INTRODUCTION
Erectile dysfunction (ED) adversely impacts men’s physical and mental health, as well as their intimate relationship. Studies have demonstrated that ED significantly increases the risk of cardiovascular diseases, stroke, and all-cause mortality. Age is one of the most important risk factors among the various etiologies of ED. In adult men, erectile function tends to decline with age, especially after the age of 40 years, even though they are in their prime. Undoubtedly, the aging process leads to the occurrence of age-related erectile dysfunction (ARED), which is also associated with the development and progression of various medical comorbidities that worsen the degree of ED. Studies have shown that most elderly men remain interested in sexual intimacy, but many cannot complete sexual life due to ED. The clinical entity of ARED is closely related to a variety of factors, such as a decline in androgen levels, fibrosis or apoptosis of the corpus cavernosum smooth muscle, reduction of nerve fibers, and presence of endothelial dysfunction., At present, the treatment of ARED mainly relies on phosphodiesterase 5 inhibitors (PDE5is) and testosterone supplementation in men with proven hypogonadism, but the efficacy is still poor for many ARED patients., More recently, there has been considerable interest in regenerative technology, such as stem cell or platelet-rich plasma injections and low-intensity extracorporeal shock wave therapy, which can be used as an alternative therapy for ED., However, due to their advanced age and the presence of medical comorbidities, ARED patients are not suitable for or unlikely to respond to these treatments.
In recent years, photobiomodulation therapy (PBMT), also known as low-level laser therapy (LLLT), has garnered considerable attention for its ability to induce cell proliferation and enhance stem cell differentiation. Its non-invasive nature, coupled with its ability to reduce pain and inflammation and promote tissue repair, has led to its adoption and increasing application across various medical fields. A plethora of studies have shown PBMT to be an effective therapeutic option in many medical conditions, such as diabetes mellitus, brain and spinal cord injuries, dermatological disorders, and dental issues. Near-infrared (NIR) laser, renowned for its superior tissue penetration capabilities, has emerged as one of the most utilized lasers in PBMT. There is compelling evidence that low-power NIR-PBMT can ameliorate endothelial dysfunction by increasing the bioavailability of nitric oxide (NO), paving a viable new avenue in treating cardiovascular diseases. It is hypothesized that NIR-PBMT may effectively restore erectile function associated with corpus cavernosum endothelial dysfunction. Recently, research has confirmed that 808 nm NIR-PBMT can significantly improve erectile function in rats with diabetes mellitus-induced erectile dysfunction (DMED) induced by streptozotocin (STZ), with minimal side effects. Another study discovers that the combination of red-light-controllable nitric oxide donor (NORD-1) and red-light irradiation can ameliorate erectile function in DMED rats. A more recent study shows that PBMT employing light-emitting diodes (LEDs) at wavelengths of 660 nm and 830 nm, and a combination of both wavelengths, significantly improve erectile function in a mouse model of cavernous nerve injury (CNI).
This study aims to assess the therapeutic potential of NIR-PBMT in a mouse model of ARED, with a particular focus on its impact on endothelial cells. We hypothesize that NIR-PBMT can enhance NO signaling, reduce cellular senescence, and improve endothelial function, thereby ameliorating ARED.
MATERIALS AND METHODS
Ethics statement and animal study design
This study was conducted in accordance with the ethical standards of the Institutional Review Board (IRB) of Peking University Third Hospital (Beijing, China), and the protocol was approved by the same ethical committee (Approval No. LM2022341). All clinical procedures were performed with the utmost respect for the ethical principles governing human experimentation, including the principles of autonomy, beneficence, and justice. Written informed consent was obtained from all participants involved in the study, ensuring that they were fully informed about the study’s objectives, procedures, potential risks, and benefits before their voluntary participation. Confidentiality and privacy of the participants were strictly maintained throughout the study, and their rights to withdraw from the study at any time without any consequences were clearly communicated and upheld. The study adhered to the Declaration of Helsinki and all applicable regulatory requirements for the protection of human subjects in medical research.
The animal study was performed following approval from the Laboratory Animal Ethics Committee of Peking University Third Hospital (License No. LA2025067) and the Animal Research: Reporting of In Vivo Experiments (ARRIVE) guideline. The Office of Academic Research and the Ethics Committee of Peking University Third Hospital have authorized this study, and all animal experiments were conducted in line with animal ethics. This study used a total of 40 C57BL/6J strain 3-month-old male mice and 20-month-old male mice. All mice were maintained in plastic cages with an ad libitum diet in a climate-controlled environment with a 12-h light and 12-h dark photoperiod. Mice at the age of 3 months are designated as young, while those at 20 months are classified as aged. Subsequently, mice from both age groups were randomized into two groups, with one group receiving natural light exposure and the other receiving NIR-PBMT (in the dark). Natural light refers to the visible light emitted by a xenon lamp and then filtered through an SP710 nm short-wave pass filter (which can effectively absorb near-infrared light with wavelengths of 710 nm and above; Gengxu Optoelectronic Technology Co., Ltd., Shenzhen, China). In NIR-PBMT, we selected an 808 nm wavelength fiber-coupled laser device (Vivolight Medical Device and Technology Co., Ltd., Shenzhen, China). The core of the fiber is 0.2 mm in diameter and can generate a maximum power of 800 mW. The laser therapy was conducted using an 808 nm near-infrared laser at an irradiance of 4 J cm−2 based on previous research, with treatments administered every 48 h over 2 weeks (Figure 1a). During treatment, we also used a skin thermometer (Hikmicro, Hangzhou, China) to monitor the skin temperature. Mice were euthanized by rapid cervical dislocation following isoflurane anesthesia, ensuring a swift and humane procedure. Confirmation of death was ascertained by the absence of respiratory and cardiac activity.
