Introduction
Dementia is a general health issue around the globe. The prevalence continues growing because of the aging population, leading to a greater amount of disability and dependency of the elderly. According to a published report by the Ministry of Health and Welfare (MOHW) on 08 August 2018, the dementia population in Taiwan exceeded 270,000 at the end of 2017 and is anticipated by a threefold increase in 50 years, thus adding challenge to the current dementia care.
Dementia is a syndrome caused by various brain illness that influences thinking, behavior, memory and ability to perform daily activities. Even though age is the strongest known risk factor for dementia, it is not an ordinary part of aging. Many people experience mild cognitive changes and memory loss when they begin to move into their 50 s. One of the most visible indicators of Frontotemporal dementia (FTD) is the speed of progression. For Alzheimer’s disease, regular mental decline associated with aging is ordinarily a gradual loss of memory or attention span with little impact on their daily performance. Dementia, on the other hand, is often characterized by a rapid, sudden, and severe change in memory, intellect or personality which interferes with their works, hobbies, social activities and family relationships. Unfortunately, there is no treatment available to cure dementia or to alter its progressive course. Therefore, efforts to develop methods that detect dementia at its earliest stage are crucial in slowing the progression of the devastating effects of dementia.
In the most clinical setting, performance-based assessments are usually administrated to assess the cognitive deficit status of the patients such as Mini-Mental State Examination (MMSE), - the Montreal Cognitive Assessment (MoCA) , and the clock drawing test (CDT). ,, The MMSE has a ceilting effect that makes it less sensitive in detecting the earliest signs of dementia, particularly in highly educated individuals. , Recent studies found that the MoCA was better at distinguishing normal cognition from MCI than the MMSE, , however some of the items included in the MoCA can influence the results when conducting to patients with severe motor symptoms. There are various ways to score performance on CDT but none of them delivered high sensitivity and specificity rates to demonstrate that the CDT is a reliable examining assessment for MCI.
Informant-based assessments may preferably than other screening methods as they can be completed by informants in a short time and determine whether to seek further diagnostic. Examples of informant-based assessments are the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE) - to evaluate cognitive decline from a pre-morbid level on informant reports, the Ascertain Dementia 8 (AD8), , and the Symptoms of Early Dementia-11 Questionnaire (SED-11Q) , for the ealy dementia detection. Recently, machine learning approach has been used for informant-based diagnosis. ,
For capturing early cognitive change, an expeditious examining test in clinical settings would be intensely beneficial as it could assist general practitioners to determine whether a more in-depth clinical evaluation is necessary under time constraints. A perfect practical examining test should be administered with effortless within tight time and minimally affected by educational background and socioeconomic status. In this respect, a brief and concise assessment with global staging scale which possesses high validity and reliability as an excellent tool.
In the present study, history-based dementia data has been collected according to a modified version of the Clinical Dementia Rating (CDR) questionnaire. It assesses intra-individual change from the individual’s prior cognitive and functional abilities. The questionnaire has been completed by indicating the degree of impairment regarding cognitive, behavioral and functional changes resulting from the cognitive loss in each of the 7 categories: Memory, Orientation, Judgment and problem solving, Community affairs, Home and hobbies, Personal care and Language. Subsequently, the severity of symptoms of dementia is quantified according to the global CDR score, which is widely used in rating the severity degree of dementia (from 0 = no dementia to 3 = severe dementia).
The purpose of this study is to develop a predictive model that helps to understand and classify the severity of symptoms of dementia based on the dementia screening questionnaire in Taiwan. The predictive model could assist clinicians in predicting the severity of dementia under time constraints before undergoing a more in-depth clinical evaluation or even enable a non-clinical trained person to detect dementia symptoms among elderly family members. The proposed modified CDR may be indicated for epidemiological study and provide a solid foundation to develop a machine-learning derived screening instrument to detect dementia symptoms.
