Introduction
When assessing general cognitive ability, psychologists often use the Wechsler series of intelligence tests, which include the Wechsler Adult Intelligence Scale (WAIS; ) and the Wechsler Intelligence Scale for Children (WISC; ). Surveys of clinicians indicate that these tests are the most commonly used neuropsychological assessments (), and have a number of routine uses, including to characterize cognitive functioning in people with neurodevelopmental conditions like autism and attention deficit hyperactivity disorder (ADHD; ). In the United Kingdom, the National Institute of Health and Care Excellence () recommends that a diagnostic assessment for autism must construct a profile of the person’s strengths and difficulties, potentially including an individual’s intellectual ability and academic skills. Likewise, several policy documents suggest use of cognitive assessment within neurodevelopmental diagnostic services (). The purpose of this cognitive assessment may be to identify support needs for intervention—however, to assess cognition as part of a neurodevelopmental diagnostic assessment may imply that cognitive assessment is helpful for diagnosis itself. There is some uncertainty about this. On the one hand, research has suggested specific cognitive profiles on the Wechsler scales in autism and ADHD (; ; ). However, research has not always supported the idea of specific profiles on the Wechsler tests (). Due to this uncertainty, the present review will assess the state of evidence surrounding this question, asking whether autism and ADHD are associated with a distinctive cognitive profile on the Wechsler scales. This is likely to have clinical relevance, as these are the most commonly used cognitive tests (; ).
Autism and ADHD are both neurodevelopmental conditions diagnosed on the basis of behavioral features that emerge in childhood (). In the case of autism, these include social/communication difficulties and restricted and repetitive behaviors and interests. In the case of ADHD, individuals show a pattern of inattention and/or hyperactivity and impulsivity. These behavioral features are thought to result from differences in genetically mediated brain development, although evidence suggests subtle differences only discernible at the group level. One of the most replicated findings in autism is of accelerated brain growth early in life (), which appears to result in changes in connectivity with reduced long-range connectivity and, potentially, enhanced local connectivity (). In the case of ADHD, there also appear to be alterations in connectivity, specifically affecting cortical attention and inhibitory control networks, alongside subtle structural differences including reduced cortical surface area ().
In this review, “cognitive profile” is taken to mean a person’s pattern of performance across the different cognitive domains covered in the assessment, including their strengths and weaknesses. There are several versions of the WAIS and WISC, each incorporating a range of subtests targeting different aspects of intellectual functioning. The WAIS-IV is the latest version for adults and consists of 10 core subtests grouped under four cognitive domains (): verbal comprehension, perceptual reasoning, working memory, and processing speed. The latest version for children (WISC-V; ) measures five cognitive domains: the same as the WAIS-IV, except that visual spatial cognition and fluid reasoning replace perceptual reasoning. Standardized scores can be determined for a person’s performance on each subtest, each domain, and overall intellectual functioning (full-scale IQ, or FSIQ). By reviewing a person’s performance across subtests and indices reflecting different domains, it is possible to identify their profile of strengths and difficulties, relative to both general population norms and the person’s overall ability level. Profiles are therefore inter-individual (comparing across people) as well as intra-individual (comparing skills within a person).
There is a longstanding topic of interest that different neurodevelopmental conditions may be characterized by a distinctive “profile” of performance on the Wechsler scales. In autism, this has been described as an uneven profile with a peak on the Block Design subtest and a particular weakness in the Comprehension subtest (see for a review of early research). linked this pattern to theories of autistic cognition: (1) the strength in Block Design may relate to a cognitive preference for processing local detail and (2) the weakness in Comprehension may relate to differences in a person’s social reasoning. came to a similar conclusion in their study comparing the verbal profile on the WISC in autism to ADHD; they highlighted that the pattern of performance observed in autistic individuals across verbal subtests (strongest on Similarities, then Vocabulary, then Comprehension) may have been due to autistic difficulties with social reasoning and formulating verbal discourse. Researchers have also been interested in characterizing different profiles within autistic subgroups, in particular differentiating the presentation known as Asperger’s syndrome in DSM-IV from other autistic presentations. A somewhat stronger performance on verbal than nonverbal subtests has been identified as characteristic of Asperger’s, whereas no discrepancy exists for “high-functioning” autism (see for a meta-analysis). On the other hand, ADHD may possibly be characterized by lower scores on the working memory and/or processing speed subtests. In over 700 children with ADHD, 90% of the sample received their lowest scores in these domains (). The literature also mentions the so-called ACID profile (lower scores on Arithmetic, Coding, Information and Digit Span subtests) and SCAD profile (lower scores on Symbol Search, Coding, Arithmetic and Digit Span subtests), which appear sensitive to ADHD (). All these subtests (except Information) belong to the working memory and processing speed domains, which reinforces the idea that these domains are the most likely areas of difficulty for individuals with ADHD.
There has been some attempt to review evidence of a distinctive cognitive profile in autism, although this research is limited. found that studies tended to identify a strength in Block Design and a weakness in Coding among autistic children. However, this narrative review has several limitations, as it only focuses on certain subtests (neglecting many papers looking at the domain-level index scores), excludes adults, and lacks overall quantitative analysis or comparison with other neurodevelopmental conditions. have also reviewed research into intellectual profiles in autism, specifically whether discrepancies between verbal IQ (VIQ) and nonverbal, or performance, IQ (PIQ) distinguish Asperger’s syndrome from other forms of autism. Therefore, focus on rather a specific question (which is now less relevant as Asperger’s syndrome is no longer diagnosed under DSM-5), and it would be helpful to understand wider aspects of performance on the Wechsler scales while also investigating whether results are valid for current versions of the Wechsler scales (as this earlier review looked at earlier versions). As for ADHD, there is no existing review of Wechsler profiles in this population.
This meta-analytic review seeks to address the evidence gap, asking whether there are distinctive profiles in autism and ADHD on the latest versions of the Wechsler scales, the WAIS-IV (for adults) and the WISC-V (for children). Restricting attention to the latest versions is likely to be most relevant to current clinical practice, as guidance would be for services to use the latest assessment tools with up-to-date norms and improved psychometric properties. In addition, focusing on more recent research allows us to study neurodevelopmental populations likely to be seen now, which may differ compared to the past due to different diagnostic criteria and much increased diagnostic rates (e.g., ). This review asks two questions via meta-analysis:
(a) Are autism and ADHD associated with a cognitive profile on the Wechsler scales?
(b) Can we differentiate between autism and ADHD based on Wechsler profile?
Materials and Methods
This review included peer-reviewed papers published between 2008 (the publication year of the WAIS-IV) and 2022 where they present standardized scores on the WAIS-IV or WISC-V by a sample of children or adults diagnosed with autism or ADHD who underwent a cognitive assessment. Scores needed to be presented for all indexes of the WAIS-IV/WISC-V. A search was conducted using Ovid of databases PsycINFO, Embase and Medline on October 20, 2022 using the following search terms: (attention deficit OR attention-deficit OR hyperactivity OR ADHD* OR autis* OR ASD OR pervasive development* or Asperger*) (in the title) AND (WAIS-IV or WISC-V) (in any element of the source). Reference lists of included papers and the Wechsler manuals were also screened for additional sources.