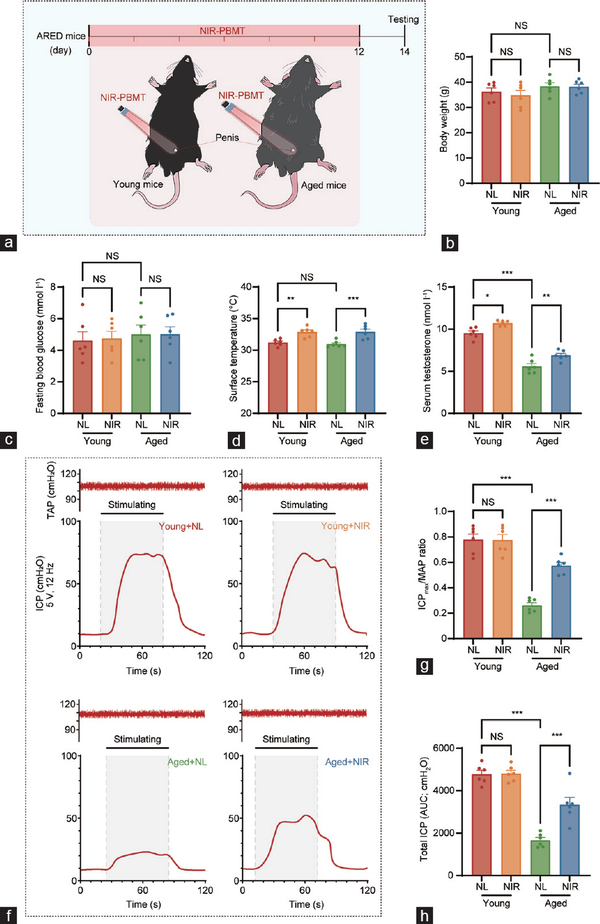
Figure 1
Schematic diagram of NIR-PBMT and erectile function analyses of the ARED animal model. (a) The penises of naturally aged mice (20 months old) were irradiated with a NIR at 808 nm and 4 J cm−2 for 2 weeks, with treatment every 48 h. A comparison of the general conditions between young mice (3 months) and aged mice (20 months) treated with natural light and NIR (mean±s.e.m.; n=6 per group). (b) Body weight, (c) fasting blood glucose, (d) surface temperature at the treatment site, and (e) serum testosterone levels were measured for each group of mice. (f) Measurement of erectile function via electrical stimulation of the cavernous nerve. Representative ICP tracings were obtained following cavernous nerve stimulation at 12 Hz and 5 V for 60 s, respectively, in the young with NL, young with NIR, aged with NL, and aged with NIR. The orange line segment represents the duration of electrical stimulation (60 s). (g) Mean ICPmax was calculated for each group. (h) Total ICP (ICP AUC) was calculated for each group. Data are expressed as the mean±s.e.m. of 6 mice. Statistical analysis was performed using an unpaired t-test. *P<0.05, **P<0.01, and ***P<0.001. NS: not significant; NIR-PBMT: near-infrared photobiomodulation therapy; ARED: age-related erectile dysfunction; ICP: intracavernous pressure; ICPmax: maximum ICP; MAP: mean arterial pressure; NL: natural light; AUC: area under the curve; s.e.m.: standard error of the mean; TAP: tail arterial pressure.
Erectile function evaluation
Erectile capacity was assessed by measuring the ratios of maximum intracavernosal pressure)/mean arterial pressure (ICPmax/MAP ratio) in response to electrical stimulation of the cavernous nerve (CN). Briefly, mice were anesthetized by intraperitoneal injection of sodium pentobarbital (35 mg kg−1). Then, a 32-gauge needle was inserted into the tail artery, and a 25-gauge needle was inserted into the left penile crura. The needles were connected to a pressure transducer of PowerLab workstation (AD Instruments Co., Ltd., Sydney, Australia) to measure arterial pressure and ICP, respectively. The CN was electrically stimulated at 5 V and 12 Hz with a pulse width of 1 ms for 1 min at 3-min intervals by PowerLab workstation. During tumescence, the ICPmax and total ICP reflected by the area under the curve (AUC) were recorded. Variations in tail artery pressure were normalized by calculating the ICPmax/MAP ratio.
Masson’s trichrome and immunohistochemistry (IHC) staining
The corpus cavernosum tissue of the penis after the removal of the glans was divided into 2 halves. The distal half was used for tissue protein extraction, and the proximal half was used to prepare paraffin sections after fixation with 4% paraformaldehyde for 48 h and embedded in paraffin blocks. Sections were cut at 5 μm thickness from each block and used for staining smooth muscle and collagen using Masson’s trichrome. The Fiji: ImageJ software was used to capture and measure the contents of smooth muscle and collagen in the corpus cavernosum of these mice.
IHC of penile tissue was performed as follows. Penile tissue sections initially stored at 4°C were warmed at 65°C for 2 h, dehydrated using immunohistochemical ascending solution, and washed twice with phosphate-buffered saline (PBS). The sections were then immersed in citric acid repair solution for 10 min, followed by consecutive washing with PBS and peroxide solution. The enzyme-locking agent was titrated for 10 min, after blocking with 10% normal goat serum for 1 h, the sections were incubated with primary antibodies, including rabbit anti-p21 (GB11153; 1:200; Servicebio, Wuhan, China), rabbit anti-phosphorylated H2A histone family member X (anti-γ-H2A.X; 1:200; GB111841; Servicebio), and rabbit anti-endothelial nitric oxide synthase (anti-eNOS; 1:200; GB115277; Servicebio), overnight at 4°C. Following three 5-min washes in PBS-Tween (0.05%), sections were incubated with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG secondary antibody (1:200; 33101ES60; Yeasen, Shanghai, China) for 60 min at room temperature. After thorough washing, immunoreactivity was visualized using 3,3’-diaminobenzidine (DAB) substrate (ZLI-9017; Zhongshan Jinqiao, Beijing, China) for 5–8 min, followed by counterstaining with hematoxylin. Following IHC staining and dehydration, the slides were sealed with neutral resin and observed by NanoZoomer S60 scanner and NDP.view2 Image viewing software (Hamamatsu Photonics, Hamamatsu, Japan).
Western blotting
Western blotting was performed according to the manufacturer’s protocol (Bio-Rad, Hercules, CA, USA). The polyvinylidene fluoride membrane was incubated with primary antibodies, including mouse anti-alpha-smooth muscle actin (anti-αSMA; 1:2000; ab7817; Abcam, Cambridge, UK), rabbit anti-eNOS (1:1000; #32027; CST, Danvers, MA, USA), rabbit anti-γ-H2A.X (1:4000; ab11175; Abcam,), rabbit anti-p21 (1:2000; 10355-1-AP; Proteintech, Wuhan, China), rabbit anti-p16inhibitor of cyclin-dependent kinase 4 (INK4A)(1:3000; 10883-1-AP; Proteintech), rabbit anti-interleukin (IL)-6 (1:3000; 66146-1-Ig; Proteintech), and rabbit anti-IL-8 (1:1000; 27095-1-AP; Proteintech), at 4°C overnight. Bound antibodies were detected using anti-mouse and anti-rabbit IgG-HRP (incubated at room temperature for 1 h) and the super-enhanced chemiluminescent (ECL) detection reagent system (36208ES76; Yeasen). Results were quantified by densitometry and normalized to α-tubulin (1:5000; YM3035; Immunoway).
