Key points
Virtual reality may reduce the severity of behavioural and psychological symptoms of dementia, particularly in reducing aggression, depressive symptoms, and apathy.
Favourable acceptability and minimal side effects were observed across various clinical settings.
The findings are constrained by the limited number of studies available, varied methodology and quality limitations, highlighting the need for future robust research.
Introduction
Dementia is a complex syndrome marked by a progressive decline in the cognitive domains required for independent functioning []. The term behavioural and psychological symptoms of dementia (BPSD) is used to describe the range of non-cognitive symptoms commonly experienced by people living with dementia. BPSD is a widely recognised term in clinical practice, dementia care guidelines, and health policies [, ]. BPSD encompasses various neuropsychiatric symptoms, including agitation, apathy, psychosis, aggression, and sexual disinhibition []. Up to 80% of people living with dementia may develop BPSD over their disease course []. These symptoms can lead to physical injuries, psychological distress and increased caregiver burden, leading to higher rates of institutionalisation and poorer quality of life [].
While antipsychotic medications are often used to manage BPSD in clinical practice, they have limited efficacy and potentially serious side effects []. A recent Cochrane review found weak evidence for the use of antipsychotics in reducing agitation and psychosis in dementia, with observed effectiveness likely reflecting natural symptom improvement as observed in the placebo group []. Due to the risks of somnolence, extrapyramidal features and even death associated with antipsychotics [], there is growing emphasis on exploring non-pharmacological approaches to reduce BPSD.
Non-pharmacological therapies are the preferred first-line treatment for BPSD in most dementia management guidelines []. Interventions ranging from traditional approaches with behavioural therapy, caregiver training and psychosocial interventions to newer therapies incorporating art, music and technology all show promising results []. However, despite several meta-analyses, there is no consensus on the most effective non-pharmacological approach to managing BPSD [, ]. Barriers to broader implementation of non-pharmacological therapies persist due to funding constraints, staffing challenges and reluctance from family or staff []. Therefore, exploring non-pharmacological interventions that are easy to implement, resource-efficient, and effective in reducing BPSD is crucial.
Over the past decade, virtual reality (VR) technologies have emerged as a novel therapy for dementia. VR is a computer-generated three-dimensional (3D) simulation that allows users to experience alternate physical spaces []. Fully immersive VR is accessed through computers with 3D screen displays or head-mounted displays (HMD) with headphones and movement sensors []. The virtual environment updates in real time to reflect the user’s actions []. This immersive experience encourages high engagement with new experiences and social interactions [], which can otherwise be challenging to achieve for those with dementia due to impaired communication skills.
The clinical potential of VR in dementia is broad, encompassing enhanced mobility, falls prevention, cognitive training [], and early identification of cognitive decline []. The feasibility and acceptability of older people with frailty wearing HMD have been shown regardless of mobility or cognitive abilities []. A recent systematic review [] demonstrated that VR improved cognition, quality of life and activities of daily living for older people with dementia. However, there is limited literature on the effect of VR on BPSD, and no systematic review has assessed VR as a non-pharmacological therapy for reducing BPSD severity. Therefore, this systematic review aimed to evaluate the effectiveness of VR in reducing BPSD severity, as well as exploring its acceptability, safety and optimal dosage.
Methods
This systematic review was reported according to the Preferred Reporting Items for Systematic Review and Meta-analysis (PRISMA) statement []. The protocol was registered in the International Prospective Register of Systematic Reviews database (CRD42024523848). Changes to the registered protocol include an additional focus on secondary outcomes of acceptability and adverse effects of VR.
Search strategy
A search strategy was developed for MEDLINE and CINAHL, which was then adapted for MEDLINE, EMBASE, and SCOPUS (see Appendix 1 in the Supplementary Data section for the full search strategy). Search terms included VR, dementia, BPSD. Other keywords describing BPSD, such as challenging behaviour, agitation, apathy and disinhibition, were also used. Reference lists of related sources were screened to identify additional relevant studies.
Inclusion criteria
Only studies published in the English language between 2014 and 2024 were included due to the rapid evolution of VR technology. The inclusion criteria were (i) Participants: studies which included people with dementia of any stage and aetiology who experience BPSD; (ii) Intervention: VR interventions including fully, semi- and non-immersive forms; (iii) Comparator: studies with or without control groups were included; (iv) Outcome: any measure of change in BPSD from baseline following intervention; (v) Study types: experimental studies including RCTs and quasi-experimental studies. These inclusion criteria were kept purposely broad, given the limited literature and relative infancy of research in this area, to capture a breadth of research about the intervention of interest in this population group.
Study selection
All retrieved papers were managed using Covidence software []. After removing duplicates, two independent reviewers (LW and AM) screened the titles, abstracts and full texts of selected articles based on the inclusion criteria. Any disagreements between reviewers at any stage were addressed through discussion or referred to a third reviewer for resolution through consensus (GM).