Methods
Data Source
The newly developed History-based Artificial Intelligent Clinical Dementia Diagnosis System (HAICDDS) ,, is embedded in a dementia registry database which is currently applied in 3 regional hospitals of the Show Chwan Healthcare System, 2 located in central Taiwan and 1 in southern Taiwan. The HAICDDS questionnaire tailored to the Taiwan population and is different from Morris’s study by including the evaluation of language function and neuropsychiatric symptoms. The language is usually related to FTD, which is a variant of Alzheimer’s disease even though it is a rare incident. Since January 2016, it had collected more than 7000 patients with more than 14000 detailed records. In the database, dementia staging was assessed using the CDR, daily function was assessed using the Instrumental Activities of Daily Living (IADL) Scale. Cognitve fucntion was assessed using the Cognitive Abilities Screening Instrument (CASI) and the Montreal Cognitive Assessment (MoCA). HAICDDS questionnaire and cognitive tests for all patients were performed by trained neuropsychologists and the completion of each record takes about 90-120 minutes. For HAICDDS, it takes about 15-20 minutes performed by well-trained neuropsychologists.
There were 6,328 patients from the database voluntarily participated in this study. There are 2,651 male participants and 3,677 female participants involved in this research with age ranged from 50 and 102. Each informant of the participant with subjective memory complaints was required to answer a Chinese version of the HAICDDS. These participants were labeled according to one of the dementia severity levels such as Normal, MCI, Mild, Moderate or Severe according to the global CDR scale. , The modified CDR was applied in each participant using the existed CDR staging rule. The levels generated using the existing algorithm from CDR sum scores, which trained neuropsychologists rate each domain based on their interviews using all CDR items. A sum score (0, 0.5, 1, 2, or 3) is then calculated using weighted question score as well as domain scores, and a level of severity is solely based on the sum score. The weighting of each question was given according to a non-AI correlation study of each question with severity of dementia before the current study. Each domain score was summation of all relative questions in the same domain. However, the clinicians could change the final score if there is a conflict. CDR staging method is a golden standard for dementia due to Alzheimer’s disease (AD) however, it is not generable to non-Alzheimer’s dementia. In this study, AD and non-AD dementia were studied and analyzed. Therefore, in this study, the CDR staging system is regarding as a reference instead of a golden standard classification. CDR 0 indicates no dementia and CDR 0.5 represents MCI. The CDR 1, 2 and 3 correspond to mild, moderate and severe dementia, respectively.
The questionnaire consists of 50 questions in 7 domains; namely 11 questions on memory (M), 5 questions on orientation (O), 6 questions on judgment and problem solving (J), 9 questions on community affairs (C), one question on home and hobbies (H), 10 questions on personal care (P) and 8 questions on Language (L). Most of the questions have dummy coding except domain H uses a scale from 0 to 5, and other domains have either dummy code, scale from 0 to 2, or scale from 0 to 3. For all types of coding, a smaller value indicates positive or healthier conditions of the participant. CDR and HAICDDS questionnaires of each patient was rated by 7 trained neuropsychologist from 3 regional hospitals with intra-class correlation coefficient of 0.8330 from interrater reliability test.
The 6,328 cases consisting of 453 cases with no dementia, 1,573 MCI, 2,391 mild dementia, 1,257 moderate dementia and 654 severe dementia cases were analyzed. This dataset was then split into a training set and a testing set using stratified random sampling. The training set consisted of 70% of the data which was used to train the linear discriminant model. Whereas, the performance of the model was validated by the testing set consisting of the remaining 30% of the data. Additionally, k-means clustering was employed to partition all patients from training and testing datasets into different severity of dementia. All analyses were conducted using IBM SPSS Statistics, version 22.
Statistical Methods
Fisher’s linear discriminant analysis (LDA) , is employed in this study to construct the classification equations for classifying individuals into different severity levels of dementia (groups) based on a set of significant HAICDDS questions (variables).
k-Means clustering is also employed to identify the dementia symptoms for different severity of dementia. k-Means , is an unsupervised clustering method where a prototype member of each cluster is identified (called a centroid) which somehow represents that cluster.