Single-cell sequencing analysis of corpus cavernosum endothelial cells in aged rats
The rat corpus cavernosum tissues were collected from 10 rats (5 young rats and 5 aged rats) according to the study of Liu et al. The experimental procedures were approved by the Committee of Animal Care and Use at Sun Yat-sen University (Guangzhou, China). Utilizing the ggplot2 package, we visualized the differentially expressed genes of endothelial cells, with a log fold change (log2FC) threshold set at 0.5 and an adjusted P-value threshold at 0.05. A mountain range plot was employed to visualize the gene set enrichment analysis (GSEA) results related to mitochondria. Gene Ontology (GO) enrichment analysis was performed using the clusterProfiler package, and the results were sorted based on the GeneRatio. The top 20 biological process (BP) terms were visualized using a bubble chart. Additionally, Kyoto Encyclopedia of Genes and Genomes (KEGG) enrichment analysis was conducted with the clusterProfiler package, and all KEGG terms with a P < 0.05 were sorted according to the GeneRatio and visualized using a bubble chart.
β-galactosidase staining of human corpus cavernosum endothelial cells (HCCECs)
HCCECs were extracted and cultured following the protocol established by Zhao et al. HCCECs were cultured to 60%–70% confluence and subjected to senescence induction and therapy as needed. Cells were fixed in 0.5% glutaraldehyde for 10 min at room temperature, washed three times with PBS, and incubated with the β-galactosidase staining solution (C0602; Beyotime, Shanghai, China) at 37°C overnight. Stained cells were counted under a light microscope, and the percentage of positively stained cells was quantified to assess senescence.
Measurement of NO levels and eNOS activity
NO levels in cell lysates were measured using a NO detection kit (S0021; Beyotime), according to the manufacturer’s instructions. To assess the activity of eNOS, L-NAME, a specific eNOS inhibitor (ST1555; Beyotime), was utilized. The inhibition of eNOS activity by L-NAME was determined by comparing NO levels in the presence and absence of the inhibitor. The experiment was conducted in triplicate, and the results are expressed as the mean ± standard error of the mean (s.e.m.).
Mitochondrial imaging in live cells
HCCECs were cultured in confocal dishes (BS-15-GJM-B-20; Biosharp, Beijing, China) to approximately 70% confluence. Cells were then treated as per the experimental protocol. For mitochondrial imaging, Mito-Tracker (C1034; Beyotime) was diluted to a working concentration of 0.2 μmol l−1 using PBS and added to the culture medium, followed by incubation at 37°C for 20 min. Hoechst 33342 (C1028; Beyotime) was used to stain cell nuclei at a dilution ratio of 1:100. Cells were imaged using a Zeiss LSM 880 confocal microscope (Carl Zeiss, Oberkochen, Germany) with a 100× oil immersion objective. Mitochondrial networks were analyzed using Fiji: ImageJ with the “Mitochondria Analyzer” plugin.
Angiogenesis assay with Calcein AM staining
For the assessment of angiogenic activity, HCCECs were suspended in serum-free medium and plated onto Matrigel (C356234; Corning, New York, NY, USA) within μ-slide 15 well three-dimensional (3D) chambers (81506; ibidi, Munich, Germany) at a density of 5000 cells per well, followed by incubation at 37°C for 45 min. To visualize the nascent vascular networks, cells were stained with Calcein AM (C2012; Beyotime) for 4 h to initiate tube formation. The intensity of angiogenesis was quantitatively analyzed using Fiji: ImageJ with the “Angiogenesis Analyzer” plugin.
Scratch wound healing assay
HCCECs were seeded in 35-mm dishes with ibidi Culture-Insert 2 Well (80209; ibidi) and allowed to reach confluence. Once a monolayer was formed, a 500 μm single scratch was made between the 2 wells by the insertion. After scratching, cells were gently washed with PBS to remove debris, and fresh media were added. Cells were then allowed to migrate into the scratched area, and images were captured at 0 h and 8 h post-wounding using an inverted microscope (Axio Observer Inverted Fluorescence Microscope; Carl Zeiss). Wound closure was analyzed by measuring the area of the scratch at different time points, and the migratory ability of cells was quantified by calculating the percentage of wound healing relative to the initial scratched area.
Statistical analyses
Data were presented as mean ± s.e.m. The data were tested for normal distribution and homogeneity of variance and were analyzed with analysis of variance (ANOVA) or the Kruskal–Wallis test if appropriate. Statistical analysis was performed using GraphPad Prism version 9.0 for Windows (www.graphpad.com) and R software version 4.2.1 (R Foundation for Statistical Computing, Vienna, Austria), with P < 0.05 denoting statistical significance.
RESULTS
NIR device and treatment
Figure 1a illustrates the conceptual framework of NIR-PBMT for the treatment of the mouse model of ARED. The NIR-PBMT device utilized in this study is a low-light delivery apparatus that administers near-infrared laser irradiation to the ventral abdominal region of mice, with a control group exposed to natural light for an equivalent duration. Body weights (Figure 1b) and fasting blood glucose levels (Figure 1c) of each mouse were assessed before treatment to ensure the absence of underlying diabetes in the study subjects.
Throughout the treatment period, the maximum surface temperature in the irradiated area was recorded to determine whether the heat generated by the laser could cause thermal injury to the treatment site. The results indicated that NIR-PMBT indeed significantly elevated the surface temperature at the irradiated site; however, the temperature increase was limited, with an approximate rise of 2°C (Figure 1d).
Interestingly, following a treatment cycle, aged mice exhibited a significant decline in serum testosterone levels compared to young mice (Figure 1e). However, after the complete NIR treatment, there was a significant increase in testosterone levels in both young and aged mice compared to those in mice exposed to natural light.