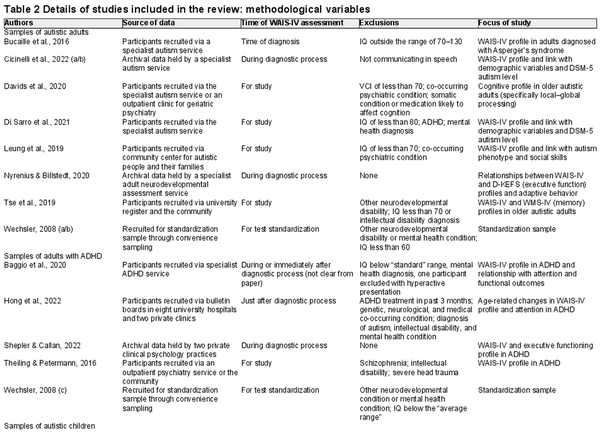
Papers were screened for inclusion in two steps. In the first step, titles and/or abstracts were screened to ensure the paper reported on original research in a sample of people diagnosed with autism and/or ADHD. In the second step, abstracts and full papers were screened to ensure the paper included index and/or subtest scores on the WAIS-IV/WISC-V. Papers were included if there was a sample of people with a clinical diagnosis of autism or ADHD with standardized scores on all indexes of a fully administered WAIS-IV/WISC-V. Note that some studies administered the WAIS-IV or WISC-V to some/all participants, but only used FSIQ scores to check eligibility for the study, characterize the sample, match clinical participants with control participants, or use as a covariate in analysis. As index scores were not available for these papers, the papers were not included in analysis. Where papers were not published in English, the plan was to translate sections of relevant peer-reviewed papers. This was only required for one paper published in German (), with support of a German-speaking psychologist.
The following background information was recorded from papers deemed eligible for inclusion: sample size, demographic information about the sample (including age, sex, and race), clinical details about the sample (diagnostic information, age at diagnosis, co-occurring diagnoses, and use of psychostimulants), country of study, purpose of study, method of recruitment/data collection, timing of cognitive assessment in relation to diagnosis, and eligibility criteria for participation. The key study data used in the meta-analysis included means and standard deviations for standardized scores on each index and optionally on each subtest too if subtest-level data were presented by the paper. Index scores were recorded as standard scores (mean = 100; SD = 15) and subtest scores as scaled scores (mean = 10; SD = 3). In addition, any information about WAIS-IV/WISC-V scores and (1) their sensitivity/specificity to neurodevelopmental conditions and (2) their relationships with clinical variables/outcomes was also noted. This data coding process was completed with support of a second rater who coded a random 25% of the data set, with full agreement with the original coding. The risk-of-bias of studies included in the review was also considered, drawing on the Observational Study Quality Evaluation (OSQE; ). Using this tool, factors were identified a priori that are most likely to introduce bias: eligibility criteria reducing representativeness, selection bias in recruitment, unsatisfactory neurodevelopmental assessment, and biases in administration/scoring of cognitive assessments. Papers were reviewed in relation to these factors. See Appendix A for a summary of this risk-of-bias assessment.
All papers provided index scores, and these were analyzed through multilevel meta-analyses using R package metafor () in the R computing environment (). For each index (VCI, PRI, VSI, FRI, WMI, PSI, and FSIQ), means were aggregated across four neurodivergent subgroups (autistic children, children with ADHD, autistic adults, and adults with ADHD). As some papers contributed more than one sample, multilevel models were specified with random effects of the sample embedded in paper. Restricted maximum likelihood estimation was used to generate estimates and error terms. These meta-analyses gave an overall mean score for each neurodivergent subgroup for each index. Scores were labeled according to the qualitative descriptors set out by the American Academy of Clinical Neuropsychology (); for example, standard scores of 90–110 are “Average.”
Having calculated mean index scores, differences between and within the subgroups were tested in three ways. In each case, the size of effects was quantified according to the guideline by , and alpha levels were adjusted for multiple comparisons. The first analysis asked whether mean index scores significantly differed from the normative mean of 100 in the four neurodivergent subgroups (children/adults diagnosed with autism/ADHD). For this analysis, the multilevel meta-analyses were rerun with index scores of all samples centered at zero (by subtracting 100), and the significance of the intercept was tested. As this analysis was carried out for each index in the four subgroups, a correction for multiple comparisons was used for each subgroup. For adult samples, the alpha level was set to .01 (.05 divided by 5, as the WAIS-IV has five indexes), and .008 in child samples (.05 divided by 6, as the WISC-V has six indexes). The second analysis explored whether index scores differed between diagnostic groups. For each index, multilevel meta-analyses were rerun for children and adults across diagnostic groups, including Diagnosis (autism vs. ADHD) as a fixed effect. The significance of this fixed effect allowed testing for group differences between people diagnosed with autism and ADHD. The alpha level was set to the same thresholds as earlier to allow for multiple comparisons. The third analysis shifted from inter-group comparisons to intra-group comparisons. This allows us to assess whether index scores significantly differ from each other within the four neurodivergent subgroups (i.e., to reflect a pattern of relative strengths and difficulties specific to the neurodevelopmental condition). For this analysis, meta-analyses were run within each of the four neurodivergent subgroups, with index as a fixed effect and with a multi-level structure (i.e., random effects clustered indexes within samples). Nonverbal ability was used as a reference level of the fixed effect (PRI or FRI, depending on age), which was compared to each other’s index one at a time. It should be noted that error terms will not be independent in this analysis (as each sample contributed an error term for more than one index). However, the covariance for these error terms is unknown (as papers have no reason to report correlations between indexes in their samples), so cluster robust estimation was used, as suggested by . For these final meta-analyses, alpha levels were left uncorrected at .05, as these analyses were likely underpowered and more exploratory.
When carrying out the first analysis described previously (quantifying overall means for each index in each neurodivergent subgroup), heterogeneity was assessed across samples contributing to these means. The I2 value (percent of variance attributable to variance in true effects of samples) was calculated for each multilevel meta-analysis. It was hypothesized that general cognitive ability would influence index scores across samples, so a measure of nonverbal ability (PRI or FRI, depending on age) was included as a moderator in follow-up multilevel meta-analyses, and the I2 value was recalculated. Analyses were also tested for publication bias by including sample size as a moderator in other follow-up meta-analyses.
In addition to these detailed quantitative analyses of index scores, data were synthesized at the subtest level in the few studies that presented this information. Overall means across studies were computed using random effects meta-analysis, and these summary means (with 95% CIs) are presented in charts showing performance across each subtest. Some papers offered further investigation into cognitive profiles in autism and ADHD, including psychometric properties, ability to discriminate between groups, and correlations with other phenotypic variables. This information is presented as a narrative synthesis in Appendix B.
Results
Participants
A literature search generated 306 sources, of which 16 were eligible for inclusion. Two papers passing initial screening stages were excluded as they included data overlapping with other sources (; ). One additional paper was identified through review of reference lists of included papers (), and standardization data were included from the WAIS-IV and WISC-V (however, as used standardization data in their analysis, the WISC-V had effectively already been counted as a data source). This gave a total of 18 sources of cross-sectional data included in the meta-analysis. Figure 1 shows a flow chart presenting this screening process.
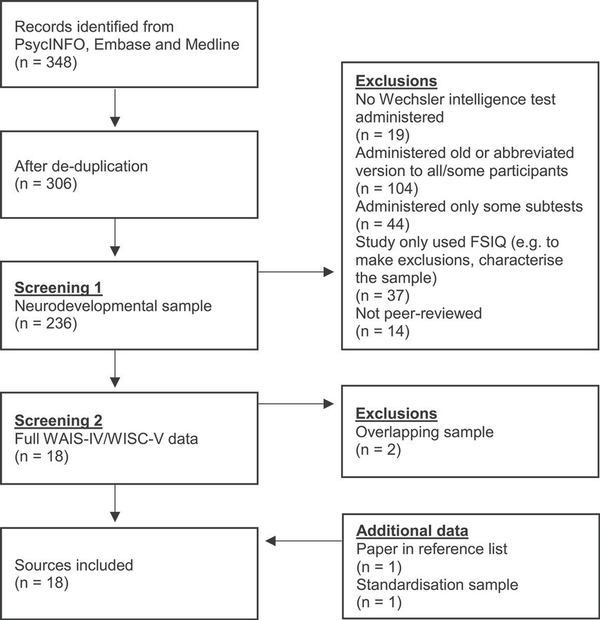
Fig. 1
Flow chart showing movement of sources through the screening process of the literature search.