Quality assessment
The methodological quality of the included studies was assessed using the JBI critical appraisal checklists for RCTs [] and quasi-experimental studies [] by two independent reviewers (LW and AM). The checklist consisted of thirteen and nine questions, respectively, with four possible answers (yes, no, unclear and not applicable). These questions assessed the quality, validity and applicability of the studies. Disagreements between the two reviewers (LW and AM) were resolved by discussing or involving a third reviewer (GM).
Data extraction
Data items were extracted from full-text articles using a modified extraction template on Covidence [] and included participant characteristics, details of the interventions, acceptability, any adverse events, as well as significant outcomes (Table 1). One reviewer (LW) extracted and entered the data into the template form, while the other (AM) cross-checked the data. Any errors were corrected through consensus.
Data synthesis and presentation
Findings were presented through a narrative synthesis. Meta-analyses were conducted using JBI SUMARI [] on studies that had accessible data and reported comparable outcomes. Study authors were contacted via email to request relevant missing data; however, some responses were not received in time for publication. The inverse variance method using a fixed-effects model was applied to calculate the mean difference (MD) and the standardised mean difference (SMD), since the same outcomes were assessed using different tools in the included studies []. A fixed-effects model was selected due to the limited number of studies in each analysis []. Statistical heterogeneity was evaluated using chi-squared and I-squared tests.
Results
Selected studies
The database search yielded 112 papers, including 37 duplicates. Review of the titles and abstracts of the remaining 75 papers identified that 53 did not meet the inclusion criteria and so were removed. This left 22 papers that were retrieved and reviewed in full-text. After screening these papers, 10 studies were found to meet the inclusion criteria. See Appendix 2 in the Supplementary Data section for PRISMA diagram of study selection.
Study characteristics
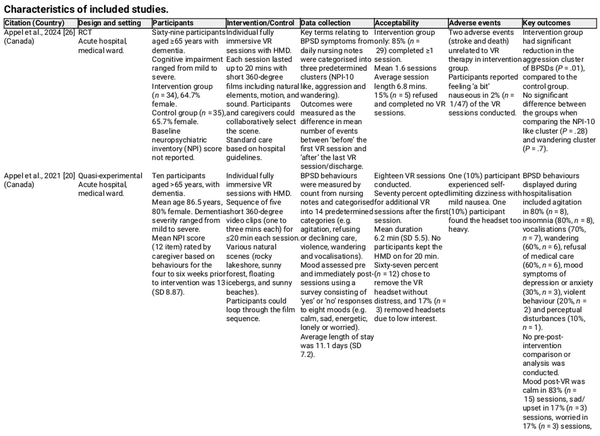
The ten included studies consisted of one RCT [] and nine quasi-experimental studies [, , , ] (Table 1).
Setting
Studies were conducted across seven countries, including Canada (n = 3, 30%), Australia (n = 2, 20%), Cyprus (n = 1, 10%), Portugal (n = 1, 10%), Spain (n = 1, 10%), Taiwan (n = 1, 10%) and the United Kingdom (n = 1, 10%). Two (20%) studies were conducted in a hospital setting with acute inpatients in a medical ward [, ]. Six studies (60%) were undertaken in an out-of-hospital care facility, with three (30%) in long-term residential aged care facilities (RACF) [, , ], two (20%) in dementia care units [, ] and one (10%) in a dementia-specific RACF []. The remaining two studies (20%) were delivered in the community, one (10%) by a local service provider to older people [] and the other by a day-stay unit [].
Participants
Studies involved participants with mean ages ranging from 73 to 89 years, except one study where the mean age was not specified []. All studies had more than 50% female participants. Only three (30%) studies included participants with dementia diagnoses based on guidelines, including the Aged Care Funding Instrument [], the National Institute on Aging-Alzheimer’s Association Revised Clinical Criteria for Alzheimer’s Disease [] and the Minimum Data Set—Cognitive Performance Scale Score []. The other seven (70%) studies included people with a clinical or documented diagnosis of dementia. The severity of cognitive impairment and scores used to assess cognitive ability varied between studies; however, most included people with dementia of varying severity, from mild to severe.
Usage and dosage of VR
The duration, frequency and length of VR sessions varied between the studies. Nine (90%) studies used individual VR sessions, whereas one (10%) performed group VR sessions []. The mean session length varied from 1 min [] as part of standard occupational therapy sensory sessions to 22 min []. The frequency of sessions also varied from one session to a defined frequency of two to five times a week.
Most studies (n = 9, 90%) used fully immersive VR characterised by HMD, with only one (10%) study using a projector displaying 360-degree video recordings []. The VR experience consisted of a series of short 360-degree films from a pre-selected video pool. The VR scenes showcased natural scenery like forests, lakeshores, and beaches and specific locations such as streets, squares, historical landmarks, or travel destinations. Specific scenes were able to be selected based on participant preference in seven (70%) studies [, , ], with two (20%) studies specifically creating videos based upon information provided by caregivers seeking to elicit positive memories [, ].