NIR-PBMT improves erectile function in ARED mice
Post-treatment assessments were conducted by measuring the ICPmax/MAP ratio to confirm the therapeutic efficacy of NIR-PBMT in ARED mice, thereby evaluating erectile function during cavernous nerve stimulation (Figure 1f). The mean ICPmax in aged mice was significantly lower than that in young mice, indicating a marked improvement of erectile function in aged mice following NIR-PBMT.
Furthermore, erectile function in mice was characterized by examining the ICPmax/MAP ratio (Figure 1g) and the total arterial pressure (Figure 1h). It was observed that erectile function in aged mice was significantly diminished relative to that in young mice. However, NIR-PBMT intervention demonstrated a capacity to ameliorate erectile function in ARED mice to a certain extent. Additionally, the arterial pressure in aged mice was not significantly higher than that in young mice, thereby excluding the interference of hypertensive ED.
NIR-PBMT restores smooth muscle contents impaired by aging
Aging is associated with a reduction in smooth muscle contents and a concurrent significant increase in collagen levels in the penile corpora cavernosum. Following NIR-PBMT, an elevated smooth muscle content and a decreased collagen level were observed in the corpora cavernosum of aged mice (Figure 2a and 2b). These histological changes were corroborated with Masson’s trichrome staining, revealing a reversal of age-related tissue alterations.
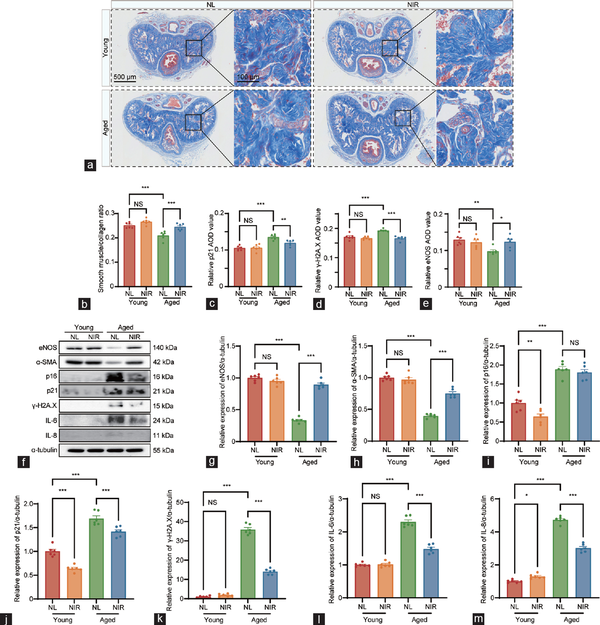
Figure 2
Histological analysis of corpus cavernosum tissue fibrosis and comprehensive analysis of erectile and senescence markers contents in different groups. (a) Penis samples in the young with NL, young with NIR, aged with NL, and aged with NIR were prepared for the detection of corpus cavernosum tissue fibrosis using Masson’s trichrome staining. Semi-quantitative image analysis of (b) smooth muscle/collagen ratio, (c) p21 expression, (d) γ-H2A.X expression, and (e) eNOS expression in corpus cavernosum tissues. (f) Representative western blotting images depicting the protein bands of interest. Quantitative assessment of relative protein expression levels for (g) eNOS, (h) α-SMA, (i) cell cycle inhibitor p16, (j) cell cycle inhibitor p21, (k) γ-H2A.X, (l) IL-6, and (m) IL-8. Data are expressed as the mean±standard error of the mean of 6 mice. Statistical analysis was performed using an unpaired t-test. *P<0.05, **P<0.01, and ***P<0.001. NS: not significant; NL: natural light; NIR: near-infrared; γ-H2A.X: phosphorylated H2A histone family member X; eNOS: endothelial nitric oxide synthase; α-SMA: alpha-smooth muscle actin; IL-6: interleukin-6; IL-8: interleukin-8; AOD: average optical density.
NIR-PBMT alleviates cellular senescence and enhances eNOS expression in mice with ARED
The immunohistochemical examination has yielded profound insights into the age-associated alterations within the penile corpora cavernosum, with a particular focus on the expression of cellular senescence markers. A marked diminution in the immunopositive areas for two senescence-associated proteins (p21 and γ-H2A.X) was observed in aged mice compared to young mice (Figure 2c and 2d). Following NIR laser therapy, a robust decrease in the contents of p21 and γ-H2A.X was observed within the cavernosum tissue of aged mice (Figure 2c and 2d, and Supplementary Figure 1). The IHC staining revealed a significant reduction in the positive areas for eNOS within the penile corpora cavernosum of aged mice compared to young mice. Following NIR laser therapy, a pronounced increase in the protein contents of eNOS was observed in the cavernosum tissue of aged mice (Figure 2e and Supplementary Figure 1).
Supplementary Figure 1
Immunohistochemistry image of corpus cavernosum tissue, cell cycle inhibitor p21, phosphorylated H2A histone family member X (γ-H2A.X), and endothelial nitric oxide synthase (eNOS) in different groups.
Molecular regulations of NIR laser therapy in ARED
To delineate the molecular underpinnings of NIR laser therapy for treating ARED, we conducted western blotting to observe the expression levels of erectile function markers (eNOS and α-SMA), senescence markers (γ-H2A.X, p16INK4A, and p21), and cytokines of the senescence-associated secretory phenotype (SASP; IL-6 and IL-8), with α-tubulin serving as the loading control (Figure 2f–2m). In aged mice, the expression levels of eNOS and α-SMA were significantly diminished compared to those in young mice, while the expression levels of (γ-H2A.X, p16INK4A, and p21) were markedly elevated. After a cycle of NIR treatment, the detrimental alterations in the cavernosum tissue were effectively mitigated in aged mice.
Differences in corpus cavernosum endothelial cells upon aging
Using the data on differentially expressed genes (DEGs) of endothelial cells from the corpus cavernosum provided by Liu et al. with the log2FC threshold at 0.5 and the adjusted P-value at 0.05, we identified 53 upregulated genes and 151 downregulated genes (Figure 3a). We further conducted a GSEA analysis of these DEGs, focusing on those related to mitochondria. With a criterion of adjusted P < 0.05 and false discovery rate (FDR) < 0.25, we found that these DEGs were significantly enriched in the terms “mitochondrial protein import” and “mitochondrial translation” (Figure 3b).