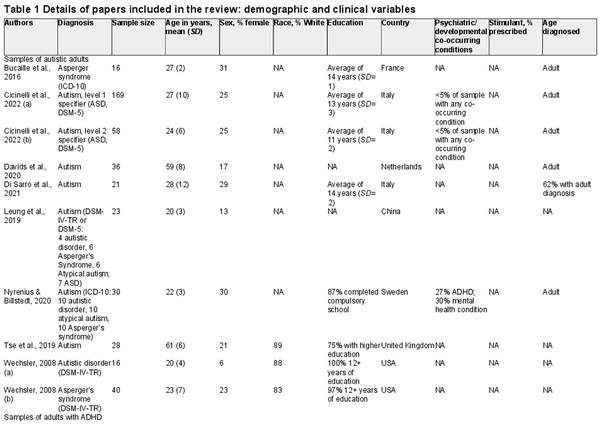
Across the 18 sources of data, there were 24 separate samples of individuals, comprising 1,842 individuals in total. Of these, 968 were adults assessed with the WAIS-IV: 437 came from samples focused on autism (mean age = 30 years; 23% female), and 527 came from samples focused on ADHD (mean age = 29 years; 35% female). In addition, there were 856 children aged between 6 and 16 assessed with the WISC-V: 630 came from samples focused on autism (mean age = 10 years; 18% female), and 226 came from samples focused on ADHD (mean age = 11 years; 34% female). Information on race/ethnicity was not consistently available across studies, although most participants were White where this information was given. Tables 1 and 2 present full information on the papers included in this review.
Neurodevelopmental diagnoses were based on DSM-IV, DSM-5, or ICD-10. Among autistic adults, most had a DSM-5 diagnosis (n = 234); others had a DSM-IV diagnosis of Asperger’s syndrome (n = 72), autistic disorder (n = 30), or atypical autism (n = 16). For some autistic adults, the criteria for diagnosis were not specified in the paper (n = 85). Autistic adults were largely diagnosed as adults (322 vs. 8 diagnosed as children), although for 107, age of diagnosis was not specified. Most papers excluded autistic adults with co-occurring diagnoses. In the few studies with more relaxed eligibility criteria, papers reported on co-occurring diagnoses (ADHD had been diagnosed in 10 autistic adults). In the samples of adults with ADHD, 103 people were diagnosed with the inattentive subtype, 349 with the combined subtype, and 8 with hyperactive subtype; for 61, information was not available about the subtype. The majority of adults with ADHD were diagnosed as adults (445 vs. 28 diagnosed as children and 44 with age of diagnosis unspecified).
Moving on to the child samples, 183 of the autistic children had a DSM-5 diagnosis, but for many, diagnostic criteria were not specified (n = 447). For a large number of children, there were no exclusionary criteria regarding co-occurring diagnoses and information was not reported about co-occurring diagnoses. In 183 autistic children for whom this information was available, 21 had co-occurring ADHD. Among children in the ADHD samples, information was not given on subtype, although 178 were diagnosed using ICD-10 criteria and 48 with DSM-5 criteria. Looking across all samples of people with autism or ADHD, the majority of papers excluded individuals with intellectual disability and/or FSIQ below a certain threshold (varying between 60 and 80).
Risk-of-Bias Assessment
See Appendix A for a table showing ratings for risk-of-bias for each paper. A summary of issues is provided here, as all papers showed the same limitations that could introduce systematic bias:
There was no detailed assessment for co-occurring conditions in any paper. Some papers screened for co-occurring conditions, but this was limited to asking if diagnoses had been made, which will not pick up undiagnosed conditions or subthreshold co-occurring traits. This was the case in studies excluding individuals with co-occurring conditions, as well as studies without these exclusion criteria. Across all samples, only 1% of individuals had autism and ADHD diagnoses, which may underestimate true co-occurring traits. Rates of co-occurrence vary widely in the literature (depending on the sample characteristics), but, for the purpose of illustration, a meta-analysis found 40% of autistic people met criteria for ADHD (). However, co-diagnosis of autism and ADHD is a complex task, as both conditions present with traits that may look like the other condition. For instance, attentional differences appear to be an early precursor to core processes affected in autism (including joint attention, arousal regulation, and perceptual processing; ). Similar traits may lead to an overestimation of co-occurring autism and ADHD within the wider literature; however, a co-occurrence rate of just 1% in the studies reviewed here seems low. If papers have not fully assessed for co-occurring conditions, it may be difficult to conclude whether a cognitive profile is explained by the condition of interest or other unassessed co-occurring features.
FSIQ thresholds were often used for inclusion, possibly biasing the cognitive profiles present in the samples, especially as some cognitive domains contribute more strongly to FSIQ than other domains. Only one study () included a significant proportion of individuals meeting criteria for intellectual disability.
Researchers were not blind to the diagnostic status of participants. Where cognitive assessments occurred following diagnosis, this may have biased administration/scoring of assessments if assessors had preconceived ideas about the possible performance of participants.
Quantitative Analyses
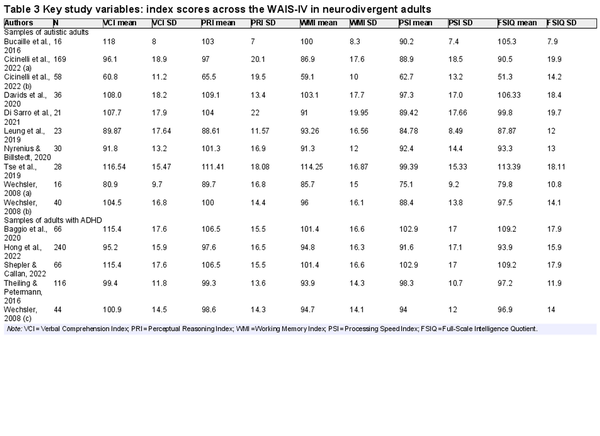
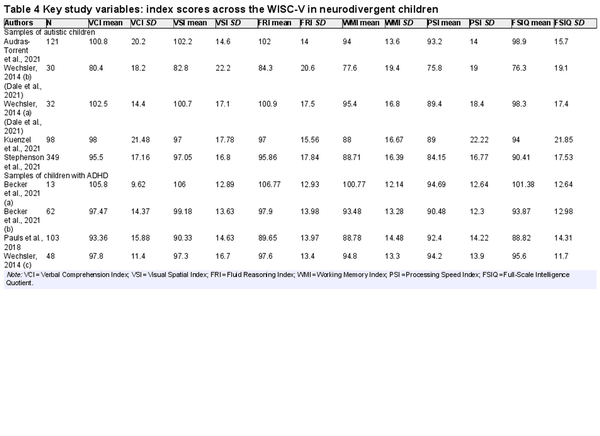
Tables 3 and 4 show the key variables included in the meta-analyses that follow. These include the mean scores on the indices of the WAIS-IV or WISC-V for each sample included in the review.
Meta-Analysis 1. Comparing index scores to the normative mean
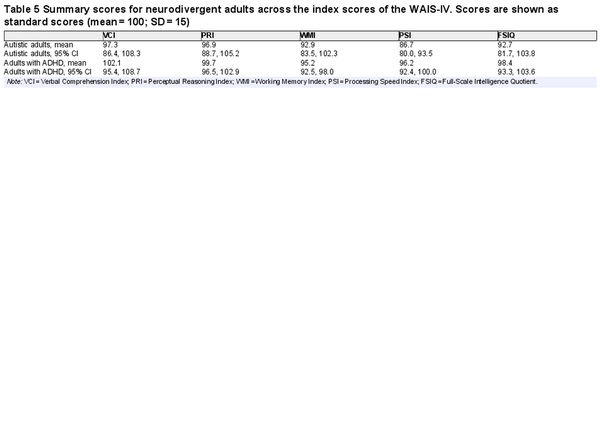
Table 5 shows mean WAIS-IV index scores generated through meta-analysis across the samples of neurodivergent adults. All mean index scores were in the Average range, except for PSI in autistic adults which was Low Average. Most mean index scores did not differ significantly from the normative mean of 100, indicating that we cannot reliably distinguish adult neurodivergent groups from the general population for most indexes. Among autistic adults, only PSI was significantly lower than the normative mean, p < .001; this was by 13.3 points. Given that 15 points is 1 SD, this represents a large effect size (). Among adults with ADHD, WMI was slightly lower than the normative mean, p < .001, although the effect size was small (4.8 points below the normative mean).