Quality assessment
Due to the absence of a clear cut-off for exclusion based on quality criteria, the limited number of included studies and their overall good methodological quality, no papers were excluded based on quality assessment. Seven studies (70%) were rated as high quality (‘yes’ responses to ≥70%) [, , ] and three studies (30%) were of medium quality (‘yes’ responses to 50%–69%) [, , ] (see Appendix 3 and 4 in the Supplementary Data section for the full details of the quality assessment). Only two studies had a control group [, ], limiting the ability to compare intervention effects against standard care or other interventions.
Review findings
The findings are presented in two sections: (i) the impact of VR on BPSD, and (ii) acceptability, optimal dosage, and safety of VR.
Impact of VR on BPSD
All ten studies assessed BPSD outcomes in participants. Each study examined different combinations of BPSD symptoms, including overall BPSD scores, aggression, agitation, depression, anxiety and apathy.
Overall BPSD scores
Overall BPSD scores were collected in four (40%) studies [, , , ], using the neuropsychiatric inventory (NPI) as the basis for assessing overall changes in BPSD. However, each study employed slightly differing methodology [, , , ]. The standardised NPI scale was used by Coelho et al. [], while the NPI-questionnaire (NPI-Q) was used by Sanchez-Nieto et al. []. Appel et al. [] used key terms from daily nursing notes on BPSD symptoms to categorise into three predetermined behaviour clusters (NPI-10-like, violence and wandering) based upon NPI-10 and the difference in mean number of events was analysed. Clay et al. [] reviewed clinical records retrospectively and recorded neuropsychiatric symptoms using the NPI as its framework. None of the four studies revealed statistically significant differences in overall NPI pre and post-VR intervention [, , , ]. Despite all studies using NPI as the basis for their measurements, additional data needed for standardisation was unavailable. Pooled results from the two studies with available data [, ] revealed no effect on overall BPSD (SMD −0.06, 95% −0.58 to 0.46, P = .82) with minimal heterogeneity (Figure 1).
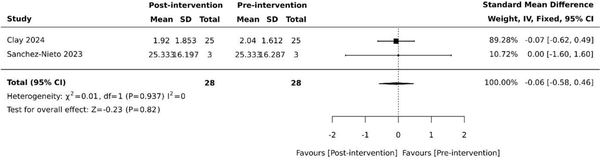
Figure 1
Effect of VR on overall BPSD based on NPI. SD: standard deviation; CI: confidence intervals.
Aggression
Three studies (30%) reported the impact of VR on aggression [, , ]. Aggression was evaluated using the Overt Aggression Scale—Modified for Neurorehabilitation (OAS-MNR) [] and categorising aggressive symptoms into predetermined NPI clusters []. Appel et al. [] found a reduction in physically aggressive behaviours and loud vocalisations in the intervention group versus the control group in an acute inpatient setting (P = .01). Similarly, Matsangidou et al. [] noted reduced frequency and severity of aggression from an aggregate OAS-MNR aggression score of 9 at baseline to 0 during and immediately after VR therapy (no p-value was reported). However, Brimelow et al. [] found no difference in the frequency of behavioural incidents when analysing the behaviour report logs in their electronic medical records (P = .864).
Agitation
Agitation was evaluated using the Cohen-Mansfield Agitation Inventory (CMAI) following intervention periods of three weeks and two weeks in two (20%) studies, respectively [, ]. Pooled studies revealed no effect on agitation (MD 1.87, 95% −1.38 to 5.13, P = .26) with moderate heterogeneity (Figure 2).
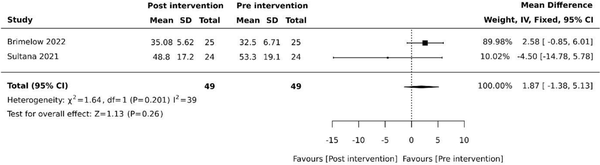
Figure 2
Effect of VR on agitation. SD: standard deviation; CI: confidence intervals.
Depression
Three (30%) studies examined the effects of VR on depression [, , ]. Of these studies, two (20%) used the Cornell Scale for Depression in Dementia (CSDD) [, ] and one (10%) used the Centre for Epidemiological Studies Depression Scale (CESD) []. Pooled results revealed reduced depressive symptoms following VR therapy (SMD −0.38, 95% −0.72 to −0.05, P = .026) with minimal heterogeneity (Figure 3).