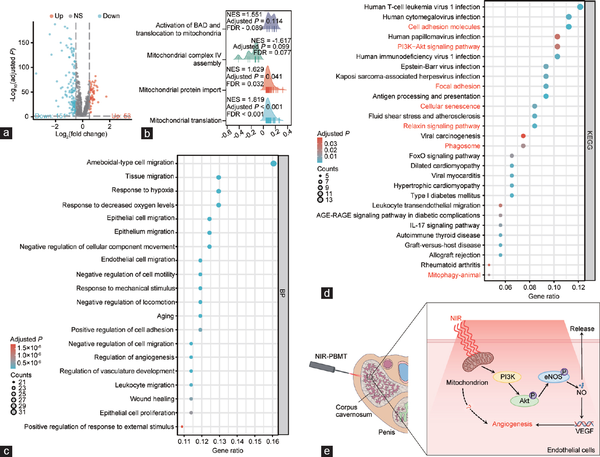
Figure 3
Secondary analysis of aged rat’s CCECs in aged rats. (a) Volcano plot (–log10[adjusted P] versus log2[fold change]; young to aged) illustrating all age-dependent changes of specific cell genes. Genes in grey are not distinctly altered with aging. (b) GSEA analysis of all CCECs using mitochondria-related DEGs. (c) GO-BP enrichment analysis of all CCECs using DEGs. (d) KEGG enrichment analysis of all CCECs using DEGs. (e) The potential mechanism of NIR in treating ARED. CCEC: corpus cavernosum endothelial cell; GSEA: gene set enrichment analysis; DEG: differentially expressed gene; KEGG: Kyoto Encyclopedia of Genes and Genomes; GO-BP: Gene Ontology-Biological Process; FoXO: forkhead box O; AGE-RAGE: Advanced Glycation End products-Receptor for Advanced Glycation End products; NIR-PBMT: near-infrared photobiomodulation therapy; VEGF: vascular endothelial growth factor; PI3K: phosphatidylinositol 3-kinase; Akt: serine/threonine kinase; eNOs: endothelial nitric oxide synthase; NO: nitric oxide.
Subsequently, we performed GO enrichment analysis of these DEGs and presented the top 20 BP terms sorted by gene ratio, which predominantly involved several biological processes, such as cell migration, aging, and angiogenesis (Figure 3c). KEGG enrichment analysis revealed all pathways with an adjusted P < 0.05, sorted by gene ratio, such as cellular adhesion, cellular senescence, mitophagy, relaxin signaling pathway, and phosphatidylinositol 3-kinase (PI3K)-protein kinase B (Akt) signaling pathway (Figure 3d). Based on the results above, we proposed a hypothesis for potential mechanisms underlying NIR in treating ARED (Figure 3e).
Verification of endothelial functionality of HCCECs and efficacy of NIR-PBMT
HCCECs were isolated, cultured, and identified by endothelial cell markers CD31 and von Willebrand factor (vWF; Supplementary Figure 2). Cellular senescence and repair were assessed using a β-galactosidase assay (Figure 4a). The proportion of SA-β-gal-positive cells, indicative of cellular aging, significantly increased following induction with 200 μmol l−1 H2O2. Treatment with NIR-PBMT substantially reduced the aging of HCCECs, demonstrating the capacity of NIR-PBMT to ameliorate H2O2-induced senescence in endothelial cells (Figure 4b).
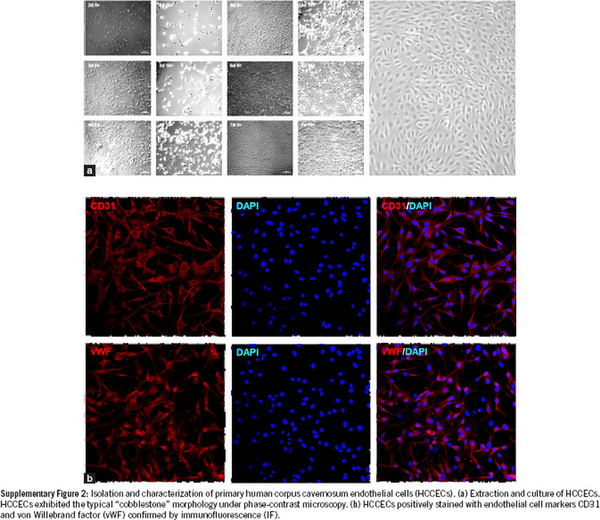
Supplementary Figure 2
Isolation and characterization of primary human corpus cavernosum endothelial cells (HCCECs). (a) Extraction and culture of HCCECs. HCCECs exhibited the typical “cobblestone” morphology under phase-contrast microscopy. (b) HCCECs positively stained with endothelial cell markers CD31 and von Willebrand factor (vWF) confirmed by immunofluorescence (IF).
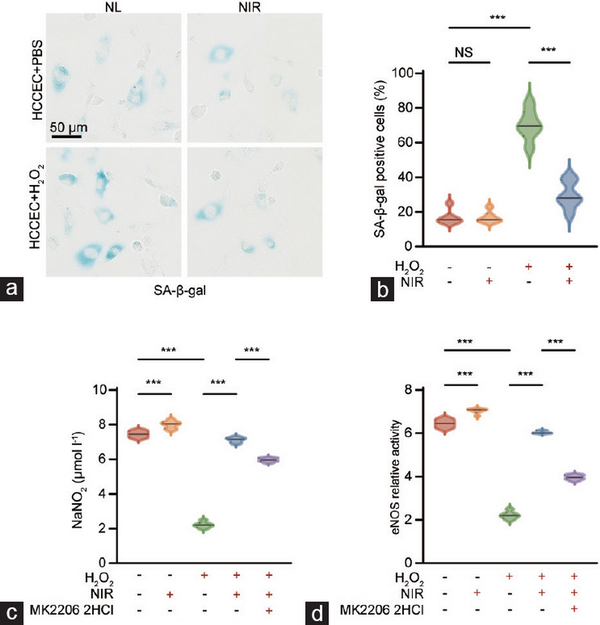
Figure 4
In vitro assessment of cellular senescence and injury repair in primary HCCECs. (a) Representative images of β-galactosidase staining in HCCECs. (b) Quantification of SA-β-gal-positive cells. (c) NIR therapy promotes NO release and (d) eNOS activity in aged HCCECs, whereas this effect is partially abrogated by the Akt inhibitor MK-2206 2HCl. Data are expressed as the mean±standard error of the mean. Statistical analysis was performed using an unpaired t-test. ***P<0.001. NS: not significant; HCCEC: human corpus cavernosum endothelial cell; NIR: near-infrared; NO: nitric oxide; eNOS: endothelial nitric oxide synthase; Akt: protein kinase B.