There was significant heterogeneity in WAIS-IV index scores in neurodivergent adults. I2 values varied between 96.2% and 98.3% across the five indexes in autistic adults and between 75.3% and 96.1% in adults with ADHD. It was hypothesized that heterogeneity was likely influenced by general ability in the samples (i.e., samples of higher ability likely performed more highly on all the indexes). Nonverbal ability (PRI) was specified as a moderator, and all meta-analyses (except for the ones modeling PRI and FSIQ) were rerun with PRI as a moderator. This time, I2 values varied between 53.7% and 88.8% in autistic adults and between 7.5% and 70.4% in adults with ADHD (in adults with ADHD, WMI that had a much lower heterogeneity when controlling for FRI, whereas other I2 values remained high). Overall, general ability explained some of the heterogeneity among neurodivergent adults, but there was still considerable variability across the samples included in the meta-analyses, suggesting variable patterns of performance across the WAIS-IV in the different samples. The possibility of publication bias was also assessed for these meta-analyses. Sample size was not associated with index scores in neurodivergent adults, all ps > 0.200, indicating no evidence for publication bias.
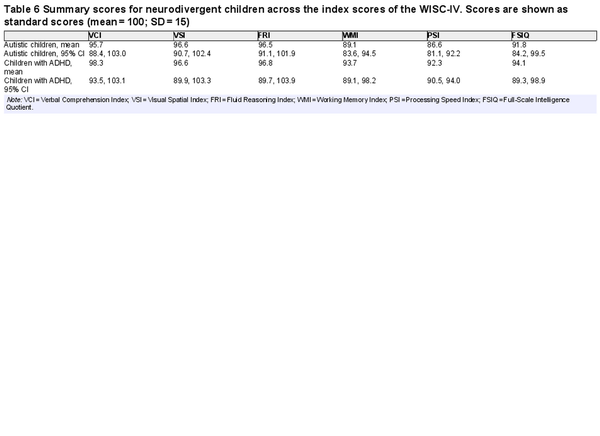
Table 6 shows mean WISC-V index scores generated in meta-analysis for neurodivergent children. Most mean index scores were in the Average range, except for WMI and PSI in autistic children, which were Low Average. Controlling for multiple comparisons, autistic children scored significantly lower than the normative mean on WMI (by 10.9 points, a medium effect), p < .001, and PSI (by 13.4 points, a large effect), p < .001. This was also the case in children with ADHD, who scored lower than the normative mean on WMI (by 6.3 points, a small effect), p = .006, and PSI (by 7.7 points, a medium effect), p < .001.
There was significant heterogeneity in WISC-V index scores in neurodivergent children. I2 values varied between 92.1% and 95.6% for indexes in autistic children and between 0% and 88.7% in children with ADHD. As with the adult samples, it was hypothesized that heterogeneity was likely influenced by general ability in the samples. Nonverbal ability (FRI) was specified as a moderator and all meta-analyses were rerun (except for the ones modeling FRI and FSIQ) with FRI as a moderator. This time, I2 values were 0% across all child samples for every index. This indicates that, when controlling for general cognitive ability, patterns of index scores were similar across the samples included in this meta-analysis. The possibility of publication bias was also assessed. Sample size was not associated with index scores in autistic children, all ps > 0.600, but was in children with ADHD for all indexes except PSI, p > 0.001. Larger samples were associated with lower scores in children diagnosed with ADHD, so the overall scores may slightly overestimate ability in children with ADHD. This is likely due to a methodological artifact rather than publication bias per se, as one of the two samples contributed by was very small and only consisted of children with no evidence of specific learning difficulties, whereas eligibility criteria in other samples were more inclusive.
Meta-Analysis 2. Comparing index scores between autism and ADHD
There were no significant differences in index scores when comparing people diagnosed with autism to those with ADHD, although we should note that this analysis was not well powered to detect differences due to the relatively small groups and large variances. Among adults, PSI tended to be lower in autistic adults compared to adults with ADHD, 9.6 points [−.4, 19.5]; however, this was not statistically significant, p = .059. Differences in other index scores were more convincingly nonsignificant among adults, all ps > 0.450. Neurodivergent children showed a similar pattern to adults, with similar scores for most indexes, all ps > 0.180, except PSI, which showed a trend for lower scores in autistic children compared to children with ADHD, 5.9 points [−.3, 12.1], p = .062.
Meta-Analysis 3. Comparing index scores within groups
Lastly, index scores were compared within groups to explore whether there was a pattern of relative strengths and difficulties for each group. Among autistic groups, there were difficulties in working memory and processing speed in the context of relative strengths in verbal and nonverbal reasoning. Compared to their PRI, autistic adults had significantly lower WMI, 5.84 points [1.9, 9.8], p = .010, and lower PSI, 9.2 points [6.1, 12.4], p < .001. PRI and VCI did not differ in autistic adults, p = .692. Compared to their FRI, autistic children showed lower WMI, 7.6 points [6.2, 9.0], p < .001, and lower PSI, 10.4 points [6.7, 14.0], p = .003. VCI and VSI did not differ from FRI, ps > 0.250.
ADHD samples showed less marked patterns than autistic groups. Compared to their PRI, adults with ADHD had lower WMI by 4.2 points [1.5, 6.8], p = .015. There was a trend for PSI to be lower by 3.6 points [−1.3, 8.5], but this was nonsignificant, p = .103. PRI and VCI did not differ among adults with ADHD, p = .780. Among children with ADHD, there were no statistical differences between FRI and any other index, all ps > 0.180.
Subtest performance
See Fig. 2a–d for subtest scores. These plots report data from 95 autistic adults, 532 autistic children, 160 adults with ADHD, and 151 children with ADHD.
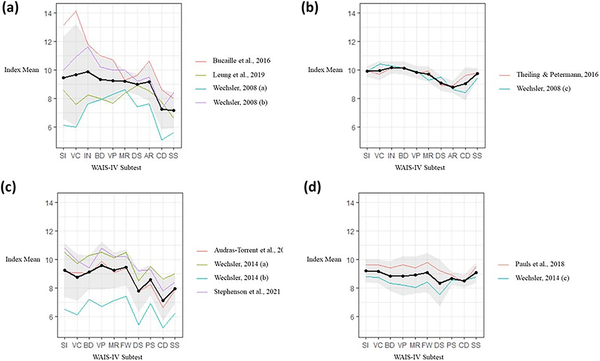
Fig. 2
(a) Plot showing subtest scaled scores of autistic adults on the WAIS-IV. (b) Plot showing subtest scaled scores of adults with ADHD on the WAIS-IV. (c) Plot showing subtest scaled scores of autistic children on the WISC-V. (d) Plot showing subtest scaled scores of children with ADHD on the WISC-V. (a-d) In all plots, the thick black line represents summary means calculated through meta-analysis. The thinner coloured lines represent means from the individual studies. Note: SI = Similarities; VC = Vocabulary; IN = Information; BD = Block Design; VP = Visual Puzzles; MR = Matrix Reasoning; DS = Digit Span; AR = Arithmetic; CD = Coding; SS = Symbol Search; FW = Figure Weights; PS = Picture Span.
