Introduction
Diffuse large B-cell lymphoma (DLBCL) is the most common subtype of non-Hodgkin lymphoma (NHL) with an aggressive behavior []. Five-year overall survival rates range from 30 to 50%, with approximately 60% of patients remaining disease free following standard immunochemotherapy. However, 20–25% of the patients relapse after an initial response within 2 years [-]. Currently, the only 2 agents approved for relapsed/refractory (R/R) DLBCL by the US Food and Drug Administration are chimeric antigen receptor T (CAR-T) cells and polatuzumab vedotin (in combination with bendamustine and rituximab). The European Medicines Agency has recently approved CAR-T-cell therapy and has also granted standard approval for the cytotoxic aza-anthracenedione pixantrone, which has been proven reduce the potential for cardiotoxicity with maintained antitumor activity []. Few real-world data have been published on the use of pixantrone in every day clinical practice [-]. Therefore, a retrospective study across Spain and Italy was conducted to assess the efficacy and safety of the drug in a real-life population of patients with multiple R/R aggressive B-cell NHL [].
Here, we focus on the Italian patient subset of this study because, in Europe, DLBCL is a rare disease, whereas, in Italy, it is not, and real-life data are needed to allocate pixantrone in the therapeutic algorithm of R/R DLBCL [].
Methods
A retrospective, international, multicenter study was conducted to assess the efficacy and safety of pixantrone in a real-life population. Briefly, patients were included in the study if they were ≥18 years old, with aggressive relapsed or refractory B-cell NHL on ≥2 prior lines of therapy. Only patients who had received pixantrone in accordance with the approved prescribing information were eligible for inclusion []. The Italian patient subset was extracted from the global registry for these post hoc analyses. The complete inclusion/exclusion criteria list, study design, methods, and treatment have been previously reported in Sancho et al. []. Additional considerations about the disease molecular profile were made in the Italian subset, focusing on both overall response rate (ORR) and best response rate (i.e., achieved at any cycle of pixantrone).
Results
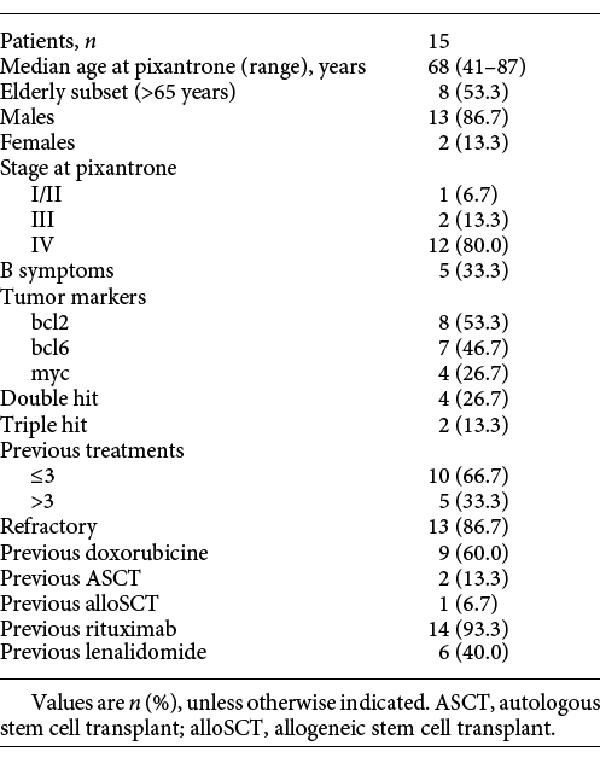
Fifteen DLBCL patients (13 males and 2 females) underwent treatment with pixantrone for a median of 2 cycles (range 1–6) between November 2014 and May 2017. Median age was 68 years (range 41–87, with 8 patients aged >65 years). Regarding the disease molecular profile: 8 patients were bcl2 positive, 7 bcl6 positive, and 4 myc positive; 4 patients were diagnosed as double-hit and 2 as triple-hit DLBCL. In general, patients were heavily pretreated, and 5 (33.3%) had received >3 therapeutic approaches before pixantrone.
Thirteen patients (86.7%) were defined as refractory, with 14 who had previous rituximab treatment, 6 with previous lenalidomide treatment (all refractory to this drug), 9 with previous doxorubicin treatment, and 3 with a previous stem cell transplant (SCT; 2 autologous [ASCT] and 1 allogeneic [alloSCT]). No patient had received CAR-T-cell therapy. Ten patients had comorbidities, of which 5 were cardiovascular ones.
Complete patient characteristics are reported in Table 1.
