. Eltrombopag had the lowest platelet response probability. The differences in clinical efficacy of the TPO-RAs may be related to their pharmacological mechanisms. Eltrombopag is the first orally self-administered, small-molecule, non-peptide TPO-RA for ITP, but it interacts with metal iron and cannot be taken with food. Concomitant administration of eltrombopag with high-calcium food or an antacid containing aluminum and magnesium was associated with significantly reduced systemic exposure, and then leading to a low clinical efficacy []. Hetrombopag has the same situation with eltrombopag, but avatrombopag and romiplostim have no drug-food interaction. Although there is no significant difference between avatrombopag and romiplostim in platelet response rate, avatrombopag is preferred because it is orally taken and more convenient. In addition, Ran Yang et al. [] found avatrombopag produced the highest SUCRA value for early response, followed by romiplostim and eltrombopag. Therefore, in terms of efficacy, avatrombopag may be the most reasonable TPO-RA as the second-line therapy for adult ITP.
In terms of safety, previous meta-analyses and network meta-analyses have compared incidence of AEs of these TPO-RAs, but few studies have compared incidence of TRAEs of TPO-RAs for ITP. In this study, we compared the incidence of TRAEs among avatrombopag, eltrombopag, romiplostim, and placebo, and no significant difference was found except for the comparison between eltrombopag and placebo. Further SUCRA analysis showed that avatrombopag had the lowest risk of TRAEs among the three TPO-RAs. In addition, hepatic toxicity events, such as increased ALT concentration and increased AST concentration, were found in patients receiving eltrombopag [, , , ]. There is no study to report TRAEs of hetrombopag or rhTPO monotherapy in treating ITP, but hetrombopag is a TPO-RA produced by the structural modification of eltrombopag to enhance potency and minimize toxicity []. Thus, it can be inferred that the incidence of TRAEs of hetrombopag is not higher than that of eltrombopag. However, hetrombopag cannot totally avoid the hepatic toxicity of eltrombopag, and the hepatic toxicity rate of hetrombopag increases as the drug-taken time is prolonged []. One RCT demonstrated that rhTPO combined with danazol was well tolerated, with an incidence of TRAEs of 13.6% at a 28-day follow-up []. Therefore, it can be believed that most TPO-RAs are tolerated, and eltrombopag may have the highest risk of TRAEs.
Even though TPO-RAs were determined to be effective as the second-line treatment for adult ITP, the price of these drugs is relatively expensive, which led to a limited and relatively later uptake. Although most TPO-RAs have been listed in the medical insurance directory, and most Chinese residents have insurance, the reimbursement rate is limited. Therefore, it is necessary to reduce the cost to improve the coverage of TPO-RAs and then reduce the burden of adult ITP.
To our knowledge, this study is the first network meta-analysis to comprehensively analyze the efficacy and safety of five TPO-RAs in adult patients with ITP. Results of this study will provide evidence for the choice of TPO-RAs in clinical practice. However, our study also has several limitations. First, only a few RCTs were included for some treatment arms. For hetrombopag and rhTPO, only one RCT reported their platelet response, and no RCT reported TRAEs. This limitation would result in a low power to detect differences. Second, due to the lack of primary data, the treatment effect of TPO-RAs was quite uncertain, which was a key weakness in the understanding of these drugs. Third, dose and the duration of treatment were not controlled in this analysis. In most of the RCTs included, drug doses for individual patients were allowed to be adjusted, which might lead to some differences in platelet outcomes. In addition, among the RCTs we included, patients were treated by rhTPO only for 2 weeks while treated by other TPO-RAs such as eltrombopag for up to 6 months. The relative imbalance of treatment duration might also lead to clinically unimportant differences in platelet outcomes.
In conclusion, the systematic review and network meta-analysis data demonstrated that avatrombopag appeared to be the better therapeutic strategy as a second-line treatment within TPO-RAs in treating adult ITP. Further head-to-head RCTs including our tested regimens, particularly hetrombopag and rhTPO, are critical to provide more evidence validating our results and supporting the optimal therapy for adult ITP patients.
Statement of Ethics
This article does not contain any studies with human participants performed by any of the authors.
Conflict of Interest Statement
All of the authors have no competing interests to declare that are relevant to the content of this article.
Funding Sources
This work was funded by the Science and Technology Department of Henan Province (RKX202102011).
Author Contributions
Yin Liu had the primary responsibility for the work and publication. Xi Zhang contributed to the design of the study, to the statistical analysis, and to the interpretation of the results and wrote this manuscript together with Yin Liu. Juan Su, and Qing-Chao Geng performed searching strategy and searching process. Xin Lin and Chen-Xi Feng contributed to statistical analysis and interpretation of results. All authors reviewed and approved the final manuscript.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its supplementary material. Further inquiries can be directed to the corresponding author.
References
- 1. Kelton JG, Gibbons S. Autoimmune platelet destruction: idiopathic thrombocytopenic purpura. <X00_Journal>Semin Thromb Hemost</X00_Journal>. 1982;8(2):83–104.