Discussion
There was evidence of a particular cognitive profile on the Wechsler intelligence tests in autistic groups, both in children and adults. This was characterized by verbal and nonverbal reasoning in the Average range, with slightly lower working memory and more significantly reduced processing speed. For both autistic children and adults, mean processing speed was Low Average (i.e., below the 25th percentile). Among individuals with ADHD, there was less robust evidence of a profile. Means of all index scores were in the Average range for people with ADHD, with a pattern of slightly lower working memory in adults and children. Processing speed was possibly slightly reduced in children with ADHD compared to the norm, but not necessarily in relation to their own ability. It remains to be seen whether the differences in groups with ADHD are clinically meaningful. Subtest-level analyses gave a similar pattern of results. There were flat profiles in the typical range for the ADHD samples, and a more uneven profile in the autistic samples, with lowest scores in subtests requiring time-pressured efficient processing of information. However, these results need to be held in mind with several factors. First, the quantitative analyses showed significant variability in performance across samples, as shown by the heterogeneity and large confidence intervals around values. In addition, several papers attempted to use cognitive profile on the Wechsler tests to distinguish individuals with an autism diagnosis from those without a neurodevelopmental diagnosis, but with limited success (e.g., ; see Appendix B for a narrative review of papers relevant to this issue). Importantly, as highlighted by , there may also be psychometric issues with conducting fine-grained analysis of profiles, as domain-level indexes show poor reliability after accounting for variance associated with FSIQ. This is a key issue that needs to be further researched, as fine-grained analysis of an individual’s performance on a cognitive assessment is commonplace in clinical practice.
Despite these factors, reduced processing speed among autistic people does stand out, given the extent of this reduction compared to the norm. Across samples of autistic children and adults, mean processing speed was ~1 SD lower than the normative mean, and lower than participants’ mean verbal and nonverbal reasoning by ~10 points. Studies reporting subtest-level data also support this picture, as autistic children and adults tended to receive their lowest scores on processing speed tests (especially Coding). This is consistent with previous research, as autistic people have been reported to score low on the Coding subtest in older versions of the Wechsler tests (see for a review) as well as on other measures of speed including simple reaction time (). Lower processing speed is also consistent with biological accounts of the autistic brain, which indicates reduced long-range connectivity in the brain (in particular, between frontal and posterior regions of cortex; ). However, low processing speed would not be appropriate as a diagnostic marker for autism, as this is found across different neurological and psychiatric conditions (). Nonetheless, low processing speed is likely to be common enough in autistic people that it may be useful to screen for these difficulties, as they have real-world impacts. For instance, lower processing speed has been found to correlate with adaptive communication skills () and predicts academic underachievement () in autistic children. However, the extent to which low processing speed predicts real-world impacts, independently of other Wechsler indices, is unclear from the literature and would be worth further investigation.
It is worth bearing in mind what “low processing speed” might mean, as it may or may not reflect slower mental processing. The motor demand in tasks may partly explain the differences between autistic and nonautistic groups in PSI, as increasing the motor requirement increases group differences on speeded tasks () and pure inspection time (without a motor requirement) may represent a relative strength for autistic people (). Thus, we should be cautious about necessarily equating lower PSI with lower mental speed. As noted by , Wechsler processing speed tests are not pure measures of speed, but tap multiple processes including visual scanning, fine motor skills, short-term memory, interference control, and choice of strategies to optimize speed-accuracy trade-offs—in essence, these tests are measures of complex processing capacity. Another issue is that “processing speed” may or may not be the same construct when measured in autistic compared to neurotypical people using the Wechsler tests, as these tests were not devised with the autistic brain in mind. It is notable, for instance, that the WISC was found to underestimate autistic intelligence in relation to another intelligence test (Raven’s Progressive Matrices) whereas this was not the case in neurotypical controls ().It would be helpful, therefore, to test the equivalence of the “processing speed” construct between neurodivergent and neurotypical groups to ensure that differences are not due to a measurement bias.
As noted previously, there was less robust evidence of a specific profile in ADHD. WMI was the one index where there was a reliable difference from the norm in both children and adults, but this was a modest effect size (about half an SD or lower). This is similar to existing literature, which has documented working memory difficulties in individuals with ADHD of a similar effect size (e.g., see for a review). The lack of a reliable difference in PSI is surprising, as there seems to be a view in the literature that significantly reduced processing speed is common in ADHD. For instance, start from the assumption that processing speed is significantly reduced in ADHD in their review of functional correlates of lower processing speed in ADHD. However, this may not be a key feature in ADHD. Where there are slightly lower scores among groups with ADHD, we also need to be mindful of possible co-occurring specific learning difficulties that may account for scores (e.g., ). Overall, the rather flat cognitive profiles of the ADHD groups echo previous research that has highlighted the questionable utility of neuropsychological assessment in the diagnosis of ADHD (; ). We might conclude, therefore, that the Wechsler tests are not helpful in diagnosing ADHD, although this is not to underplay that the tests will help identify any individual’s personal strengths and weaknesses, which may support their learning and occupational functioning. For instance, the indexes appear to be associated with various measures of adaptive behavior across different papers reviewed in the narrative synthesis. This can likely be understood in the context of the well-established finding that FSIQ predicts a variety of outcomes (). Therefore, a cognitive assessment can tell us something about a person’s functioning whether or not they have a neurodevelopmental condition. One thing that remains unclear is whether an assessment actually improves a person’s functioning (e.g., through building insight into their personal cognitive profile). This has only received limited attention in the research literature, and no studies have evaluated the added value of a cognitive assessment for autistic people ().
Limitations
There are several limitations to hold in mind about this review. The papers included are all subject to various limitations including (i) use of convenience samples, (ii) poor characterization of co-occurring neurodevelopmental traits, which makes it difficult to be sure that an observed profile is truly related to autism or ADHD and not a co-occurring condition (e.g., dyslexia), and (iii) arbitrary exclusion criteria based on cognitive functioning (i.e., individuals with an IQ below a certain threshold were not included in many studies). These factors may bias estimates in this review. For instance, the FSIQ exclusion criteria may have meant individuals with lower scores on certain indexes were more likely to be excluded (as subtests within the verbal and perceptual domains contribute more strongly to FSIQ than those within the working memory and processing speed domains). These criteria may have biased the results—although the large reduction in processing speed in autistic people found in this review was possibly too large to be simply an artifact of this sampling approach. Certainly, the FSIQ exclusion criteria do mean that a large population of autistic individuals has been undersampled in this review—those with co-occurring intellectual disability. In addition, autistic adults diagnosed as children have been undersampled in this review, as most adults were diagnosed as adults, which is important, as age at diagnosis is likely to influence presentation (). This review has also undersampled females and individuals with diverse gender identities. Although the ratio of males to females in this review (~3:1) is largely representative of neurodevelopmental conditions (), the small numbers do make it difficult to know how well the results specifically apply beyond males.
This paper has been restricted to the latest versions of the Wechsler tests (WAIS-IV and WISC-V). The purposes of this were to optimize the relevance of the review for clinicians practicing today, as well as to restrict the amount of research to review—the Wechsler tests are so widely used in research that a review covering all versions would be impractical and overly heterogeneous. There was also the related issue that even when looking at just these two versions of the Wechsler tests, use of the tests is very variable across the research literature, especially when the tests are just used to characterize the sample or apply eligibility criteria (in these cases, it was common for only certain participants to be tested or only certain subtests to be given). This meant it was necessary to approach the review in a principled manner, only including papers where the 10 core subtests were administered and the main indexes reported, which had the positive effect of making the review manageable but with the possible side-effect of reducing overall sample size. This means the overall sample size, while reasonably big, is not well powered for some analyses (such as directly comparing the autistic and ADHD samples).
In addition, an inherent weakness of this type of meta-analysis is the lack of analysis at the individual level. Although the studies showed evidence of cognitive profiles at a group level, it is unclear how common these are on the individual level. In addition, it is plausible that there may be cognitive markers on the individual level that get obscured at the group level (i.e., individuals with neurodevelopmental differences may be more likely to show a variable profile, but this may be distinct to the individual rather than the condition). We know that variability is an element in the cognitive profile of neurodevelopmental conditions (e.g., there is greater variability in reaction time across tasks in people diagnosed with autism and ADHD; ), but we don’t know how this may (or not) present on the Wechsler tests. It is not possible to assess for these issues when only dealing with summary statistics rather than raw data. An interesting suggestion that variability may be a part of the cognitive profile was the heterogeneity analyses among adults diagnosed with autism or ADHD. These analyses showed that patterns of performance across the indices varied across samples. This may have been influenced by variability on the individual level.