Exploratory analysis of the mechanism behind NIR-PBMT for ARED
First, we assessed the NO-releasing capacity of HCCECs by measuring the concentration of NaNO2 in the medium and further calculated the relative activity of eNOS following treatment with L-NAME (an eNOS inhibitor; Figure 4c and 4d). The results indicated a significant reduction in NO release and eNOS activity in H2O2-induced senescent HCCECs, which could be markedly restored by NIR-PBMT. Moreover, in the presence of MK-2206 2HCl, a specific Akt inhibitor capable of suppressing the PI3K/Akt/eNOS pathway, NIR-PBMT could still ameliorate the bioavailability of NO in aged HCCECs. These results suggest that NIR-PBMT may improve endothelial cell function in ARED through molecular mechanisms beyond the PI3K/Akt/eNOS signaling pathway.
Subsequently, we employed the mitochondrial probe Mito-tracker 633 to evaluate the morphology and function (focusing on the network structure) of mitochondria in live HCCECs. We observed a significant decrease in the total branch length and branch junctions of the mitochondrial network in aged HCCECs, which was substantially restored by NIR-PBMT. Notably, inhibition of the PI3K/Akt pathway by MK-2206 2HCl did not affect the mitochondrial functional recovery induced by NIR (Figure 5a–5c).
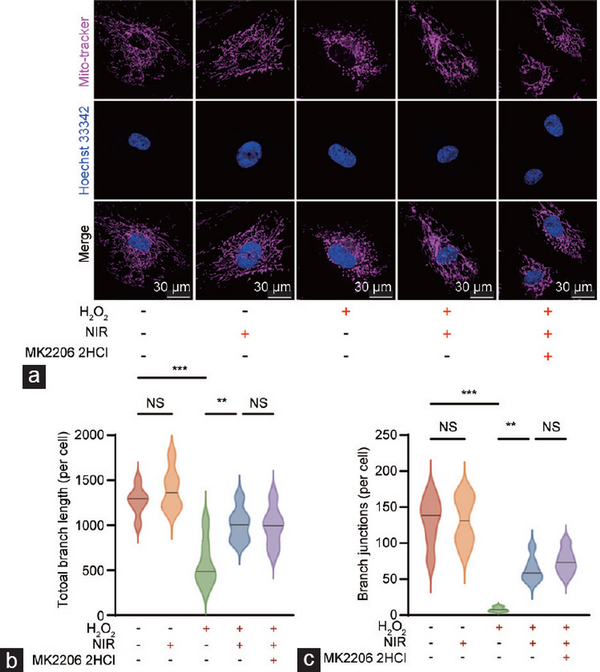
Figure 5
Comprehensive analysis of mitochondrial morphology and function in HCCECs. (a) Visualization of mitochondrial structures in HCCECs under different experimental conditions. (b) Quantitative assessment of the total branch length of the mitochondrial network. (c) Quantitative assessment of mitochondrial network branch junctions. Data are expressed as the mean±santard error of the mean. Statistical analysis was performed using an unpaired t-test. **P<0.01, ***P<0.001. NS: not significant; NIR: near-infrared; HCCEC: human corpus cavernosum endothelial cell.
Furthermore, we observed whether NIR-PBMT could affect the tube-forming capacity and migratory ability of endothelial cells. Angiogenesis assays revealed a marked reduction in the tube-forming ability of senescent HCCECs (Figure 6a), and wound healing assays indicated a substantial decline in their migratory capacity (Figure 6b). Interestingly, NIR-PBMT notably rejuvenated the tube-forming capacity and migratory ability of aged HCCECs. Notably, the blockade of the PI3K/Akt signaling pathway significantly counteracted the pro-angiogenic and pro-migratory effects of NIR-PBMT (Figure 6c and 6d).
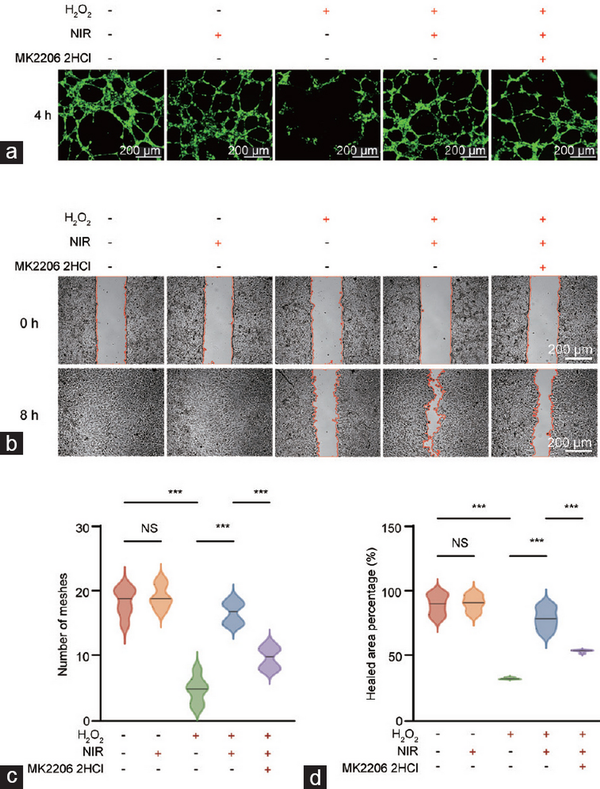
Figure 6
Enhancement of NIR-PBMT on angiogenic and migratory abilities of aged HCCECs in wound healing assays. NIR-PBMT (a) stimulates angiogenic and (b) enhances the migratory ability capabilities in aged HCCECs. Evaluation of (c) angiogenesis by counting the number of meshes formed in the vascular network and (d) migratory ability by measuring the percentage of the healed area. Data are expressed as the mean±standard error of the mean. Statistical analysis was performed using an unpaired t-test. ***P<0.001. NS: not significant; NIR-PBMT: near-infrared photobiomodulation therapy; HCCEC: human corpus cavernosum endothelial cell.