In order to evaluate the performance of the LDA model, a multiple-classes confusion matrix will be constructed based on the outputs from the testing set. Three types of performance measure, i.e. sensitivity, specificity and average class accuracy are calculated from the confusion matrix.
Results
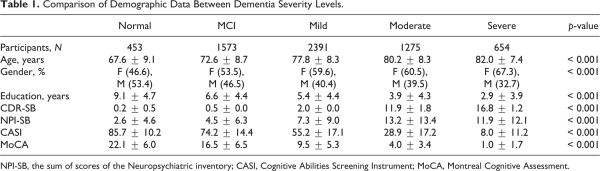
A comparison of demographic among patients from different dementia severity levels revealed a significant difference in all parameters (p-value < 0.001) (Table 1). The severity of dementia increased as the patients’ getting older and the number of years of education is lesser. There is also a gender discrepancy where more female experienced serious dementia while male has higher percentage in light dementia or non-dementia cases.
1. Classification of the dementia severity levels using the HAICDDS
The first analysis is to build Fisher’s linear discriminant functions that can classify the patients into one of the severity dementia levels based on the HAICDDS questions. Based on the Wilks’ lambda stepwise selection with and , it is observed that only 36 out of 50 HAICDDS questions (variables) are significant at .
The Fisher’s linear discriminant outputs were analyzed. There are 7 questions indicating different symptoms deliver a significant impact on the Memory domain, namely M01, M05, M06, M07, M09, M10 and M12. A patient is most likely to have moderate or severe dementia if all these 7 symptoms appeared. A mild dementia can be differentiated from moderate and severe dementia if the patient does not forget something very familiar (M12). A normal patient can remember what he or she said recently (M06), does not repeat the same questions or says the same things repeatedly (M09), and does not think of the past frequently (M10). Contrarily, MCI dementia will repeatedly ask the same questions (M09) but does not forget something they familiar (M12).
For the Orientation domain, all 5 questions (O02, O03, O05, O06 and O07) are significant. If a patient exhibits all these 5 symptoms, then he or she may have moderate or severe dementia. The patient is likely to have mild dementia if he or she does not recognize a wrong person constantly (O07). In addition to O07, if the patient does not have difficulty to remember the dating time (O03) and does not get lost in a familiar environment (O05), then he or she may have MCI dementia. Normal patient sometimes will get lost in a familiar environment.
There are 4 significant symptoms (J01, J03, J05 and J06) in Judgment & Problem-Solving domain. A patient who exhibits all these 4 symptoms may have moderate dementia. Whereas, a patient is more likely to be considered as normal, MCI or mild dementia if he or she does not find it more difficult to operate daily necessities than before (J06). Surprisingly, the severe dementia patients indicated that they are capable to handle complex financial matters (J03) as compare to other levels of dementia.
There are 9 “Community Affairs” symptoms asked in the CDR questionnaire and 8 of them are significant other than C04. A normal patient has the ability to handle money including the banking stuffs (C02) as compare to dementia patient. While all levels of dementia patient responded that they need help when liaising with the bank. For those with severe dementia, they claimed that their incapability to perform daily activities is mainly caused by physical disability.
There is only one significant symptom (H01) in Home & Hobbies domain. This symptom has positive coefficients for all dementia severity levels. The magnitude of the coefficients indicates that the engagement of hobbies or interests among the patients is gradually reduced as the severity level increases.
Seven symptoms (P01, P03-P06, P08 & P09) in the Personal Care domain are significant, while 3 symptoms (P02, P07 & P10) are not significant. It is observed that all significant symptoms contain positive coefficients, except “able to walk over 50 meters on the ground” (P08) for severe dementia. This indicates that patients with severe dementia are capable to walk individually on the flat ground for more than 50 meters, while the normal-MCI, mild and moderate group may require assistance.
For the language domain, 4 out of 5 questions (L02, L03, L05 & L06) are significant. The patient with severe dementia responded that they have problems in understanding what other people says (L02), express their idea fluently (L03), talks very little (L05) and unable to recall the nouns or names (L06). Patients with moderate dementia are better in recalling the nouns or names of a person and place. While patients from mild dementia, MCI dementia and normal groups are also able to understand what other people says beside they can remember the names of a person and place.