Clinical Implications
This review suggests there is no clinically significant cognitive profile that can be detected in ADHD using a WISC-V or WAIS-IV assessment. In the case of autism, processing speed is reduced in both children and adults with a large effect size, which might be important to bear in mind when working with this population. It should be noted that processing speed differences are neither sufficiently sensitive nor specific to use for diagnostic purposes, and there would be a danger of biasing diagnostic decisions if reduced processing speed was considered a cognitive marker of autism. Nonetheless, there is good evidence for a pattern of strengths and difficulties on the Wechsler tests among autistic people, defined by relatively good reasoning skills in the presence of reduced processing speed. This is a pattern that can be supported with appropriate adjustments (e.g., more time for tasks), and the recognition of strengths may improve self-confidence and other people’s understanding of the issues, as well as prompt ideas for person-centered compensatory strategies.
In summary, this review investigated evidence for a distinctive cognitive profile in people with neurodevelopmental conditions on the most recent editions of the Wechsler intelligence tests, the WAIS-IV and WISC-V. Test performance was collated from over 1,800 individuals with a diagnosis of autism or ADHD reported in 18 different sources of data. Among autistic children and adults, there was a consistent pattern of verbal and nonverbal reasoning representing relative strengths, with a significant weakness in processing speed and slight weakness in working memory. People diagnosed with ADHD tended to show slightly weaker working memory than their other abilities, but this was a modest difference. Based on the review, it is unlikely to be helpful to conduct a cognitive assessment for diagnostic purposes, but an assessment may be helpful in determining an individual’s profile of strengths and difficulties, especially among autistic people.
Conflict of Interest
None declared.
Appendix A. Risk-of-bias assessment
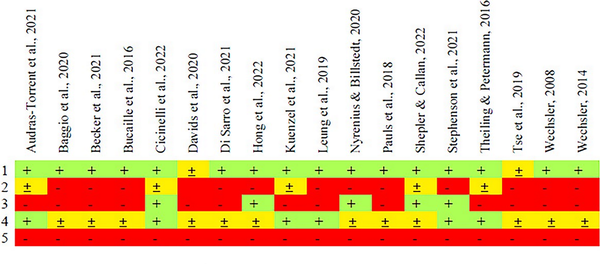
Studies are rated for high (-), medium (±), and low (+) risk of bias for the following qualities:
Internal Validity of the Sample I. Are we confident the study recruited individuals meeting criteria for the relevant neurodevelopmental diagnosis?
Internal Validity of the Sample II. Are we confident that co-occurring conditions have been adequately assessed? As this study compares autism and ADHD, it is important to know the extent to which these might co-occur at clinical or subclinical levels in the sample.
External Validity of the Sample I. Are we confident the sample is inclusive of individuals of varying intellectual ability? Given the focus of this study on cognitive abilities, we required studies to be inclusive of individuals regardless of IQ to avoid biasing profiles (although we allowed for exclusion of individuals unable to complete a cognitive assessment).
External Validity of the Sample II. Are we confident that the sample is a relatively random community/clinical sample? For this, we disregarded IQ eligibility criteria as that was considered in (3), and considered issues such as other eligibility criteria, missing data, recruitment pathway, and site of recruitment.
Validity of Assessment. Are we confident that assessors were blinded to avoid bias in profiling the abilities of neurodivergent people?
Appendix B. Narrative summary
A few studies investigated aspects of cognitive profile beyond index and subtest means, including whether information from more than one index could distinguish neurodivergent from control participants on the individual level. found that index scores were only effective in distinguishing autistic children with a co-occurring language impairment, suggesting that the WISC-V was sensitive to language/learning difficulties rather than autism. found that WMI was moderately effective in differentiating adults with ADHD from those with a mental health diagnosis; however, the WAIS-IV was administered during the diagnostic process, so there is a significant risk of bias (i.e., participants scoring lower on the WAIS-IV may have been more likely to receive an ADHD diagnosis). Two studies looked at discrepancies between indexes (; ), and both studies’ discrepancies were more common in autistic adults (approximately twice as common as the norm). However, both studies had small samples, and it is unclear whether these were planned analyses, so there is a high risk of type I error. Overall, studies present limited and weak evidence for using a specific profile to distinguish neurodivergent individuals from the general population at the individual level.
Several studies found that Wechsler index scores correlated with adaptive behavior in autistic children () and adults (; ). In autistic children, this included moderate correlations between all WISC-V indexes and communication skills (.31 < r < .50) and small correlations between VSI, FRI, and FSIQ and daily living skills (r = .20 or .21), but no correlations with social skills (). In autistic adults, there were moderate correlations between FSIQ, WMI, and PSI and adaptive behavior in the general, conceptual, and practical domains (.40 < r < .49; ). found that parent- and self-rated social skills correlated in some cases with WAIS-IV indexes, but many correlations were computed, and so, there is a risk of false positives. In adults with ADHD, found that lower FSIQ was associated with lower probability of higher education and currently being in work/education and greater probability of repeating a grade at school (N = 66). Overall, there is evidence that cognitive scores correlate with adaptive skills, but these relationships do not seem specific to any one index.
looked at psychometric properties of the WISC-V in autistic children (N = 349). They found that the WISC-V did not have the same psychometric structure across autistic and nonautistic groups; specifically, autistic children performed lower on the Digit Span and Coding subtests than would be expected based on their performance across all other subtests. In addition, the researchers found limited reliability of the domain-level indexes after accounting for variance relating to FSIQ. Overall, results from this paper suggest that the WISC-V may detect some autism-specific cognitive differences on particular subtests, but this should be interpreted cautiously on the individual level as such differences may not be psychometrically reliable.
References
- Aiello R., Ruble L., Esler A. (2017). National study of school psychologists’ use of evidence-based assessment in autism spectrum disorder. Journal of Applied School Psychology, 33(1), 67–88. https://doi.org/10.1080/15377903.2016.1236307.
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (5th ed.). Washington, D.C.: American Psychiatric Publishing. https://doi.org/10.1176/appi.books.9780890425596.
- Audras-Torrent L., Miniarikova E., Couty F., Dellapiazza F., Berard M., Michelon C., et al (2021). WISC-V profiles and their correlates in children with autism spectrum disorder without intellectual developmental disorder: Report from the ELENA cohort. Autism Research, 14(5), 997–1006. https://doi.org/10.1002/aur.2444.
- Baggio S., Hasler R., Deiber M. P., Heller P., Buadze A., Giacomini V., et al (2020). Associations of executive and functional outcomes with full-score intellectual quotient among ADHD adults. Psychiatry Research, 294, 113521. https://doi.org/10.1016/j.psychres.2020.113521.
- Barbeau E. B., Soulières I., Dawson M., Zeffiro T. A., Mottron L. (2013). The level and nature of autistic intelligence III: Inspection time. Journal of Abnormal Psychology, 122(1), 295–301. https://doi.org/10.1037/a0029984.
- Becker A., Daseking M., Kerner auch Koerner J. (2021). Cognitive profiles in the WISC-V of children with ADHD and specific learning disorders. Sustainability, 13(17), 9948. https://doi.org/10.3390/su13179948.
- Bölte S., Neufeld J., Marschik P. B., Williams Z. J., Gallagher L., Lai M. C. (2023). Sex and gender in neurodevelopmental conditions. Nature Reviews. Neurology, 19(3), 136–159. https://doi.org/10.1038/s41582-023-00774-6.
- Bucaille A., Grandgeorge M., Degrez C., Mallégol C., Cam P., Botbol M., et al (2016). Cognitive profile in adults with Asperger syndrome using WAIS-IV: Comparison to typical adults. Research in Autism Spectrum Disorders, 21, 1–9. https://doi.org/10.1016/j.rasd.2015.09.001.
- Charman T., Pickles A., Simonoff E., Chandler S., Loucas T., Baird G. (2011). IQ in children with autism spectrum disorders: Data from the special needs and autism project (SNAP). Psychological Medicine, 41(3), 619–627. https://doi.org/10.1017/S0033291710000991.