DISCUSSION
Our study evaluated the therapeutic potential of NIR-PBMT in a mouse model of ARED. The findings indicate that NIR-PBMT significantly improves erectile function, restores smooth muscle content, alleviates cellular senescence, and enhances eNOS expression in aged mice. These improvements suggest a multifaceted beneficial effect of NIR-PBMT on ARED.
Previous studies have shown that aging is a significant risk factor for ED, and conventional treatments such as PDE5is and testosterone supplementation have limited efficacy in elderly patients. The improvements in erectile function following NIR-PBMT may be attributed to the therapy’s ability to enhance NO signaling, reduce cellular senescence, and improve endothelial functions. Our data suggest that NIR-PBMT increases NO bioavailability and eNOS expression, which are critical for maintaining erectile function. Additionally, the restoration of smooth muscle content and the reduction of collagen deposition in the penile corpus cavernosum further support the therapeutic effects of NIR-PBMT on ARED.
Furthermore, the curtailment of cellular senescence markers, evidenced by the reduced expression of p21 and γ-H2A.X proteins, suggests molecular regeneration of corpus cavernosum tissue following NIR-PBMT. This is important since cellular senescence has been increasingly considered a therapeutic bullseye in age-related conditions.
In this study, bioinformatics analysis indicates that ARED is significantly correlated with cellular senescence, cell adhesion, angiogenesis, mitochondrial function, and the PI3K-Akt signaling pathway. Moreover, in vitro validation with primary HCCECs emphasizes the role of cellular senescence and mitochondrial dysfunction in the pathogenesis of ARED. We demonstrated that NIR-PBMT effectively combats the aging in HCCECs induced by oxidative stress and enhances the bioavailability of NO in these cells. These in vitro studies align with research highlighting the potential of photobiomodulation in strengthening NO signaling and improving endothelial function. The restoration of mitochondrial network structure and function in aged HCCECs post-NIR-PBMT suggests that this therapy may target various cellular pathways related to aging and endothelial dysfunction. In endothelial function experiments, NIR-PBMT revived the migratory and angiogenic abilities of senescent HCCECs. Although inhibiting the PI3K/Akt/eNOS pathway by MK-2206 2HCl significantly diminished the effectiveness of NIR-PBMT, a partial effect persisted, indicating that besides the PI3K/Akt/eNOS pathway, other pathways may also be involved in the molecular mechanisms of NIR-PBMT for treating ARED. This result suggests a more intricate mechanism of action than previously comprehended for NIR-PBMT, such as regulating mitochondrial function and dynamics independently of the PI3K/Akt/eNOS pathway. Our findings echo other studies on light-based therapies for ED, especially ED induced by diabetes mellitus and CNI.
Our research highlights the molecular renewal within the penile corpus cavernosum following NIR-PBMT, echoing the finding of physiological enhancements as reported by Yang et al. in a diabetic rat model. This congruence is of note, given the high prevalence of diabetes in ARED, and our study broadens the therapeutic horizon of NIR-PBMT to encompass the aging population.
Anita et al. used photobiomodulation in a CNI model and presented a stark contrast to our study. While their research CNI-induced ED, our study focuses on age-related degeneration. Yet, both studies intersect in their affirmation of light therapy’s therapeutic efficacy, suggesting a universal mechanism in ED amelioration, irrespective of the underlying etiology.
Our findings on the upregulation of NOS post-NIR-PBMT are similar to the synergistic effect of NORD-1 treatment in combination with red-light irradiation reported by Hotta et al. which presents a complementary mechanism to our observed molecular enhancements, opening avenues for a multi-prolonged therapeutic strategy.
Our study distinguishes itself by providing a detailed molecular analysis, which contrasts with previous studies that focus mainly on physiological outcomes. We have meticulously measured the expression levels of key markers associated with erectile function, oxidative stress, and cellular senescence. This approach allows us to elucidate the complex molecular changes induced by NIR-PBMT, providing a nuanced understanding of its therapeutic effects. By examining these specific markers, we bridge the molecular and physiological effects and offer a clearer picture of the underlying mechanisms at play.
We acknowledged our study’s limitations. The molecular mechanisms through which NIR-PBMT modulates the expression of target proteins are yet to be fully explained. Future studies incorporating transcriptomic and proteomic analyses may yield further insights into the relevant molecular pathways. Additionally, as with other preclinical studies, the generalizability of our findings to clinical settings is provisional and requires proper validation through human trials. The transition from bench to bedside is contingent upon translational research that affirms the efficacy and safety of NIR-PBMT in treating ARED. What’s more, we lack an analysis of cavernous vessels in the model animals, which may reflect the injection and excretion of nutrients and metabolites. In addition, we did not evaluate the expression of elastin, collagen, and pan-actin, which may contribute to the assessment of cavernosum fibrosis.
In conclusion, our study forges ahead in the molecular narrative of NIR-PBMT as a therapeutic intervention for ARED. The molecular restorations, in tandem with the physiological improvements observed by others, collectively press for an intensified exploration and application of light-based therapies in ED.
AUTHOR CONTRIBUTIONS
ZJZ conducted animal experiments, performed mechanistic investigations, and drafted the manuscript. YC participated in animal experiments and contributed to manuscript drafting. QXC and WHT executed bioinformatics analysis and statistical modeling. EC and KH collaborated on manuscript writing and critical revision. SDZ and HCL conceptualized the study, oversaw its design and coordination, and assisted in manuscript revision. All authors read and approved the final manuscript.
COMPETING INTERESTS
All authors declare no competing interests.
ACKNOWLEDGMENTS
This research is financially supported by the National Science Foundation of China (No. 82371633 and No. 81601272), Beijing Municipal Natural Science Foundation (No. 7212134), the Fundamental Research Funds for the Central Universities: Peking University Clinical Scientist Program (BMU2023PYJ H012), and the Clinical Cohort Construction Project C of Peking University Third Hospital (BYSYDL2024011).
Supplementary Information is linked to the online version of the paper on the Asian Journal of Andrology website.
REFERENCES
1.
Yafi FA, Jenkins L, Albersen M, Corona G, Isidori AM, et al. Erectile dysfunction. Nat Rev Dis Primers 2016; 2: 16003.2.