Figure 1 shows the group means and disperse of the individual observations in the discriminant coordinate system. The “black star” points indicates the centroid of each cluster. It is observed that the group centroid for mild dementia, moderate dementia and severe dementia groups are far from each other; however, the normal group and MCI group are closer to each other. This suggests that the developed HAICDDS questionnaire is more effective to classify the severe, moderate and mild dementia cases as compared to normal and MCI cases. This may partly because the symptoms for normal aging and MCI are quite similar, thus it is difficult to differentiate the onset of these 2 groups.
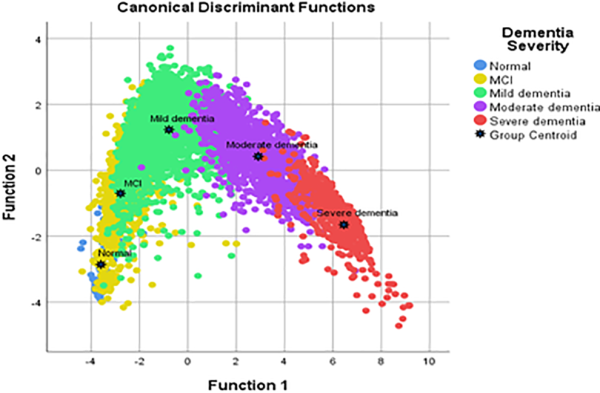
Figure 1
Discriminant space of 5-dementia levels.
2. Performance of the developed Fisher’s LDA
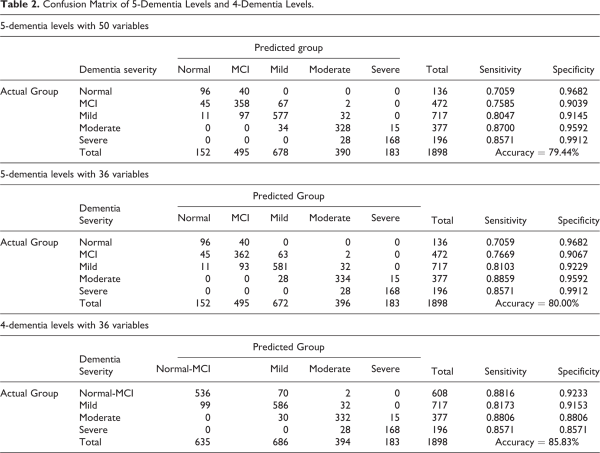
The LDA model was then applied to classify the patients in testing dataset. For comparison purpose, 3 LDA models will be constructed, namely the 5-dementia levels with 50 variables and with the reduced 36 variables, and the 4-dementia levels (after combining normal and MCI dementia) with 36 reduced variables. The classification results are presented in the confusion matrix showed in Table 2 in which the respective performance measures such as accuracy, sensitivity and specificity were calculated.
Table 2(a) provides the classification results using 50 variables (questions) with 5-dementia levels. The average accuracy is 79.44% while the sensitivity and specificity ranged from 0.7059 to 0.8700 and from 0.9039 to 0.9912, respectively. Generally the severe and moderate dementia groups have better classification outputs with high sensitivity (0.8571 and 0.7800) and high specificity (0.9912 and 0.9592) values. The normal and MCI groups have lowest sensitivity of 0.7059 and 0.7585, respectively. Table 2(b) displays the classification results using reduced 36 variables with 5-dementia levels. The accuracy is 80.00%, about the same with the full model, while the sensitivity and specificity for MCI, mild and moderate dementia groups are only slightly increases. Therefore, the model with reduced 36 variables is recommended as it will reduce the time taken by the patients to answer the questionnaire.