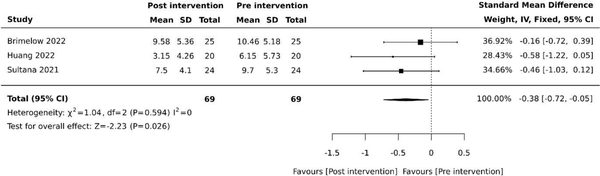
Figure 3
Effect of VR on depression. SD: standard deviation; CI: confidence intervals.
Anxiety
Three (30%) studies assessed the impact of VR on anxiety using different tools: General Anxiety Disorder 7 item (GAD-7) [], the Hamilton Anxiety Rating Scale (HARS) [] and the Observed Emotion Rating Scale (OERS) []. Brimelow et al. [] showed no change in anxiety with GAD-7 scores of 4.27 before and 4.06 after the 3-week intervention period (P = .681). GAD-7 is typically used for screening with cut-off scores of 10 for generalised anxiety disorder, which indicates no anxiety at baseline in this study. Similarly, Sanchez-Nieto et al. [] showed no difference in both the psychological and somatic anxiety items using the Hamilton Anxiety Rating Scale (HARS) between baseline and week five of the study after three weeks of VR intervention (P > .05). However, Matsangidou et al. [] demonstrated reduced anxiety symptoms using OERS, from a rating of 2.65 before to 1.2 immediately after VR exposure based on a dementia-specific RACF (P < .001).
Apathy
Two (20%) studies examined the effects of VR on apathy [, ]. Both studies assessed apathy using the Person Environmental Apathy rating (PEAR) scale [, ]. The multiple-session group VR programme conducted by Brimelow et al. [] demonstrated a reduction in apathy immediately after each VR session (P < .001). Meanwhile, Moyle et al. [] demonstrated no change in apathy with a PEAR apathy score of 18.3 before and 18.7 after the singular VR session (P > .05). However, there was reduced apathy during the VR session compared to baseline (P = .01) [].
Acceptability, optimal dosage and safety of VR
Acceptability was reported in nine (90%) studies [, , , , , ] and safety outcomes were reported in six (60%) studies [, , ]. Acceptability was reported by either the percentage of participants who completed their proposed session numbers [, , ] or intended VR exposure duration [], recording any participant requests for more sessions or longer sessions [, ] or the mean duration of VR sessions completed [, , , , ]. The intended total of four and nine VR sessions was completed by 78% [] and 100% [] of participants in each study, respectively. 85% of participants completed the full intended VR exposure duration of 15 min []. 70% of participants opted for extra sessions during their hospital stay [], while 85% of participants requested longer exposure times than the planned 15 min []. The actual mean duration of VR sessions tolerated by patients ranged from 6.2 to 22 min in the five (50%) studies that reported this outcome [, , , , ].
Adverse events related to VR intervention were uncommon and mild, with two cases of eye strain and head fullness [], two cases of headache and giddiness [] and two cases of mild nausea [, ]. The headset was considered too heavy in one participant [] and caused discomfort due to ill fit in two participants [].
Discussion
This systematic review has examined the effectiveness of VR as a non-pharmacological therapy for reducing the severity of BPSD and explored its acceptability, safety and optimal dosage. Despite mixed results, several studies identified potential positive benefits, including reduced aggression [, ], depressive symptoms [, ] and apathy [, ]. Favourable acceptability and minimal side effects were observed across various clinical settings, supporting VR as a potential intervention for reducing BPSD severity.
The impact of VR on overall BPSD yielded mixed findings. Several studies failed to demonstrate statistically significant changes using NPI-based tools. While NPI is a validated clinical tool for dementia-related psychopathology, it is typically used to define behavioural changes over a 4-week period []. All but one of the included studies had shorter assessment periods, which may explain the limited statistically significant results. Additionally, BPSD symptoms are complex and dynamic, often relying on proxy reporting [], which further complicates assessment with standardised tools. The NPI may not have fully captured the nuanced effects of VR on BPSD in the short term, highlighting the need for more sensitive and specific tools to measure BPSD.
Aggression and agitation are among the most challenging BPSD symptoms [], often treated with antipsychotic medications despite the increased risk of death, cerebrovascular adverse events and extrapyramidal side effects of pharmacotherapy []. Triggers such as pain, noise, and environmental stimulation in hospitals may exacerbate these behaviours []. More evidence-based non-pharmacological interventions for BPSD are needed. This review found that VR reduced aggression in both an acute hospital and dementia-specific RACF setting [, ]. VR-based relaxation devices have also alleviated discomfort, pain, stress and anxiety in ICU patients [], while VR calm rooms have successfully distracted psychiatric patients from their stressful extended hospital stays []. These findings highlight VR as a potentially safe alternative to antipsychotic medications to reduce aggression and agitation that warrants further research.