- Chiang H. M., Tsai L. Y., Cheung Y. K., Brown A., Li H. (2014). A meta-analysis of differences in IQ profiles between individuals with Asperger's disorder and high-functioning autism. Journal of Autism and Developmental Disorders, 44(7), 1577–1596. https://doi.org/10.1007/s10803-013-2025-2.
- Cicinelli G., Nobile E., Brighenti S., Bari S., Tonella E., Aresi A., et al (2022). Wechsler intelligence scale for adults - fourth edition profiles of adults with autism spectrum disorder. Epidemiology and Psychiatric Sciences, 31, e67. https://doi.org/10.1017/S2045796022000506.
- Cohen J. (1988). Statistical power analysis for the behavioral sciences. New York: Routledge.
- Cook N. E., Braaten E. B., Surman C. B. H. (2018). Clinical and functional correlates of processing speed in pediatric attention-deficit/hyperactivity disorder: A systematic review and meta-analysis. Child Neuropsychology, 24(5), 598–616. https://doi.org/10.1080/09297049.2017.1307952.
- Dale B. A., Finch W. H., Shellabarger K. A. R., Davis A. (2021). Wechsler intelligence scale for children, fifth edition profiles of children with autism spectrum disorder using a classification and regression trees analysis. Journal of Psychoeducational Assessment, 39(7), 783–799. https://doi.org/10.1177/07342829211025924.
- Dale B. A., Finch W. H., Shellabarger K. A. R., Davis A. (2022). Comparison of verbal performance of children with autism spectrum disorder on the WISC-V. Journal of Psychoeducational Assessment, 40(7), 811–824. https://doi.org/10.1177/07342829221106592.
- Davids R. C. D., Groen Y., Berg I. J., Tucha O., van Balkom I. D. C. (2020). Local-global processing approaches in older autistic adults: A matched control study using RCFT and WAIS-IV. Research in Autism Spectrum Disorders, 78, 101655. https://doi.org/10.1016/j.rasd.2020.101655.
- Dawson M., Soulières I., Gernsbacher M. A., Mottron L. (2007). The level and nature of autistic intelligence. Psychological Science, 18(8), 657–662. https://doi.org/10.1111/j.1467-9280.2007.01954.x.
- Di Sarro R., Di Santantonio A., Desideri L., Varrucciu N. (2021). Profiling planning skills and cognitive flexibility of adults with autism spectrum disorders: Preliminary results from an exploratory service-based study. International Journal of Developmental Disabilities, 68(5), 651–657. https://doi.org/10.1080/20473869.2020.1871311.
- Donders J. (2020). The incremental value of neuropsychological assessment: A critical review. The Clinical Neuropsychologist, 34(1), 56–87. https://doi.org/10.1080/13854046.2019.1575471.
- Donovan A. P., Basson M. A. (2017). The neuroanatomy of autism - a developmental perspective. Journal of Anatomy, 230(1), 4–15. https://doi.org/10.1111/joa.12542.
- Drukker M., Weltens I., van Hooijdonk C. F. M., Vandenberk E., Bak M. (2021). Development of a methodological quality criteria list for observational studies: The observational study quality evaluation. Frontiers in Research Metrics and Analytics, 6, 675071. https://doi.org/10.3389/frma.2021.675071.
- Ehlers S., Nydén A., Gillberg C., Sandberg A. D., Dahlgren S. O., Hjelmquist E., et al (1997). Asperger syndrome, autism and attention disorders: A comparative study of the cognitive profiles of 120 children. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 38(2), 207–217. https://doi.org/10.1111/j.1469-7610.1997.tb01855.x.
- Faraone S. V., Banaschewski T., Coghill D., Zheng Y., Biederman J., Bellgrove M. A., et al (2021). The world federation of ADHD international consensus statement: 208 evidence-based conclusions about the disorder. Neuroscience and Biobehavioral Reviews, 128, 789–818. https://doi.org/10.1016/j.neubiorev.2021.01.022.
- Guilmette T. J., Sweet J. J., Hebben N., Koltai D., Mahone E. M., Spiegler B. J., et al (2020). American Academy of Clinical Neuropsychology consensus conference statement on uniform labeling of performance test scores. The Clinical Neuropsychologist, 34(3), 437–453. https://doi.org/10.1080/13854046.2020.1722244.
- Happé F. G. (1994). Wechsler IQ profile and theory of mind in autism: A research note. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 35(8), 1461–1471. https://doi.org/10.1111/j.1469-7610.1994.tb01287.x.
- Hayes J., Ford T., Rafeeque H., Russell G. (2018). Clinical practice guidelines for diagnosis of autism spectrum disorder in adults and children in the UK: A narrative review. BMC Psychiatry, 18(1), 222. https://doi.org/10.1186/s12888-018-1800-1.
- Hedges L. V., Tipton E., Johnson M. C. (2010). Robust variance estimation in meta-regression with dependent effect size estimates. Research Synthesis Methods, 1(1), 39–65. https://doi.org/10.1002/jrsm.5.
- Holdnack J., Goldstein G., Drozdick L. (2011). Social perception and WAIS-IV performance in adolescents and adults diagnosed with Asperger's syndrome and autism. Assessment, 18(2), 192–200. https://doi.org/10.1177/1073191110394771.
- Hong J. S., Lee Y. S., Hong M., Kim B., Joung Y. S., Yoo H. K., et al (2022). Cognitive developmental trajectories in adult ADHD patients and controls: A comparative study. Journal of Attention Disorders, 26(3), 391–407. https://doi.org/10.1177/1087054720978548.
- Just M. A., Keller T. A., Malave V. L., Kana R. K., Varma S. (2012). Autism as a neural systems disorder: A theory of frontal-posterior underconnectivity. Neuroscience and Biobehavioral Reviews, 36(4), 1292–1313. https://doi.org/10.1016/j.neubiorev.2012.02.007.
- Kanai C., Hashimoto R., Itahashi T., Tani M., Yamada T., Ota H., et al (2017). Cognitive profiles of adults with high-functioning autism spectrum disorder and those with attention-deficit/hyperactivity disorder based on the WAIS-III. Research in Developmental Disabilities, 61, 108–115. https://doi.org/10.1016/j.ridd.2016.12.008.
- Karalunas S. L., Geurts H. M., Konrad K., Bender S., Nigg J. T. (2014). Annual research review: Reaction time variability in ADHD and autism spectrum disorders: Measurement and mechanisms of a proposed trans-diagnostic phenotype. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 55(6), 685–710. https://doi.org/10.1111/jcpp.12217.
- Keehn B., Müller R. A., Townsend J. (2013). Atypical attentional networks and the emergence of autism. Neuroscience and Biobehavioral Reviews, 37(2), 164–183. https://doi.org/10.1016/j.neubiorev.2012.11.014.
- Kenny L., Hattersley C., Molins B., Buckley C., Povey C., Pellicano E. (2016). Which terms should be used to describe autism? Perspectives from the UK autism community. Autism, 20(4), 442–462. https://doi.org/10.1177/1362361315588200.
- Kenworthy L., Yerys B. E., Weinblatt R., Abrams D. N., Wallace G. L. (2013). Motor demands impact speed of information processing in autism spectrum disorders. Neuropsychology, 27(5), 529–536. https://doi.org/10.1037/a0033599.
- Kuenzel E., Seguin D., Nicolson R., Duerden E. G. (2021). Early adversity and positive parenting: Association with cognitive outcomes in children with autism spectrum disorder. Autism Research, 14(12), 2654–2662. https://doi.org/10.1002/aur.2613.
- Lai M. C., Baron-Cohen S. (2015). Identifying the lost generation of adults with autism spectrum conditions. The Lancet Psychiatry, 2(11), 1013–1027. https://doi.org/10.1016/S2215-0366(15)00277-1.
- Lange K. W., Hauser J., Lange K. M., Makulska-Gertruda E., Takano T., Takeuchi Y., et al (2014). Utility of cognitive neuropsychological assessment in attention-deficit/hyperactivity disorder. Attention Deficit and Hyperactivity Disorders, 6(4), 241–248. https://doi.org/10.1007/s12402-014-0132-3.