Since Figure 1 suggests that the normal and MCI dementia are most likely not differentiable using the HAICDDS questionnaire, a 4-dementia levels with 36 variables combining the normal and MCI into single group are developed and the classification results are depicted in Table 2(c). It is noted that the accuracy increases to 85.83% as compare to the 5-dementia levels model. However, the 4-dementia levels model do not yielded in better sensitivity and specificity values except for the sensitivity (0.8816) for join normal-MCI dementia group. In view of the clinical needs to differentiate the normal patients from the dementia patients, the LDA model of 5-dementia levels with 36 variables will be recommended and subsequently adopted to identify the dementia symptoms for different severity of dementia.
3. Identifying Dementia Symptoms for Each Severity Level
One of the key interests for medical practitioners is to know how the patients with different severity of dementia behave within a cognitive or functional domain. By knowing the patients’ behavior, it enables the medical practitioners to understand the symptoms and provide better treatments to their patients. To do so, a k-means clustering is employed to partition all patients into 5 clusters, ideally each cluster will be associated with one type of dementia severity, namely normal, MCI, mild, moderate and severe.
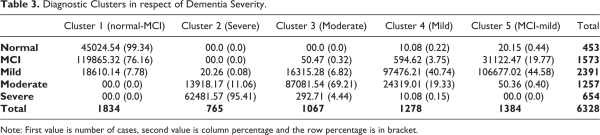
Table 3 summarizes the 5 clusters resulted from clustering procedures, with the cluster 2 consists of 81.57% are severe dementia patients, 18.17% moderate dementia patients and 0.26% mild dementia patients. Within the severe dementia patients, 95.41% are in cluster 2. Hence it is concluded that cluster 2 represents severe dementia patients. The cluster 3 has the highest number of moderate dementia patients, which is 870 persons or 81.54%, followed by mild dementia (15.28%), severe dementia (2.71%) and MCI (0.47%). Since majority (69.21%) of the moderate dementia patients are in cluster 3 and hence it is labeled as moderate dementia. Cluster 4 is labeled as mild dementia with 76.21% of its members are mild dementia patients, while 19.01% are moderate dementia. Other types of dementia severity has less than 5% in cluster 4. The grouping of cluster 1 and cluster 5 are not obvious. Cluster 1 consists of 65.32% are MCI patients, 24.54% normal patients and 10.14% mild dementia patients, without any moderate or severe dementia patients. 99.35% of the normal patients and 76.16% of the MCI patients are in cluster 1. Thus there are 2 types of dominant patients in cluster 1, namely normal-MCI. Similarly, 2 dominant severity in cluster 5 are MCI (22.47%) and mild dementia (77.02%).
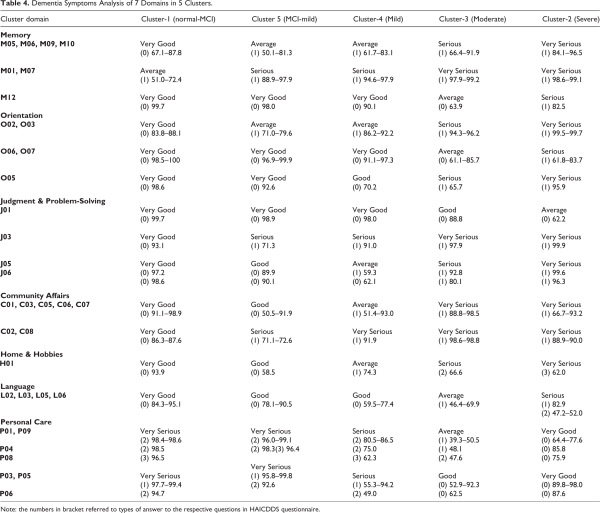
Based on the observations in Table 2, a details analysis of the dementia symptoms within the clusters is depicted in Table 4. Generally, all patients demonstrated declining abilities in cognitive and functional abilities except the Personal Care domain. In Memory domain, for example, 67.1% to 87.8% (very good) of the patients in normal-MCI cluster have no problem in recalling what they said or do, and do not repeatedly say the same thing. However, there are 50.1% to 83.1% (average) of the patients in MCI-mild or mild clusters admitted that they have such problems, and the percentage of patients have such problems increased to 66.4%-91.9% (serious) and 84.1%-96.5% (very serious) for moderate dementia cluster and severe dementia cluster, respectively. Similar patterns were observed in Orientation, Judgment and Problem Solving, Community Affairs, Home and Hobbies, and Language domains.