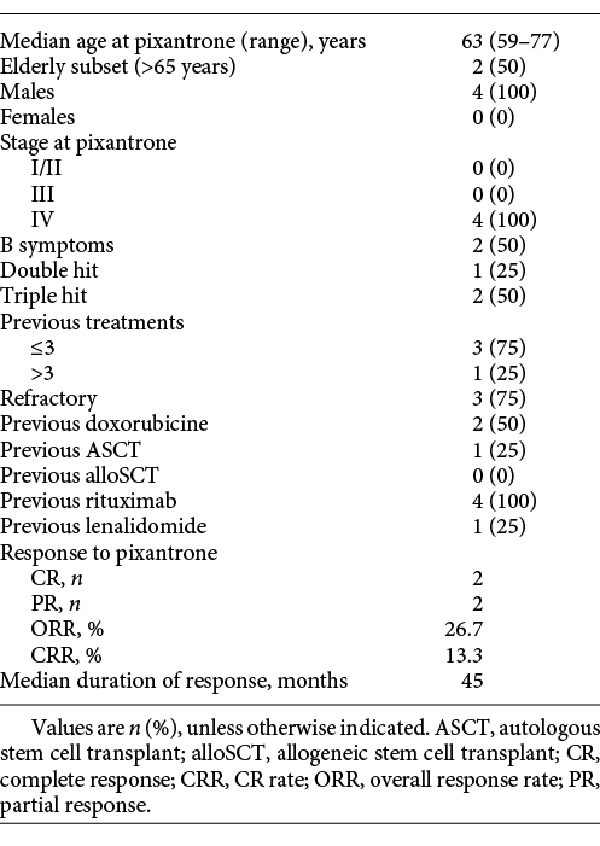
Best response rate with pixantrone was 46.7% (5 partial responses [PR] and 2 complete responses [CR]), whereas ORR was 26.7% (2 PR and 2 CR). The 2 CRs were achieved at cycle IV and VI, respectively: 1 patient directly achieved CR, while the other converted a PR to a CR between cycles II and IV. Patients who achieved CR were <65 years old. One patient who was bcl6 positive and had >3 previous therapeutic approaches including alloSCT as the last prior treatment was still in CR at the latest available follow-up (>40 months). The second CR patient had a double-hit DLBCL, received pixantrone as third-line treatment after failure of DLBCL combination therapies, consolidated with ASCT, and was in CR at >50 months. Both patients who achieved PR at the end of therapy (at III and VI cycles, respectively) had triple-hit DLBCL and maintained PR for 9.7 and 12.5 months, respectively. One of these 2 patients received pixantrone as a third-line approach with a duration of response of 12.5 months. Three of 6 patients who failed previous lenalidomide therapy had a response with pixantrone (2 PR and 1 CR). Among double-hit patients, 1 out of 4 achieved a CR (ORR and CR rate 25%) while among triple-hit DLBCL patients, 2 out of 2 achieved a PR (ORR 100% and CR rate 0%). Table 2 reports characteristics of the patients who had at least a PR at the end of treatment. At the time of writing, overall median progression-free survival was 6 months, whereas the median overall survival was 13 months.
Three patients had grade IV adverse events (AEs), namely neutropenia, which caused drug discontinuation. Four patients had 5 cases of grade III AEs, namely thrombocytopenia (n = 1), stomatitis (n = 1), and neutropenia (n = 3). One of the 2 patients who achieved a CR experienced grade III neutropenia at the fourth cycle, leading to drug interruption, but not to a loss of response. Eighteen AEs ≤ grade II occurred in 6 patients. Of note, among them, only 1 mild cardiac toxicity was possibly related to pixantrone (sinus tachycardia) for which no action was required.
Discussion
The first-line approach induces a response in the 60–70% of patients with DLBCL across all risk groups. During the past decades, most attempts to substantially increase this proportion by adding additional drugs to or by increasing doses of the classic immunochemotherapy regimen have failed. Furthermore, 20–25% of the patients relapse after initial response within 2 years [-]. As a consequence, treatment of R/R DLBCL represents a challenge for clinicians due to the lack of effective therapeutic options; prognosis in this setting often remains poor, especially for patients who are ineligible for a transplant as there is no standard-of-care treatment regimen and the therapeutic algorithm is not well established.
Pixantrone is the only single-agent treatment approved for multiple R/R aggressive B-cell NHL in Europe. In recent trials, pixantrone appeared to be effective in third- or fourth-line treatment in patients affected by aggressive B-cell NHL; in fact, it produced better ORRs compared with physician’s choice (43.6 vs. 12.8%) and can lead to long-term remission in a subset of patients, regardless of responses to previous treatments [, ].
