In at-risk medical patients, rivaroxaban after discharge did not reduce symptomatic or fatal VTE at 45 days
- Dunn, Andrew MD, MPH, MACP, SFHM
- Sacks, Henry S. PhD, MD, FACP
Question
In hospitalized medical patients at risk for venous thromboembolism (VTE), does thromboprophylaxis with rivaroxaban for 45 days after discharge reduce symptomatic or fatal VTE?
Methods
Design
Randomized placebo-controlled trial (Medically Ill Patient Assessment of Rivaroxaban versus Placebo in Reducing Post-Discharge Venous Thrombo-Embolism Risk [MARINER] trial). ClinicalTrials.gov NCT02111564.
Allocation
Concealed.
Blinding
Blinded (patients, investigators, and outcome adjudication committee).
Follow-up period
45 days for efficacy; 75 days for safety.
Setting
671 centers in 36 countries.
Patients
12 024 patients ≥ 40 years of age (mean age 70 y, 52% men) who were hospitalized for 3 to 10 consecutive days with heart failure and left ventricular ejection fraction ≤ 45%, acute respiratory insufficiency, chronic obstructive pulmonary disease exacerbation, acute ischemic stroke, or acute infectious or inflammatory disease; had other VTE risk factors (modified International Medical Prevention Registry on Venous Thromboembolism score ≥ 4 out of 10 [higher scores = greater VTE risk] or score 2 or 3 and plasma D-dimer level > 2 times the upper limit of normal); and had received low-molecular-weight heparin or unfractionated heparin during the hospitalization. Exclusion criteria included bleeding in the past 3 months or high risk for bleeding, active cancer, need for anticoagulant or dual antiplatelet therapy, or other contraindications to rivaroxaban.
Intervention
Rivaroxaban, 7.5 mg/d or 10 mg/d, with dose based on creatinine clearance level (n = 6007), or placebo (n = 6012) for 45 days after discharge.
Outcomes
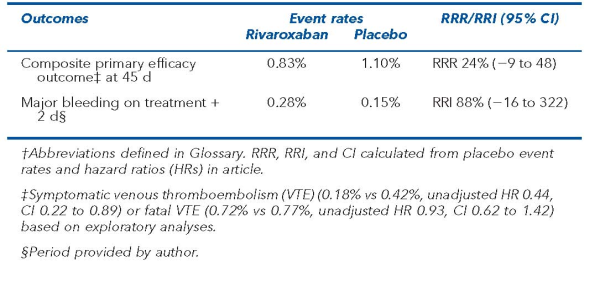
Primary outcomes were a composite of symptomatic or fatal VTE (efficacy) and major bleeding (safety). 161 primary efficacy events were needed to detect a 40% relative reduction in the primary efficacy outcome with rivaroxaban from 2.5% in the placebo group (90% power, 2-sided α = 0.05).
Patient follow-up
> 99% at 45 days (intention-to-treat analysis).
Main results
The main results are in the Table.
Conclusion
In at-risk medical patients, thromboprophylaxis with rivaroxaban after hospital discharge did not reduce a composite of symptomatic or fatal venous thromboembolism at 45 days.