- Leung C. N. W., Chan R. W. S., Fan J. T. C., Lam C. H. Y., Yau S. S. W. (2019). IQ profiling, autistic traits and social competence in Chinese adults with high-functioning ASD: A comparison between self- and parent-report. The European Journal of Psychiatry, 33(1), 24–31. https://doi.org/10.1016/j.ejpsy.2018.10.001.
- Mayes S. D., Calhoun S. L. (2004). Similarities and differences in Wechsler Intelligence Scale for Children--Third Edition (WISC-III) profiles: Support for subtest analysis in clinical referrals. The Clinical Neuropsychologist, 18(4), 559–572. https://doi.org/10.1080/13854040490888530.
- Mayes S. D., Calhoun S. L. (2006). WISC-IV and WISC-III profiles in children with ADHD. Journal of Attention Disorders, 9(3), 486–493. https://doi.org/10.1177/1087054705283616.
- Mayes S. D., Calhoun S. L. (2007). Learning, attention, writing, and processing speed in typical children and children with ADHD, autism, anxiety, depression, and oppositional-defiant disorder. Child Neuropsychology, 13(6), 469–493. https://doi.org/10.1080/09297040601112773.
- National Institute for Health and Care Excellence. (2011). Autism spectrum disorder in under 19s: Recognition, referral and diagnosis (clinical guideline CG128). https://www.nice.org.uk/guidance/cg128
- Nisbett R. E., Aronson J., Blair C., Dickens W., Flynn J., Halpern D. F., et al (2012). Intelligence: New findings and theoretical developments. The American Psychologist, 67(2), 130–159. https://doi.org/10.1037/a0026699.
- Nyrenius J., Billstedt E. (2020). The functional impact of cognition in adults with autism spectrum disorders. Nordic Journal of Psychiatry, 74(3), 220–225. https://doi.org/10.1080/08039488.2019.1694698.
- Oliveras-Rentas R. E., Kenworthy L., Roberson R. B. 3rd, Martin A., Wallace G. L. (2012). WISC-IV profile in high-functioning autism spectrum disorders: Impaired processing speed is associated with increased autism communication symptoms and decreased adaptive communication abilities. Journal of Autism and Developmental Disorders, 42(5), 655–664. https://doi.org/10.1007/s10803-011-1289-7.
- O'Reilly C., Lewis J. D., Elsabbagh M. (2017). Is functional brain connectivity atypical in autism? A systematic review of EEG and MEG studies. PLoS One, 12(5), e0175870. https://doi.org/10.1371/journal.pone.0175870.
- Pauls F., Daseking M., Jacobs C., Werpup L., Petermann F. (2018). Intelligenzdiagnostik bei Kindern und Jugendlichen mit ADHS: Eine analyse der WISC-V-Leistungsprofile (Measuring intellectual performance of children and adolescents with ADHD: An analysis of WISC-V profiles). Kindheit und Entwicklung, 27(3), 165–174. https://doi.org/10.1026/0942-5403/a000256.
- Pettersson R., Söderström S., Nilsson K. W. (2018). Diagnosing ADHD in adults: An examination of the discriminative validity of neuropsychological tests and diagnostic assessment instruments. Journal of Attention Disorders, 22(11), 1019–1031. https://doi.org/10.1177/1087054715618788.
- R Core Team (2022). R: A language and environment for statistical computing. Vienna: R Foundation for Statistical Computing. https://www.R-project.org/
- Rabin L. A., Paolillo E., Barr W. B. (2016). Stability in test-usage practices of clinical neuropsychologists in the United States and Canada over a 10-year period: A follow-up survey of INS and NAN members. Archives of Clinical Neuropsychology, 31(3), 206–230. https://doi.org/10.1093/arclin/acw007.
- Ramos A. A., Hamdan A. C., Machado L. (2020). A meta-analysis on verbal working memory in children and adolescents with ADHD. The Clinical Neuropsychologist, 34(5), 873–898. https://doi.org/10.1080/13854046.2019.1604998.
- Rommelse N., Luman M., Kievit R. (2020). Slow processing speed: A cross-disorder phenomenon with significant clinical value, and in need of further methodological scrutiny. European Child & Adolescent Psychiatry, 29(10), 1325–1327. https://doi.org/10.1007/s00787-020-01639-9.
- Rong Y., Yang C.-J., Jin Y., Wang Y. (2021). Prevalence of attention-deficit/hyperactivity disorder in individuals with autism spectrum disorder: A meta-analysis. Research in Autism Spectrum Disorders, 83, 101759. https://doi.org/10.1016/j.rasd.2021.101759.
- Russell G., Stapley S., Newlove-Delgado T., Salmon A., White R., Warren F., Pearson A., Ford T. (2022). Time trends in autism diagnosis over 20 years: A UK population-based cohort study. Journal of Child Psychology and Psychiatry, and Allied Disciplines, 63(6), 674–682. https://doi.org/10.1111/jcpp.13505.
- Shepler D. K., Callan P. D. (2022). Differences in executive functioning between adults with ADHD and those diagnosed with other psychiatric diagnoses: Utility of the CTMT and the WAIS-IV. Applied Neuropsychology Adult. https://doi.org/10.1080/23279095.2022.2102923
- Snow J. B., Sapp G. L. (2000). WISC-III subtest patterns of ADHD and normal samples. Psychological Reports, 87(3 Pt 1), 759–765. https://doi.org/10.2466/pr0.2000.87.3.759.
- Stephenson K. G., Beck J. S., South M., Norris M., Butter E. (2021). Validity of the WISC-V in youth with autism spectrum disorder: Factor structure and measurement invariance. Journal of Clinical Child and Adolescent Psychology, 50(5), 669–681. https://doi.org/10.1080/15374416.2020.1846543.
- Takayanagi M., Kawasaki Y., Shinomiya M., Hiroshi H., Okada S., Ino T., et al (2022). Review of cognitive characteristics of autism spectrum disorder using performance on six subtests on four versions of the Wechsler Intelligence Scale for Children. Journal of Autism and Developmental Disorders, 52(1), 240–253. https://doi.org/10.1007/s10803-021-04932-x.
- Theiling J., Petermann F. (2016). Neuropsychological profiles on the WAIS-IV of adults with ADHD. Journal of Attention Disorders, 20(11), 913–924. https://doi.org/10.1177/1087054713518241.
- Theiling J., Petermann F., Daseking M. (2013). Zusammenhang zwischen selbsteingeschätzter ADHS-Symptomatik und der Leistungsfähigkeit in der WAIS-IV (Relationship between self-reported ADHD symptoms and WAIS-IV performance). Gesundheitswesen, 75(11), 768–774. https://doi.org/10.1055/s-0033-1357163.
- Tse V. W. S., Crabtree J., Islam S., Stott J. (2019). Comparing intellectual and memory abilities of older autistic adults with typically developing older adults using WAIS-IV and WMS-IV. Journal of Autism and Developmental Disorders, 49(10), 4123–4133. https://doi.org/10.1007/s10803-019-04122-w.
- Viechtbauer W. (2010). Conducting meta-analyses in R with the metafor package. Journal of Statistical Software, 36(3), 1–48. https://doi.org/10.18637/jss.v036.i03.
- Wechsler D. (2008). Wechsler adult intelligence scale—Fourth edition (WAIS-IV). San Antonio: Psychological Corporation.
- Wechsler D. (2014). Wechsler intelligence scale for children—Fifth edition (WISC-V). Bloomington: Psychological Corporation.
- Zapparrata N. M., Brooks P. J., Ober T. M. (2022). Slower processing speed in autism spectrum disorder: A meta-analytic investigation of time-based tasks. Journal of Autism and Developmental Disorders. https://doi.org/10.1007/s10803-022-05736-3.
- Zayat M., Kalb L., Wodka E. L. (2011). Brief report: Performance pattern differences between children with autism spectrum disorders and attention deficit-hyperactivity disorder on measures of verbal intelligence. Journal of Autism and Developmental Disorders, 41(12), 1743–1747. https://doi.org/10.1007/s10803-011-1207-z.