In the Personal Care domain, most of the severe dementia patients claimed that they are able to take care of themselves such as consume foods, using washroom, walk individually, take care of personal hygiene, take showers, walk up/down the stairs, and control defecation. Contrarily, patients with lower dementia levels admitted that they can only handle these activities partially or need help from someone. This findings is contradict with the general perceptions on the dementia patients. One possible reason for this could be the more serious dementia the less likely they are to accept that they need help from others. The question C09 is removed from this analysis because it is regarding the physical disability of the patients.
Discussion and Conclusion
In this study, a LDA model of 5 dementia levels with 36 variables has been developed with 80.00% of average accuracy of classification. This model acts as a rapid screening test in predicting the degree of dementia such as normal, MCI, mild, moderate or severe dementia under time constraints before undergoing a more in-depth clinical examination. HAICDDS questions that significantly related to each severity level of dementia were also identified within the cognitive and functional domains. The developed HAICDDS questionnaire excels in classifying mild, moderate and severe dementia but acceptably to discriminate MCI from the normal group as these patients do not exhibits much different in their behavior. Nevertheless, the ability of HAICDDS is still outperformed the Global Deterioration Scale (GDS) , in which the later is unable to distinguish MCI group from the normal group. The peformance of LDA model on HAICDDS are consistent with those previous studies using Bayesian-network classifier and support vector machine, , LDA and artificial neural networks, LDA and random forests, and Naïve Bayes. It shows that the proposd modified CDR can be used to develop a machine-learning derived screening instrument.
Besides, clustering analysis confirmed that the normal-MCI group has the strongest cognition and functionality in memory, orientation, judgment & problem solving, community affairs, home & hobbies and language domains, followed by mild dementia, moderate dementia and severe dementia. These findings can be applied to epidemiological studies. However, the clustering analysis presents contradictory results in respect of the personal care category, and hence further investigations are necessary. There are 2 key limitations in this study. Firstly, social, cultural and educational elements may impact some categories of the CDR scale although the format of the interview with both the patient and informant is flexible and suited to diverse socioeconomic groups and cultures. In addition, current HAICDDS scoring procedures are much more weighted toward the memory category.
For clinical pracice, an easy and simple screening tool for precision and efficiency in distinguishing normal/MCI/dementia is important. For the early detection of MCI or mild dementia, we have published a research article which used the same questionnaire and developed a novel simple questionnaire namely the NMD-12. Finding of the article revealed that it provides healthcare professionals with a simple and practical screening tool which accurately differentiates NC, MCI, VMD, and dementia. Differently, purpose of this study is to provide a precision and efficiency in discrimination all different stages of CI or dementia within a single questionnaire and we believed it is also important for clinical practice as well as caregiver system.
In conclusion, a modified and an abridged version of the CDR questionnaire, a 36-question screening tool, could be used to detect different types of dementia severity. Inclusion of language and psychiatric symptoms in the modified CDR provides a support for patients care and follow-up if it is correctly applied with necessary complementary assessment. However, there is a risk of failure to diagnose the early stages of dementia which leads to the neglecting of the early treatment of dementia. Hence, it is noteworthy to find out the specific attributes to widen the marked differences between normal cognition and MCI in a near future study in order to improve the classification accuracy. Furthermore, GDS is suggested to assess dementia stages as it encloses the whole range of pathology in central nervous system aging and progressive dementia while the CDR does not refer to Abou-Saleh et al (2011). A detailed assessment is required for existing staging tools such as GDS or FAST staging to develop a tailored care plan, subtyping and differential diagnosis.