- 2. McFarland J. Pathophysiology of platelet destruction in immune (idiopathic) thrombocytopenic purpura. <X00_Journal>Blood Rev</X00_Journal>. 2002;16:1–2.
- 3. Terrell DR, Beebe LA, Vesely SK, Neas BR, Segal JB, George JN. The incidence of immune thrombocytopenic purpura in children and adults: a critical review of published reports. <X00_Journal>Am J Hematol</X00_Journal>. 2010;85(3):174–80.
- 4. Provan D, Stasi R, Newland AC, Blanchette VS, Bolton-Maggs P, Bussel JB, et al. International consensus report on the investigation and management of primary immune thrombocytopenia. <X00_Journal>Blood</X00_Journal>. 2010;115(2):168–86.
- 5. Cuker A, Cines DB, Neunert CE. Controversies in the treatment of immune thrombocytopenia. <X00_Journal>Curr Opin Hematol</X00_Journal>. 2016;23(5):479–85.
- 6. Jenkins JM, Williams D, Deng Y, Uhl J, Kitchen V, Collins D, et al. Phase 1 clinical study of eltrombopag, an oral, nonpeptide thrombopoietin receptor agonist. <X00_Journal>Blood</X00_Journal>. 2007;109(11):4739–41.
- 7. Wang B, Nichol JL, Sullivan JT. Pharmacodynamics and pharmacokinetics of AMG 531, a novel thrombopoietin receptor ligand. <X00_Journal>Clin Pharmacol Ther</X00_Journal>. 2004;76(6):628–38.
- 8. Shirley M. Avatrombopag: first global approval. <X00_Journal>Drugs</X00_Journal>. 2018;78(11):1163–8.
- 9. Sabath DF, Kaushansky K, Broudy VC. Deletion of the extracellular membrane-distal cytokine receptor homology module of Mpl results in constitutive cell growth and loss of thrombopoietin binding. <X00_Journal>Blood</X00_Journal>. 1999;94(1):365–7.
- 10. Zufferey A, Kapur R, Semple JW. Pathogenesis and therapeutic mechanisms in immune thrombocytopenia (ITP). <X00_Journal>J Clin Med</X00_Journal>. 2017;6(2):16.
- 11. Cipriani A, Higgins JP, Geddes JR, Salanti G. Conceptual and technical challenges in network meta-analysis. <X00_Journal>Ann Intern Med</X00_Journal>. 2013;159(2):130–7.
- 12. Deng J, Hu H, Huang F, Huang C, Huang Q, Wang L, et al. Comparative efficacy and safety of thrombopoietin receptor agonists in adults with thrombocytopenia: a systematic review and network meta-analysis of randomized controlled trial. <X00_Journal>Front Pharmacol</X00_Journal>. 2021;12:704093.
- 13. Wojciechowski P, Wilson K, Nazir J, Pustułka I, Tytuła A, Smela B, et al. Efficacy and safety of avatrombopag in patients with chronic immune thrombocytopenia: a systematic literature review and network meta-analysis. <X00_Journal>Adv Ther</X00_Journal>. 2021;38(6):3113–28.
- 14. Yang R, Lin L, Yao H, Ji O, Shen Q. Therapeutic options for adult patients with previously treated immune thrombocytopenia - a systematic review and network meta-analysis. <X00_Journal>Hematology</X00_Journal>. 2019;24(1):290–9.
- 15. Giardina TD, Royse KE, Khanna A, Haskell H, Hallisy J, Southwick F, et al. Health care provider factors associated with patient-reported adverse events and harm. <X00_Journal>Jt Comm J Qual Patient Saf</X00_Journal>. 2020;46(5):282–90.
- 16. Hu J, Zhang HN, Li B, Feng S, Ha YX, Wei CY, et al. [Analysis of adverse events and influencing factors in randomized controlled trials of oral Chinese medicine published in English]. <X00_Journal>Zhongguo Zhong Yao Za Zhi</X00_Journal>. 2020;45(8):1948–52.
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. <X00_Journal>PLoS Med</X00_Journal>. 2009;6(7):e1000097.
- 18. Higgins JP, Altman DG, Gøtzsche PC, Juni P, Moher D, Oxman AD, et al. The cochrane collaboration’s tool for assessing risk of bias in randomised trials. <X00_Journal>BMJ</X00_Journal>. 2011;343:d5928.
- 19. Crepaz N, Baack BN, Higa DH, Mullins MM. Effects of integrated interventions on transmission risk and care continuum outcomes in persons living with HIV: meta-analysis. <X00_Journal>AIDS</X00_Journal>. 2015;29(18):2371–83.
- 20. Jurczak W, Chojnowski K, Mayer J, Krawczyk K, Jamieson BD, Tian W, et al. Phase 3 randomised study of avatrombopag, a novel thrombopoietin receptor agonist for the treatment of chronic immune thrombocytopenia. <X00_Journal>Br J Haematol</X00_Journal>. 2018;183:479–90.
- 21. Kuter DJ, Bussel JB, Lyons RM, Pullarkat V, Gernsheimer TB, Senecal FM, et al. Efficacy of romiplostim in patients with chronic immune thrombocytopenic purpura: a double-blind randomised controlled trial. <X00_Journal>Lancet</X00_Journal>. 2008;371(9610):395–403.
