2020 - In COVID-19, adding lopinavir–ritonavir to usual care did not shorten time to clinical improvement
- Yang, Philip MD
- Tekwani, Seema MD, MHA
- Martin, Greg S. MD, MSc
Question
In adults hospitalized with coronavirus disease 2019 (COVID-19), what are the efficacy and safety of adding oral lopinavir–ritonavir to usual care?
Design
Randomized controlled trial (RCT) (Lopinavir Trial for Suppression of SARS-CoV-2 in China [LOTUS China] trial).
Blinding
Treatment allocation concealed; unblinded.*
Setting
Jin Yin-Tan Hospital, Wuhan, Hubei Province, China.
Patients
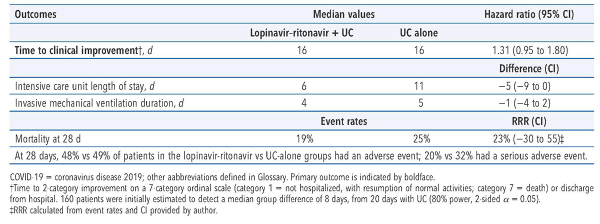
199 patients ≥ 18 years of age (median age 58 y, 60% men, median 13 d from symptom onset to randomization) who had a respiratory tract sample with a reverse transcriptase polymerase chain reaction assay positive for severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2)infection, pneumonia confirmed with chest imaging, and oxygen saturation ≤ 94% when breathing ambient air or a ratio of Pao2:Fio2 ≤ 300 mg Hg. Key exclusions: severe liver disease, HIV infection, or pregnancy or breastfeeding.
Interventions
Oral lopinavir–ritonavir, 400 mg/100 mg twice daily, plus usual care, which could include ventilation, supplemental oxygen, extracorporeal membrane oxygenation, vasopressor support, antibiotics, and renal replacement therapy (n = 99), or usual care alone (n = 100), for 14 days.
Funding
Major Projects of National Science and Technology on New Drug Creation and Development; Chinese Academy of Medical Sciences Emergency Project of Covid-19; National Science Grant for Distinguished Young Scholars.
* See Glossary.
Bottom line:
In adults hospitalized with COVID-19, adding oral lopinavir–ritonavir to usual care did not shorten time to clinical improvement.