Authors’ Note Y.F. Chang conducted the data analysis, edited author contributions, and was mainly responsible for revisions and drafts of the manuscript. W.Y. Loi conducted the literature search and data analysis. P.Y. Chiu participated in the data collection, revisions and final draft of the manuscript. H.N. Huang contributed to the revisions of the manuscript. The HAICDDS project was reviewed by the Medical Research Ethics Committee of Show Chwan Memorial Hospital and approved by the Data Inspectorate. The participants were selected from a register-based database of Show Chwan Health System and the data were analyzed anonymously.
Declaration of Conflicting Interests The authors declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Dr. Chiu’s work has been partly supported by Show Chwan Memorial Hospital and the work of Prof. Huang-Nan Huang is partially supported by the Ministry of Science and Technology of Taiwan under the grant number MOST 107-2115-M-029-003. The study was funded by Show Chwan Memorial Hospital (No. RD-105032) and the Ministry of Science and Technology of Taiwan (MOST 107-2115-M-029-003).
Yun Fah Chang
https://orcid.org/0000-0003-1582-4734
Pai-Yi Chiu
https://orcid.org/0000-0003-2552-4516
References
- 1.
- 2.
- 3.
- 4. Folstein MF, Folstein SE, McHugh PR. Mini-mental state—a practical method for grading the cognitive state of patients for the clinician. J Psychiatr Res. 1975;12(3):189–198.
- 5. Anthony JC, LeResche L, Niaz U, Von Korff MR, Folstein MF. Limits of the ‘Mini-Mental State’ as a screening test for dementia and delirium among hospital patients. Psychol Med. 1982;12(2):397–408.
- 6. Tombaugh TN, McIntyre NJ. The mini-mental state examination: a comprehensive review. J Am Geriatr Soc. 1992;40(9):922–935.
- 7. Andrew EB, Paul RS. Memory Loss, Alzheimer’s Disease, and Dementia: A Practical Guide for Clinicians. 2nd ed. Elsevier; 2015.
- 8. Nasreddine ZS, Philips NA, Bedirian V, et al. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53(4):695–699.
- 9. Tsai CF, Lee WJ, Wang SJ, Shia BC, Nasreddine Z, Fuh JL. Psychometrics of the Montreal Cognitive Assessment (MoCA) and its subscales: validation of the Taiwanese version of the MoCA and an item response theory analysis. Int Psychogeriatr. 2012;24(4):651–658.
- 10. Howard MF, Kenneth R, John Y. Brocklehurst’s Textbook of Geriatric Medicine and Gerontology. 8th ed. Elsevier; 2017.
- 11. Ehreke L, Luck T, Luppa M, Konig HH, Villringer A, Riedel-Heller SG. Clock drawing test—screening utility for mild cognitive impairment according to different scoring systems: results of the Leipzig Longitudinal Study of the aged (LEILA 75+). Int Psychogeriatr 2011;23(10):1592–1601.
- 12. Wind AW, Schellevis FG, Van Staveren G, Scholten RP, Jonker C, Van Eijk JT. Limitations of the Mini-Mental State Examination in diagnosing dementia in general practice. Int J Geriatr Psychiatry 1997;12(1):101–108.
- 13. Roalf DR, Moberg PJ, Xie SX, et al. Comparative accuracies of two common screening instruments for classification of Alzheimer’s disease, mild cognitive impairment, and healthy aging. Alzheimers Dement. 2013;9(5):529–537.
- 14. Larner AJ. ed Cognitive Screening Instruments: A Practical Approach. 2nd ed. Springer International Publishing; 2017.
- 15. Jorm AF, Korten AE. Assessment of cognitive decline in the elderly by informant interview. Br J Psychiatry. 1988;152(2):209–213.
- 16. Jorm AF, Jacomb PA. The Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): socio-demographic correlates, reliability, validity and some norms. Psychol Med. 1989;19(4):1015–1022.
- 17. Jorm AF. A short form of the Informant Questionnaire on Cognitive Decline in the Elderly (IQCODE): development and cross-validation. Psychol Med. 1994;24(1):145–153.
- 18. Galvin JE, Roe CM, Powlishta KK, et al. The AD8: a brief informant interview to detect dementia. Neurology. 2005;65(4):559–564.