The introduction of anthracyclines, such as doxorubicin and daunorubicin represents a milestone in the treatment of NHL. However, the use of these compounds is limited by their cardiotoxicity and the cumulative dose allowed, which usually does not enable their (re)use at relapse. Pixantrone is an aza-anthracenedione with similarities in chemical structure to anthracyclines, but with a different mode of action and acceptable risk of cardiac toxicity [].
Here, we present Italian real-world data on the use of pixantrone in patients with R/R DLBCL, a disease that is not rare in Italy []. Post hoc analyses in a national context are important due to the differences in the disease management, drug approval timing, and reimbursement across countries; therefore, real-world data are needed to allocate pixantrone in the therapeutic algorithm of R/R DLBCL. Even with a small sample, the ORR (26.7%) and best response rate (46.7%) were satisfactory for this high-risk and difficult-to-treat subset of patients with an acceptable toxicity profile. Interestingly, 2 of 4 responder patients (1 CR and 1 PR) received pixantrone as part of their third-line therapeutic approach. These data are in line with those reported by Pettengel et al. [] in a post hoc analysis on the responder patients of the pivotal PIX 301 phase 3 study: among the 17 patients achieving a CR or an unconfirmed CR in pixantrone arm, 12 (70.6%) received the drug as a third-line treatment. Although 5 patients presented with cardiovascular comorbidities at the start of pixantrone treatment, only 1 patient had a cardiac toxicity that did not interfere with the therapeutic plan, confirming the acceptable risk of cardiac toxicity showed by this agent in previous clinical trials and real-world studies [, , ]. We also analyzed molecular data, but the importance of collecting this information is still to be established, as our study sample did not allow a clear conclusion to be drawn. Of note, 1 patient with CR had double-hit DLBCL, and the 2 patients with PR had triple-hit DLBCL.
The therapeutic algorithm for R/R DLBCL has yet to be established. Treatment with lenalidomide for patients with R/R DLBCL has shown efficacy, especially in elderly patients with manageable toxicities in the everyday clinical practice, but not for all patients []. Interestingly, in the present report, 3 out of 6 patients who were refractory to prior lenalidomide treatment had a response with pixantrone (2 PR and 1 CR).
CAR-T-cell therapy directed at the CD19 antigen on B cells has changed the therapeutic landscape and prognosis for patients with R/R B-cell NHL, and an increasing number of patients will be receiving these treatments in the near future, but real-world data are still poor [].
The application settings of pixantrone may be multiple: elderly patients with a second relapse who are not eligible for transplant or CAR-T-cell therapy, young patients refractory to 2 previous lines of therapy as a bridge to autologous transplantation, bridge to allogeneic transplantation or CAR-T-cell therapy, and salvage therapy at relapse after a SCT or CAR-T-cell approach. In particular, pixantrone could represent one of the most suitable agents to bridge patients to CAR-T-cell therapy based on its safety profile and its ability to induce a rapid response in patients who are sensitive to this agent [-]. To note, this is only a clinical reasoning, as the patients in this study were treated with pixantrone years before the introduction of CAR-T and polatuzumab. Prospective data will be needed to evaluate the hypothetic role of pixantrone as part of a bridge to a CAR-T treatment strategy.
Furthermore, future trials could evaluate the role of pixantrone in DLBCL in combination with targeted therapies as in vitro synergistic activities have already been observed, especially with ibrutinib, idelalisib, lenalidomide, etoposide, or rituximab [].
Conclusion
Clinical trials and real-world data documented clear efficacy of pixantrone in R/R DLBCL. Therefore, pixantrone is a significant treatment option in this subset of patients with an unmet clinical need.
Acknowledgment
We would like to thank Lisa Argnani for writing the outline and for the editing of the manuscript. This medical writing assistance was funded by Servier, Italy.
Statement of Ethics
The study was approved by the Ethical Committee at each site and was performed in accordance with the ethical standards as laid down in the 1964 Declaration of Helsinki and its later amendments.
Conflict of Interest Statement
The authors have nothing to disclose.
Funding Sources
The study was funded by Servier. This funding source had no role in the interpretation of the data.
Author Contributions
P.L.Z. and M.S. conception. P.L.Z., M.B., M.M., G.M., A.B., F.P., and A.P. acquisition of data. P.L.Z., M.B., M.M., G.M., A.B., F.P., and A.P. interpretation of data. P.L.Z., M.B., M.S., M.M., G.M., A.B., F.P., and A.P. revision of the manuscript. P.L.Z., M.B., M.S., M.M., G.M., A.B., F.P., and A.P. final approval of the manuscript.