Anxiety and depression are among the earliest non-cognitive expressions of BPSD []. This review found that VR could be effective in reducing depressive symptoms [, ], mirroring findings from Zhai et al. [] where VR reduced depression in RACF residents. This is relevant given the higher rates of depression and anxiety associated with moving into RACF []. VR may help ease this transition by providing new experiences and social interactions. This review showed mixed results in terms of anxiety reduction. Interestingly, there has been growing research on VR reducing perioperative anxiety by easing anticipatory fear []. During the COVID-19 pandemic, where those in COVID-19 intensive care units faced prolonged isolation periods, VR reduced perceived stress and anxiety in those with mild cognitive impairment []. This may translate to similar benefits in older people with dementia who face social isolation in hospital or institutional settings. Future studies are necessary to confirm VR’s effects on mood and emotions of older people with dementia.
This review demonstrated that people with dementia tolerated VR well across hospital, RACF and community settings, with few adverse events. Completion rates were high, even among acutely unwell medical inpatients, with comparable adherence (65%–78%) to exercise programmes for those with BPSD []. Recorded adverse events in this review were much lower than the side effects of nausea or headache for common antidepressants []. Symptoms such as eye strain, headache and mild dizziness were likely caused by motion sickness or headset weight []. Much like music therapy for BPSD, which can cause overstimulation with excess volume [], VR experiences should be carefully adjusted for comfort, particularly in those with pre-existing motion sickness or visual impairment. Encouragingly, a systematic review by Chen et al [] found no negative outcomes with VR exergames in older people, despite their demands on movement and balance. Saredakis et al. [] also reported lower levels of cybersickness in older participants. There is a need for further research to refine candidate selection and enhance VR comfort and design for broader use.
As has been seen in reviews of interventions in other disease groups [], the included studies used widely different VR protocols. Currently, there is limited guidance on dosage of VR and its impact on outcomes as no studies assessed how these variations in protocol impacted the effectiveness in reducing BPSD severity. This variability, together with the lack of standardised BPSD outcomes, complicates comparisons and determination for the ideal VR dosage. A recent meta-analysis found that the most effective VR-based rehabilitation for cognition and motor function in individuals with mild cognitive impairment or dementia was 30 min, three times per week for five to eight weeks []. However, this review [] focused on community settings, which leaves a gap for hospital-based studies that may also introduce additional variables like logistical constraints and clinical events that could affect adherence []. Additional research is needed to compare different VR dosages within the same clinical setting to establish the most effective approach.
This systematic review was the first to explore the use and efficacy of VR on BPSD. It consolidates current knowledge on how it is used, its potential impact on specific BPSD symptoms as well its acceptability and safety. The review also highlights the heterogeneity in the literature and provides valuable insights to guide future research. However, several limitations must be acknowledged. The strength of the evidence is limited by the small number of studies undertaken across limited geographical regions, additionally, issues such as small sample sizes and lack of blinding impact study quality. Nine out of ten studies were observational with no control group nor blinding, increasing the risk of bias and limiting the ability to draw conclusions. The heterogeneity of interventions, including variations in VR equipment, frequency, duration, imagery, and interventions settings, as well as differences in dementia type and severity, further compromise the generalisability of findings. Studies were short (up to three months) with limited follow-up, limiting assessment of longer-term sustained effects. Methodological limitations must be also considered. The search strategy, while comprehensive, may have excluded relevant studies due to language restrictions, as only English-language publications were included. Additionally, variations in search terms and indexing of VR-related interventions may have led to the omission of some studies. A meta-analysis could not be completed due tothe variability in study designs, outcome measures, and missing data. Where meta-analysis was performed, the absence of control groups meant that only pre-post comparisons were possible, making the results highly susceptible to bias. Although this review represents the best available evidence, given these limitations, findings should be interpreted with caution.
Conclusion
While VR may become a non-pharmacological intervention for reducing the severity of BPSD, the current evidence is insufficient to establish its effectiveness definitively. Nonetheless, VR has demonstrated an excellent acceptability and safety profile across numerous settings. The limited number of studies, varied methodology, and quality limitations constrain the findings, underscoring the need for future research with larger sample sizes, standardised VR intervention protocols and comprehensive BPSD outcome measures. Integration of VR into clinical practice could potentially alleviate the challenges faced by both patients and caregivers.
References
- 1. Gale SA, Acar D, Daffner KR. Dementia. Am J Med 2018;131:1161–9. 10.1016/j.amjmed.2018.01.022.
- 2. The Royal Australian College of General Practitioners (RACGP) (ed). Behavioural and psychological symptoms of dementia. In: RACGP Aged Care Clinical Guide (Silver Book), 5th edition, 2019. Royal Australian College of General Practitioners (RACGP), East Melbourne, Victoria, Australia.
