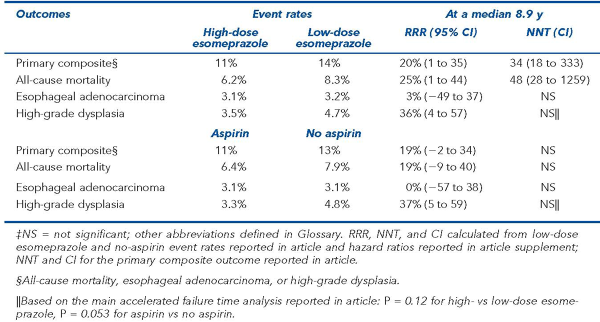
In Barrett esophagus, high- vs low-dose esomeprazole improved clinical outcomes; aspirin vs no aspirin did not
- Marshall, John K. MD, MSc, FRCPC, AGAF
Question
In Barrett esophagus (BE), does high- vs low-dose esomeprazole and aspirin vs no aspirin reduce all-cause mortality, esophageal adenocarcinoma, and high-grade dysplasia?
Methods
Design
Randomized controlled 2 x 2 factorial trial (Aspirin and Esomeprazole Chemoprevention in Barrett's metaplasia Trial [AspECT]). EudraCT 2004-003836-77.
Allocation
Concealed.
Blinding
Blinded {pathologists evaluating adenocarcinoma and dysplasia outcomes}.
Follow-up period
Maximum 10 years (median 8.9 y).
Setting
84 centers in the UK and 1 center in Canada.
Patients
2557 patients ≥ 18 years of age (median age 59 y, 80% men) with BE (≥ 1 cm of columnar-lined esophagus confirmed by histology). Exclusion criteria included esophageal carcinoma, high-grade dysplasia, or use of nonsteroidal antiinflammatory drugs (NSAIDs).
Interventions
Esomeprazole, 40 mg twice/d (high-dose, n = 1281), or 20 mg/d (low-dose, n = 1276). Patients were also randomized to aspirin, 300 to 325 mg/d (n = 1138 in analysis), or no aspirin (n = 1142 in analysis). 255 patients were ineligible for the aspirin analyses.
Outcomes
Primary outcome was a composite of all-cause mortality, esophageal adenocarcinoma, or high-grade dysplasia. Other outcomes included composite outcome components.
Patient follow-up
54% completed the trial. 99% were included in the intention-to-treat analysis.
Main results
The main results are in the Table. There was no interaction between esomeprazole and aspirin {for the primary outcome} (P = 0.281). In analyses censored at first NSAID use, aspirin vs no aspirin reduced the primary composite outcome (n = 2236, 11% vs 13%, P = 0.043).
Conclusion
In Barrett esophagus, high- vs low-dose esomeprazole reduced a composite of all-cause mortality, esophageal adenocarcinoma, or high-grade dysplasia; aspirin vs no aspirin did not.