References
- 1. Johnson NA, Slack GW, Savage KJ, Connors JM, Ben-Neriah S, Rogic S, et al Concurrent expression of MYC and BCL2 in diffuse large B-cell lymphoma treated with rituximab plus cyclophosphamide, doxorubicin, vincristine, and prednisone. J Clin Oncol. 2012;30(28):3452–9.0732-183X
- 2. Sehn LH. Paramount prognostic factors that guide therapeutic strategies in diffuse large B-cell lymphoma. Hematology (Am Soc Hematol Educ Program). 2012;2012(1):402–9.1520-4391
- 3. Péan E, Flores B, Hudson I, Sjöberg J, Dunder K, Salmonson T, et al The European Medicines Agency review of pixantrone for the treatment of adult patients with multiply relapsed or refractory aggressive non-Hodgkin’s B-cell lymphomas: summary of the scientific assessment of the committee for medicinal products for human use. Oncologist. 2013;18(5):625–33.1083-7159
- 4. Minotti G, Han H, Cattan V, Egorov A, Bertoni F. Pixantrone: novel mode of action and clinical readouts. Expert Rev Hematol. 2018;11(7):587–96.1747-4086
- 5. Eyre TA, Linton KM, Rohman P, Kothari J, Cwynarski K, Ardeshna K, et al Results of a multicentre UK-wide retrospective study evaluating the efficacy of pixantrone in relapsed, refractory diffuse large B cell lymphoma. Br J Haematol. 2016;173(6):896–904.0007-1048
- 6. Appio L, Landoni C, La Targia M, Bertolli V, Chiarucci M, Crovetti G, et al Single-agent pixantrone as a bridge to autologous stem cell transplantation in a patient with refractory diffuse large B-cell lymphoma. Chemotherapy. 2017;62(3):187–91.0009-3157
- 7. Malaspina F, Pellegrini C, Casadei B, Argnani L, Zinzani PL. Impressive response to pixantrone after allogeneic transplant in a multiple relapsed diffuse large B-cell lymphoma. Acta Haematol. 2017;137(4):191–4.0001-5792
- 8. Sancho JM, Navarro B, Soler Campos JA, de Oteyza JP, de Barrenetxea Lekue C, Bregni M, et al Efficacy and safety of pixantrone for the treatment of multiply relapsed or refractory aggressive non-Hodgkin B-cell lymphomas. Eur J Haematol. 2020;104(5):499–508.0902-4441
- 9. AIRTUM Working Group, Busco S, Buzzoni C, Mallone S, Trama A, Castaing M, et al. Italian cancer figures—Report 2015: the burden of rare cancers in Italy. Epidemiol Prev. 2016;40(1Suppl 2):1–120.1120-9763
- 10. Pettengell R, Coiffier B, Narayanan G, de Mendoza FH, Digumarti R, Gomez H, et al Pixantrone dimaleate versus other chemotherapeutic agents as a single-agent salvage treatment in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: a phase 3, multicentre, open-label, randomised trial. Lancet Oncol. 2012;13(7):696–706.1470-2045
- 11. Pettengell R, Sebban C, Zinzani PL, Derigs HG, Kravchenko S, Singer JW, et al Monotherapy with pixantrone in histologically confirmed relapsed or refractory aggressive B-cell non-Hodgkin lymphoma: post-hoc analyses from a phase III trial. Br J Haematol. 2016;174(5):692–9.0007-1048
- 12. Pettengell R, Coiffier B, Egorov A, Singer J, Sivcheva L. Long-term response and remission with pixantrone in patients with relapsed or refractory aggressive non-Hodgkin lymphoma: post-hoc analysis of the multicenter, open-label, randomized PIX301 trial. Clin Drug Investig. 2018;38(6):527–33.1173-2563
- 13. Broccoli A, Casadei B, Chiappella A, Visco C, Tani M, Cascavilla N, et al Lenalidomide in Pretreated Patients with Diffuse Large B-Cell Lymphoma: An Italian Observational Multicenter Retrospective Study in Daily Clinical Practice. Oncologist. 2019;24(9):1246–52.1083-7159
- 14. Levin A, Shah NN. Chimeric antigen receptor modified T cell therapy in B cell non-Hodgkin lymphomas. Am J Hematol. 2019;94S1:S18–23.0361-8609
- 15. Tarantelli C, Gaudio E, Cascione L, Stathis A, Zucca E, Bertoni F. In vitro demonstration of synergism with pixantrone combined with targeted agents in lymphomas. Br J Haematol. 2019;186(1):149–52.0007-1048