Pooled RCTs: Adding LABAs to ICSs do not increase serious asthma events and reduce exacerbations
- Peters, Jay I. MD
- Maselli, Diego J. MD
Question
In adolescents and adults with asthma, does adding long-acting β2-agonists (LABAs) to inhaled corticosteroids (ICSs) affect risks for serious asthma events or exacerbations?
Scope
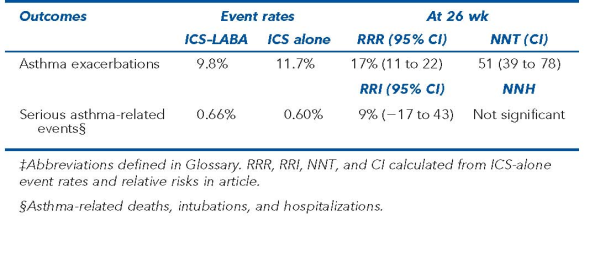
Planned pooled analysis of 4 randomized noninferiority trials (ClinicalTrials.gov NCT01444430, NCT01475721, NCT01471340, and NCT01845025) that compared ICS–LABAs with ICSs alone in adolescents ≥ 12 years of age and adults {who had asthma for ≥ 1 year, used daily asthma medication, and had ≥ 1 exacerbation in the past year but not in the past 4 weeks. Exclusion criteria included life-threatening asthma or smoking history of > 10 pack-years}. Recruitment in 1 trial was stopped early when the study drug was removed from the market. LABAs were formoterol and salmeterol; ICSs were budesonide, fluticasone, and mometasone. Primary outcome was a composite of asthma-related death or intubation. Secondary outcomes included individual components of the primary outcome, serious asthma-related events (death, intubation, and hospitalization), and asthma exacerbations.
Methods
Individual-patient data meta-analysis of 36 010 patients ({mean age 44 y}, 66% girls and women), with follow-up at 26 weeks. The meta-analysis was planned before the 4 trials were designed; data were analyzed using an intention-to-treat approach. Outcome adjudicators were blinded to group allocation.
Main results
For the primary outcome, there were 2 asthma-related deaths (ICS–LABA group) and 3 asthma-related intubations (1 in the ICS–LABA group); this outcome was considered not to have provided robust results. Results for serious asthma-related events and asthma exacerbations are in the Table.
Conclusion
In adolescents and adults with asthma, adding long-acting β2-agonists to inhaled corticosteroids did not increase risk for serious asthma-related events and reduced exacerbations.