Selvin E, Wang D, Tang O, Fang M, Christenson RH, et al. Elevated cardiac biomarkers, erectile dysfunction, and mortality in U. S. men: NHANES 2001 to 2004. JACC Adv 2023; 2: 100380.3.
Gandaglia G, Briganti A, Jackson G, Kloner RA, Montorsi F, et al. A systematic review of the association between erectile dysfunction and cardiovascular disease. Eur Urol 2014; 65: 968–78.4.
Assar ME, Angulo J, García-Rojo E, Sevilleja-Ortiz A, García-Gómez B, et al. Early manifestation of aging-related vascular dysfunction in human penile vasculature-a potential explanation for the role of erectile dysfunction as a harbinger of systemic vascular disease. Geroscience 2022; 44: 485–501.5.
Echeverri Tirado LC, Ferrer JE, Herrera AM Aging and erectile dysfunction. Sex Med Rev 2016; 4: 63–73.6.
Ferrini MG, Gonzalez-Cadavid NF, Rajfer J Aging related erectile dysfunction-potential mechanism to halt or delay its onset. Transl Androl Urol 2017; 6: 20–7.7.
Ajayi AF, Onaolapo MC, Omole AI, Adeyemi WJ, Oluwole DT Mechanism associated with changes in male reproductive functions during ageing process. Exp Gerontol 2023; 179: 112232.8.
Lee H, Hwang EC, Oh CK, Lee S, Yu HS, et al. Testosterone replacement in men with sexual dysfunction. Cochrane Database Syst Rev 2024; 1: CD013071.9.
Traish AM, Goldstein I, Kim NN Testosterone and erectile function: from basic research to a new clinical paradigm for managing men with androgen insufficiency and erectile dysfunction. Eur Urol 2007; 52: 54–70.10.
Pai R, Ory J, Delgado C, Ramasamy R Energy-based therapies for erectile dysfunction: current and future directions. Urol Clin North Am 2021; 48: 603–10.11.
Schönhofen J, Räber L, Knöchel J, Keo HH, Regli C, et al. Endovascular therapy for arteriogenic erectile dysfunction with a novel sirolimus-eluting stent. J Sex Med 2021; 18: 315–26.12.
Saffati G, Naeem T, Guhan M, Abello A, Hinojosa-Gonzalez DE, et al. Hyperbaric oxygen therapy as a treatment for erectile dysfunction: a meta-analysis. Sex Med Rev 2023; 12: 94–9.13.
Dompe C, Moncrieff L, Matys J, Grzech-Leśniak K, Kocherova I, et al. Photobiomodulation-underlying mechanism and clinical applications. J Clin Med 2020; 9: 1724.14.
Cardoso FD, Gonzalez-Lima F, Gomes da Silva S Photobiomodulation for the aging brain. Ageing Res Rev 2021; 70: 101415.15.
DE Oliveira MF, Johnson DS, Demchak T, Tomazoni SS, Leal-Junior EC Low-intensity LASER and LED (photobiomodulation therapy) for pain control of the most common musculoskeletal conditions. Eur J Phys Rehabil Med 2022; 58: 282–9.16.
Hafner D, Hrast P, Tomaževič T, Jazbec J, Kavčič M Photobiomodulation for chemotherapy-induced oral mucositis in pediatric patients. Biomolecules 2023; 13: 418.17.
Muste JC, Kalur A, Iyer A, Valentim CC, Singh RP Photobiomodulation therapy in age-related macular degeneration. Curr Opin Ophthalmol 2021; 32: 225–32.18.
Kashiwagi S, Morita A, Yokomizo S, Ogawa E, Komai E, et al. Photobiomodulation and nitric oxide signaling. Nitric Oxide 2023; 130: 58–68.19.
Yang L, Liu G, Jiang D, Lin G, Ren Z, et al. Effect of near-infrared laser treatment on improving erectile function in rats with diabetes mellitus. Andrology 2023; 11: 1472–83.20.
Hotta Y, Oyama K, Yoshida T, Ieda N, Mori T, et al. The effects of a red-light controllable nitric oxide donor, NORD-1, on erectile dysfunction in rats with streptozotocin induced diabetes mellitus. World J Mens Health 2025; 43: 197–204.21.
Anita L, Choi MJ, Yin GN, Ock J, Kwon MH, et al. Photobiomodulation as a potential therapy for erectile function: a preclinical study in a cavernous nerve injury model. World J Mens Health 2024; 42: 842–54.22.
Bae SG, Yin GN, Ock J, Suh JK, Ryu JK, et al. Single-cell transcriptome analysis of cavernous tissues reveals the key roles of pericytes in diabetic erectile dysfunction. Elife 2024; 12: RP88942.23.
Schindelin J, Arganda-Carreras I, Frise E, Kaynig V, Longair M, et al. Fiji: an open-source platform for biological-image analysis. Nat Methods 2012; 9: 676–82.24.
Liu Q, Song Y, Cui Y, Hu C, Luo Y, et al. Heterogeneity of fibroblasts is a hallmark of age-associated erectile dysfunction. Int J Biochem Cell Biol 2023; 156: 106343.25.
Zhao L, Han S, Su H, Li J, Zhi E, et al. Single-cell transcriptome atlas of the human corpus cavernosum. Nat Commun 2022; 13: 4302.26.
Johnson JM, Bivalacqua TJ, Lagoda GA, Burnett AL, Musicki B eNOS-uncoupling in age-related erectile dysfunction. Int J Impot Res 2011; 23: 43–8.27.
Sun T, Liu Y, Chen Y, Xu W, Wang T, et al. Study on the mechanism of aging-related erectile dysfunction based on bioinformatics and experimental verification. Transl Androl Urol 2023; 12: 197–208.28.
de Almeida Rezende MS, Oliveira de Almeida AJ, Gonçalves TA, de Azevedo F de LA, Dantas SH, et al. D-(+)-Galactose-induced aging: a novel experimental model of erectile dysfunction. PLoS One 2021; 16: e0249487.29.
Suryadevara V, Hudgins AD, Rajesh A, Pappalardo A, Karpova A, et al. SenNet recommendations for detecting senescent cells in different tissues. Nat Rev Mol Cell Biol 2024; 25: 1001–23.