- 22. Mei H, Xu M, Yuan G, Zhu F, Guo J, Huang R, et al. A multicentre double-blind, double-dummy, randomised study of recombinant human thrombopoietin versus eltrombopag in the treatment of immune thrombocytopenia in Chinese adult patients. <X00_Journal>Br J Haematol</X00_Journal>. 2021;195(5):781–9.
- 23. Bussel JB, Kuter DJ, Aledort LM, Kessler CM, Cuker A, Pendergrass KB, et al. A randomized trial of avatrombopag, an investigational thrombopoietin-receptor agonist, in persistent and chronic immune thrombocytopenia. <X00_Journal>Blood</X00_Journal>. 2014;123(25):3887–94.
- 24. Cheng G, Saleh MN, Marcher C, Vasey S, Mayer B, Aivado M, et al. Eltrombopag for management of chronic immune thrombocytopenia (RAISE): a 6-month, randomised, phase 3 study. <X00_Journal>Lancet</X00_Journal>. 2011;377(9763):393–402.
- 25. Shirasugi Y, Ando K, Miyazaki K, Tomiyama Y, Okamoto S, Kurokawa M, et al. Romiplostim for the treatment of chronic immune thrombocytopenia in adult Japanese patients: a double-blind, randomized Phase III clinical trial. <X00_Journal>Int J Hematol</X00_Journal>. 2011;94(1):71–80.
- 26. Huang YT, Liu XF, Chen YF, Fu RF, Liu W, Zhang L, et al. [The efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia]. <X00_Journal>Zhonghua Xue Ye Xue Za Zhi</X00_Journal>. 2018;39(1):32–6.
- 27. Yang R, Li J, Jin J, Huang M, Yu Z, Xu X, et al. Multicentre, randomised phase III study of the efficacy and safety of eltrombopag in Chinese patients with chronic immune thrombocytopenia. <X00_Journal>Br J Haematol</X00_Journal>. 2017;176(1):101–10.
- 28. Tomiyama Y, Miyakawa Y, Okamoto S, Katsutani S, Kimura A, Okoshi Y, et al. A lower starting dose of eltrombopag is efficacious in Japanese patients with previously treated chronic immune thrombocytopenia. <X00_Journal>J Thromb Haemost</X00_Journal>. 2012;10(5):799–806.
- 29. Mei H, Liu X, Li Y, Zhou H, Feng Y, Gao G, et al. A multicenter, randomized phase III trial of hetrombopag: a novel thrombopoietin receptor agonist for the treatment of immune thrombocytopenia. <X00_Journal>J Hematol Oncol</X00_Journal>. 2021;14(1):37.
- 30. Bussel JB, Kuter DJ, George JN, McMillan R, Aledort LM, Conklin GT, et al. AMG 531, a thrombopoiesis-stimulating protein, for chronic ITP. <X00_Journal>N Engl J Med</X00_Journal>. 2006;355(16):1672–81.
- 31. Bussel JB, Cheng G, Saleh MN, Psaila B, Kovaleva L, Meddeb B, et al. Eltrombopag for the treatment of chronic idiopathic thrombocytopenic purpura. <X00_Journal>N Engl J Med</X00_Journal>. 2007;357(22):2237–47.
- 32. Bussel JB, Provan D, Shamsi T, Cheng G, Psaila B, Kovaleva L, et al. Effect of eltrombopag on platelet counts and bleeding during treatment of chronic idiopathic thrombocytopenic purpura: a randomised, double-blind, placebo-controlled trial. <X00_Journal>Lancet</X00_Journal>. 2009;373(9664):641–8.
- 33. Al-Samkari H, Nagalla S. Efficacy and safety evaluation of avatrombopag in immune thrombocytopenia: analyses of a phase III study and long-term extension. <X00_Journal>Platelets</X00_Journal>. 2022;33(2):257–64.
- 34. Ghanima W, Cooper N, Rodeghiero F, Godeau B, Bussel JB. Thrombopoietin receptor agonists: ten years later. <X00_Journal>Haematologica</X00_Journal>. 2019;104(6):1112–23.
- 35. Grace RF, Neunert C. Second-line therapies in immune thrombocytopenia. <X00_Journal>Hematology Am Soc Hematol Educ Program</X00_Journal>. 2016;2016:698–706.
- 36. Williams DD, Peng B, Bailey CK, Wire MB, Deng Y, Park JW, et al. Effects of food and antacids on the pharmacokinetics of eltrombopag in healthy adult subjects: two single-dose, open-label, randomized-sequence, crossover studies. <X00_Journal>Clin Ther</X00_Journal>. 2009;31(4):764–76.
- 37. Wang S, Yang R, Zou P, Hou M, Wu D, Shen Z, et al. A multicenter randomized controlled trial of recombinant human thrombopoietin treatment in patients with primary immune thrombocytopenia. <X00_Journal>Int J Hematol</X00_Journal>. 2012;96(2):222–8.
Yin Liu and Han-Xi Zhang have contributed equally to this work.