- 3. Dementia Centre for Research Collaboration, Centre for Health Brain Ageing (DCRC) and Centre for Healthly Brain Ageing (CHeBA). Assessment and Management of Behaviours and Psychological Symptoms Associated with Dementia (BPSD): A Handbook for NSW Health Clinicians Providing Services for People Experiencing BPSD, 2022. New South Wales Ministry of Health in collaboration with University of New South Wales, Sydney, Australia. https://www.health.nsw.gov.au/mentalhealth/resources/Publications/ass-mgmt-bpsd-handbook-dec-22.pdf
- 4. Bessey LJ, Walaszek A. Management of behavioral and psychological symptoms of dementia. Curr Psychiatry Rep 2019;21:66. 10.1007/s11920-019-1049-5.
- 5. Abraha I, Rimland JM, Trotta FM, et al Systematic review of systematic reviews of non-pharmacological interventions to treat behavioural disturbances in older patients with dementia. The SENATOR-OnTop series. BMJ Open 2017;7:e012759. 10.1136/bmjopen-2016-012759.
- 6. Gaugler JE, Kane RL, Kane RA, et al Caregiving and institutionalization of cognitively impaired older people: utilizing dynamic predictors of change. Gerontologist 2003;43:219–29. 10.1093/geront/43.2.219.
- 7. Trinkley KE, Sturm AM, Porter K, et al Efficacy and safety of atypical antipsychotics for behavioral and psychological symptoms of dementia among community dwelling adults. J Pharm Pract 2020;33:7–14. 10.1177/0897190018771272.
- 8. Mühlbauer V, Möhler R, Dichter MN, et al Antipsychotics for agitation and psychosis in people with Alzheimer’s disease and vascular dementia. Cochrane Database Syst Rev 2021;12:CD013304. 10.1002/14651858.CD013304.pub2.
- 9. Li YQ, Yin ZH, Zhang XY, et al Non-pharmacological interventions for behavioral and psychological symptoms of dementia: a systematic review and network meta-analysis protocol. Front Psych 2022;13:1039752. 10.3389/fpsyt.2022.1039752.
- 10. Caspar S, Davis ED, Douziech A, et al Nonpharmacological management of behavioral and psychological symptoms of dementia: what works, in what circumstances, and why? Innov Aging 2017;1:igy001. 10.1093/geroni/igy001.
- 11. Yin Z, Li Y, Bao Q, et al Comparative efficacy of multiple non-pharmacological interventions for behavioural and psychological symptoms of dementia: a network meta-analysis of randomised controlled trials. Int J Ment Health Nurs 2024;33:487–504. 10.1111/inm.13254.
- 12. Lühnen J, Richter T, Calo S, et al Psychosocial interventions for reducing antipsychotic medication in care home residents. Cochrane Database Syst Rev 2023;2023:CD008634. 10.1002/14651858.CD008634.pub3.
- 13. Cohen-Mansfield J, Thein K, Marx MS, et al What are the barriers to performing nonpharmacological interventions for behavioral symptoms in the nursing home? J Am Med Dir Assoc 2012;13:400–5. 10.1016/j.jamda.2011.07.006.
- 14. Riva G, Botella C, Baños R, et al Presence-Inducing Media for Mental Health Applications. In: Lombard, M, Biocca, F, Freeman, J, Ijsselsteijn, W, Schaevitz, R (eds). Immersed in Media: Teleprescence Theory, Measurement and Technology. Cham, Switzerland: Springer International Publishing, 2015, 283–332. 10.1007/978-3-319-10190-3_12.
- 15. Hamad A, Jia B. How virtual reality technology has changed our lives: an overview of the current and potential applications and limitations. Int J Environ Res Public Health 2022;19:11278. 10.3390/ijerph191811278.
- 16. Clay F, Howett D, Fitzgerald J, et al Use of immersive virtual reality in the assessment and treatment of Alzheimer’s disease: a systematic review. J Alzheimers Dis 2020;75:23–43. 10.3233/JAD-191218.
- 17. Appel L, Ali S, Narag T, et al Virtual reality to promote wellbeing in persons with dementia: a scoping review. J Rehabil Assist Technol Eng 2021;8:205566832110539. 10.1177/20556683211053952.
- 18. Zhu S, Sui Y, Shen Y, et al Effects of virtual reality intervention on cognition and motor function in older adults with mild cognitive impairment or dementia: a systematic review and meta-analysis. Front Aging Neurosci 2021;13:586999. 10.3389/fnagi.2021.586999.
- 19. Mannan FA, Porffy LA, Joyce DW, et al Automatic detection of cognitive impairment with virtual reality. Sensors 2023;23:1026. 10.3390/s23021026.
- 20. Appel L, Kisonas E, Appel E, et al Administering virtual reality therapy to manage behavioral and psychological symptoms in patients with dementia admitted to an acute care hospital: results of a pilot study. JMIR Form Res 2021;5:e22406. 10.2196/22406.