- 19. Galvin JE, Roe CM, Xiong C, Morris JC. The validity and reliability of the AD8 informant interview in dementia. Neurology. 2006;67(11):1942–1948.
- 20. Yohko M, Tomoharu Y, Haruyasu Y. Symptoms of early dementia-11 questionnaire (SED-11Q): a brief informant-operated screening for dementia. Dement Geriatr Cogn Dis Extra 2013;3(1):131–142.
- 21. Bhavna S, Deepika D, Dinesh K. An exploratory study on the prevalence of early dementia among geriatric population in a rural area of district Jammu. Int J Community Med Public Health 2017;4(5):1500–1503.
- 22. Chiu PY, Wei CY, Hung GU. Preliminary study of the history-based artificial intelligent clinical dementia diagnostic system. Show Chwan Med J. 2019;18(1):018–027. doi:10.3966/156104972019061801003
- 23. Chiu PY, Tang H, Wei CY, Zhang C, Hung GU, Zhou W. NMD-12: a new machine-learning derived screening instrument to detect mild cognitive impairment and dementia. PLoS One. 2019;14(3):e0213430. doi:10.1371/journal.pone.0213430
- 24. Morris JC. The Clinical Dementia Rating (CDR): current version and scoring rules. Neurology. 1993;43(11):2412–2414.
- 25. Lin CM, Hung GU, Wei CY, Tzeng RC, Chiu PY. An informant-based simple questionnaire for language assessment in neurodegenerative disorders. Dement Geriatr Cogn Disord. 2018;46(3-4):207–216.
- 26. Hughes CP, Berg L, Danziger WL, Coben LA, Martin RL. A new clinical scale for the staging of dementia. Br J Psychiatry. 1982;140(6):566–572.
- 27. Fisher R. The use of multiple measurements in taxonomic problems. Ann Eugen. 1936;7(2):179–188.
- 28. Richard AJ, Dean WW. Applied Multivariate Statistical Analysis. 6th ed. Pearson Prentice Hall; 2007.
- 29. MacQueen JB. Some methods for classification and analysis of multivariate observations. In: Proceedings of 5-th Berkeley Symposium on Mathematical Statistics and Probability. University of California Press; 1967.
- 30. John DK, Brian MN, Aoife DA. Fundamentals of Machine Learning for Predictive Data Analytics. The MIT Press; 2015.
- 31. Lo RY. The borderland between normal aging and dementia. Tzu Chi Med J. 2017;29(2):65–71.
- 32. Reisberg B, Ferris SH, de Leon MJ, Crook T. The Global Deterioration Scale for assessment of primary degenerative dementia. Am J Psychiatry. 1982;139(9):1136–1139.
- 33. Yoon JC, Chang WW, Sunyoung K, et al. Five items differentiate mild to severe dementia from normal to minimal cognitive impairment—using the Global Deterioration Scale. J Clin Gerontol Geriatr. 2016;7(1):1–5.
- 34. Shankle WR, Mani S, Dick MB, Pazzani MJ. Simple models for estimating dementia severity using machine learning. Stud Health Technol Inform 1998;52(Pt 1):472–476.
- 35. Chen R, Herskovits EH. Machine-learning techniques for building a diagnostic model for very mild dementia. Neuroimage 2010;52(1):234–244.
- 36. French BM, Dawson MR, Dobbs AR. Classification and staging of dementia of the Alzheimer type: a comparison between neural networks and linear discriminant analysis. Arch Neurol. 1997;54(8):1001–1009.
- 37. Maroco J, Silva D, Rodrigues A, Guerreiro M, Santana I, de Mendonça A. Data mining methods in the prediction of dementia: a real-data comparison of the accuracy, sensitivity and specificity of linear discriminant analysis, logistic regression, neural networks, support vector machines, classification trees and random forests. BMC Res Notes. 2011;4:299.
- 38.
- 39. Abou-Saleh MT, Katona CL, Kumar A. Principles and Practice of Geriatric Psychiatry. 3rd ed. John Wiley & Sons; 2011.