- 21. Wen J, Yan H, Wang S, et al The effectiveness of nursing interventions for elderly dementia patients based on virtual reality technology: a systematic review and meta-analysis. Ageing Res Rev 2024;93:102135. 10.1016/j.arr.2023.102135.
- 22. Moher D, Shamseer L, Clarke M, et al Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst Rev 2015;4:1. 10.1186/2046-4053-4-1.
- 23. Covidence Systematic Review Software, Melbourne, Australia: Veritas Health Innovation, 2024.
- 24. Barker TH, Stone JC, Sears K, et al The revised JBI critical appraisal tool for the assessment of risk of bias for randomized controlled trials. JBI Evid Synth 2023;21:494–506. 10.11124/JBIES-22-00430.
- 25. Aromataris E, Munn Z. In: Aromataris E, Lockwood C, Porritt K, Pilla B, Jordan Z (eds), JBI Manuafor Evidence Synthes. JBI, Adelaide, Australia: The University of Adelaide, 2024. Available from: https://synthesismanual.jbi.global.
- 26. Appel L, Appel E, Kisonas E, et al Evaluating the impact of virtual reality on the behavioral and psychological symptoms of dementia and quality of life of inpatients with dementia in acute care: randomized controlled trial (VRCT). J Med Internet Res 2024;26:e51758. 10.2196/51758.
- 27. Brimelow RE, Thangavelu K, Beattie R, et al Feasibility of group-based multiple virtual reality sessions to reduce behavioral and psychological symptoms in persons living in residential aged care. J Am Med Dir Assoc 2022;23:831–837.e2. 10.1016/j.jamda.2021.07.026.
- 28. Clay F, Hunt R, Obiefuna N, et al The use of immersive virtual reality in sensory sessions on a specialist dementia unit: service evaluation of feasibility and acceptability. Occup Ther Health Care 2024;38:317–30. 10.1080/07380577.2023.2270052.
- 29. Coelho T, Marques C, Moreira D, et al Promoting reminiscences with virtual reality headsets: a pilot study with people with dementia. Int J Environ Res Public Health 2020;17:1–13. 10.3390/ijerph17249301.
- 30. Huang LC, Yang YH. The long-term effects of immersive virtual reality reminiscence in people with dementia: longitudinal observational study. JMIR Serious Games 2022;10:e36720. 10.2196/36720.
- 31. Matsangidou M, Solomou T, Frangoudes F, et al Affective out-world experience via virtual reality for older adults living with mild cognitive impairments or mild dementia. Int J Environ Res Public Health 2023;20:2919. 10.3390/ijerph20042919.
- 32. Moyle W, Jones C, Dwan T, et al Effectiveness of a virtual reality forest on people with dementia: a mixed methods pilot study. Gerontologist 2018;58:478–87. 10.1093/geront/gnw270.
- 33. Lawton MP, Van Haitsma K, Perkinson M, et al Observed affect and quality of life in dementia: further affirmations and problems. J Ment Health Aging 1999;5:69–81.
- 34. Sánchez-Nieto D, Castaño-Castaño S, Navarro-Martos R, et al An intervention on anxiety symptoms in moderate Alzheimer’s disease through virtual reality: a feasibility study and lessons learned. Int J Environ Res Public Health 2023;20:2727. 10.3390/ijerph20032727.
- 35. Sultana M, Campbell K, Jennings M, et al Virtual reality experience intervention may reduce responsive Behaviors in nursing home residents with dementia: a case series. J Alzheimers Dis 2021;84:883–93. 10.3233/JAD-210010.
- 36. Munn Z, Aromataris E, Tufanaru C, et al The development of software to support multiple systematic review types: the Joanna Briggs institute system for the unified management, assessment and review of information (JBI SUMARI). Int J Evid Based Healthc 2019;17:36–43. 10.1097/XEB.0000000000000152.
- 37. Andrade C. Mean difference, standardized mean difference (SMD), and their use in meta-analysis: as simple as it gets. J Clin Psychiatry 2020;81:20f13681. 10.4088/JCP.20F13681.
- 38. Tufanaru C, Munn Z, Stephenson M, et al Fixed or random effects meta-analysis? Common methodological issues in systematic reviews of effectiveness. Int J Evid Based Healthc 2015;13:196–207. 10.1097/XEB.0000000000000065.
- 39. Cummings JL, Mega M, Gray K, et al The neuropsychiatric inventory: comprehensive assessment of psychopathology in dementia. Neurology 1994;44:2308–14. 10.1212/WNL.44.12.2308.
- 40. Melander C, Sävenstedt S, Olsson M, et al Assessing BPSD with the support of the NPI-NH: a discourse analysis of clinical reasoning. Int Psychogeriatr 2018;30:581–9. 10.1017/S1041610217002009.
- 41. Akrour R, Courret-Gilgen C, Perrenoud B. Prevention and management of behavioural and psychological symptoms in patients with dementia in acute care: a best practice implementation project. JBI Evid Implement 2022;20:289–300. 10.1097/XEB.0000000000000329.
- 42. Chiu Y, Bero L, Hessol NA, et al A literature review of clinical outcomes associated with antipsychotic medication use in North American nursing home residents. Health Policy (New York) 2015;119:802–13. 10.1016/j.healthpol.2015.02.014.
- 43. Ilievski V, Barrett T, Lawson W, et al Cognitive impairment and behavioural emergencies within the acute hospital setting. BMJ Open Qual 2023;12:e002034. 10.1136/bmjoq-2022-002034.
- 44. Merliot-Gailhoustet L, Raimbert C, Garnier O, et al Discomfort improvement for critically ill patients using electronic relaxation devices: results of the cross-over randomized controlled trial E-CHOISIR (Electronic-CHOIce of a System for Intensive care Relaxation). Crit Care 2022;26:1–12.
- 45. Ilioudi M, Wallström S, Steingrimsson S, et al Patient experience of a virtual reality calm room in a psychiatric inpatient care setting in Sweden: a qualitative study with inpatients. BMJ Open 2023;13:e076285. 10.1136/bmjopen-2023-076285.
- 46. Masters MC, Morris JC, Roe CM. “Noncognitive” symptoms of early Alzheimer disease: a longitudinal analysis. Neurology 2015;84:617–22. 10.1212/WNL.0000000000001238.
- 47. Zhai K, Dilawar A, Yousef MS, et al Virtual reality therapy for depression and mood in long-term care facilities. Geriatrics (Switzerland) 2021;6:58. 10.3390/geriatrics6020058.
- 48. Creighton AS, Davison TE, Kissane DW. The prevalence of anxiety among older adults in nursing homes and other residential aged care facilities: a systematic review. Int J Geriatr Psychiatry 2016;31:555–66. 10.1002/gps.4378.
- 49. Kodvavi MS, Asghar MA, Ghaffar RA, et al Effectiveness of virtual reality in managing pain and anxiety in adults during periprocedural period: a systematic review and meta-analysis. Langenbecks Arch Surg 2023;408:301. 10.1007/s00423-023-03046-5.
- 50. Yahara M, Niki K, Ueno K, et al Remote reminiscence using immersive virtual reality may be efficacious for reducing anxiety in patients with mild cognitive impairment even in covid-19 pandemic: a case report. Biol Pharm Bull 2021;44:1019–23. 10.1248/bpb.b21-00052.
- 51. Steichele K, Keefer A, Dietzel N, et al The effects of exercise programs on cognition, activities of daily living, and neuropsychiatric symptoms in community-dwelling people with dementia—a systematic review. Alzheimers Res Ther 2022;14:97. 10.1186/s13195-022-01040-5.
- 52. Kelly K, Posternak M, Alpert JE. Toward achieving optimal response: understanding and managing antidepressant side effects. Dialogues Clin Neurosci 2008;10:409–18. 10.31887/DCNS.2008.10.4/kkelly.
- 53. Chang E, Kim HT, Yoo B. Virtual reality sickness: a review of causes and measurements. Int J Hum Comput Interact 2020;36:1658–82. 10.1080/10447318.2020.1778351.
- 54. van der Steen JT, van Soest-Poortvliet MC, van der Wouden JC, et al Music-based therapeutic interventions for people with dementia. Cochrane Database Syst Rev 2017;2017:CD003477. 10.1002/14651858.CD003477.pub3.
- 55. Chen PJ, Hsu HF, Chen KM, et al VR exergame interventions among older adults living in long-term care facilities: a systematic review with meta-analysis. Ann Phys Rehabil Med 2023;66:101702. 10.1016/j.rehab.2022.101702.
- 56. Saredakis D, Szpak A, Birckhead B, et al Factors associated with virtual reality sickness in head-mounted displays: a systematic review and meta-analysis. Front Hum Neurosci 2020;14:96. 10.3389/fnhum.2020.00096.
- 57. Rowland DP, Casey LM, Ganapathy A, et al A decade in review: a systematic review of virtual reality interventions for emotional disorders. Psychosoc Interv 2022;31:1–20. 10.5093/pi2021a8.
- 58. Ren Y, Wang Q, Liu H, et al Effects of immersive and non-immersive virtual reality-based rehabilitation training on cognition, motor function, and daily functioning in patients with mild cognitive impairment or dementia: a systematic review and meta-analysis. Clin Rehabil 2024;38:305–21. 10.1177/02692155231213476.
- 59. Shiner CT, Croker G, McGhee J, et al Perspectives on the use of virtual reality within a public hospital setting: surveying knowledge, attitudes, and perceived utility among health care professionals. BMC Digital Health 2024;2:1–14. 10.1186/s44247-024-00076-x.