Introduction
Prescription opioid analgesics were commonly implicated in overdose deaths in the first 2 decades of the 2000s in the United States (abbreviations listed in Table S1), reinvigorating pharmaceutical engineering innovations intended to reduce crushing (and injection or snorting) of tablets.- Extending back at least to the 1980s, real-world evaluations of so-called abuse-deterrent formulations (ADFs) have consistently shown mixed and limited success in reducing drug-related harms,- in part because patients prescribed the medications are somewhat distinct from those who obtain them from outside the medical system. However, starting around 2010, multiple opioid analgesic formulations were brought to market with tamper-deterring technology, including physical properties that made physical manipulation difficult or led to the release of an opioid antagonist if crushed. In 2015 and 2017, the US Food and Drug Administration (FDA) provided a Guidance to Industry on the evaluation of these products, including a pathway for label claims.,
While premarket evaluation of new putative ADFs is well defined by these guidances, real-world evaluation has proven challenging. Surveillance and localized studies of illicit drug-using populations- have generally found meaningfully lower nonmedical use of prescription opioids with ADF properties, most of which focused on the reformulation of extended-release oxycodone (OxyContin; Purdue Pharma, LP) in the United States. A notable exception was the multimodal suite of studies conducted in Australia.- However, fewer studies have examined the impact of ADF opioids on intended patient populations with painful conditions.,-
While measuring safety of ADFs in controlled trials with clear endpoints is relatively straightforward, estimating the impact of ADFs in postmarketing epidemiologic studies is more complex. As highlighted by Turk et al., crucial design considerations must be accounted for in pharmacoepidemiologic studies, particularly those that utilize insurance claims data. These studies may be subject to multiple sources of bias due to selection criteria, choice of comparators, and potential confounding by “indication.”- In 2017, the FDA convened a workshop to discuss these methodological issues. Most studies to date, often industry-sponsored, have focused on 1 ADF or had limited methods to address methodological difficulties.
In this study, we examined the relationship between ADFs and opioid-related harm (eg, opioid use disorder, opioid overdose) using rigorous epidemiologic methods to address issues of confounding by “indication” and choice of comparators that often arise in opioid research. We implemented a study design including patients with variable treatment histories,, comparing patients initiating ADFs to patients initiating, restarting, or continuing non-ADF extended-release/long-acting (ER/LA) opioids to understand the relationship between any ADF use and the risk of opioid-related harm.
Methods
Data sources
Data for this analysis were from a large private health insurance provider in North Carolina (NC), United States. The data source included pharmaceutical, inpatient, and outpatient claims and membership files for individuals with (1) a pain diagnosis using the International Classification of Diseases, Ninth Revision, Clinical Modification (ICD-9-CM) and International Classification of Diseases, Tenth Revision, Clinical Modification (ICD-10-CM); (2) surgical procedure, defined using Current Procedural Terminology (CPT) codes; or (3) outpatient opioid analgesic prescription claim, identified using National Drug Codes, from January 1, 2006, through September 30, 2018. Claims data were linked to death records from the NC Department of Health and Human Services using a hierarchical matching algorithm (Figure S1).
This analysis was part of a larger study approved by the Institutional Review Board, Office of Human Research Ethics, University of North Carolina at Chapel Hill.
Cohort selection and study design
Adult (18-64 years) patients were eligible for inclusion if they initiated ER/LA opioid analgesics (ADF or non-ADF). Patients with a documented history of opioid overdose or opioid use disorder (OUD) based on ICD-9-CM or ICD-10-CM diagnostic codes in insurance claims (Table S2) were excluded using all-available lookback. Lookback periods could extend before the introduction of ADF ER/LAs (hereafter referred to as “ADF”) in August 2010; however, to improve comparability between groups, we limited the analytic observation period to August 1, 2010, through September 30, 2018.
We implemented a study design similar to the prevalent new-user design, first proposed by Suissa et al for examining a new drug when a standard treatment already exists, where many individuals initiating this new treatment likely have a history of exposure to the comparator treatment, as is the case with individuals initiating ADFs. This design accounts for the fact that individuals might follow different treatment trajectories when initiating the drug of interest: (1) directly switch from the comparator (ie, non-ADF ER/LA) to the drug of interest (ie, ADF), (2) switch from the comparator to the drug of interest after a treatment gap (ie, delayed switch), or (3) initiate the drug of interest without prior exposure to the comparator (ie, new initiator).
This design allows us to examine our exposure of interest (ADF use) in relation to our chosen comparator (non-ADF ER/LA) through the lens of a hypothetical trial examining the following research question: “What if patients who initiated ADFs had instead initiated, continued, or re-initiated non-ADF ER/LAs at the time of ADF initiation?” To examine this question, we created 2 subcohorts: ADF and non-ADF ER/LA (comparator). For the ADF subcohort, we identified individuals’ first ADF outpatient pharmaceutical claim after August 1, 2010, that was not co-filled with a non-ADF ER/LA prescription on the same day (Figure S2). For the non-ADF ER/LA subcohort, we identified the first non-ADF ER/LA claim after August 1, 2010, for individuals who did not have a prior ADF after August 1, 2010. The ADF and non-ADF ER/LA subcohorts were not mutually exclusive; a patient could be exposed to non-ADF ER/LAs before ADF initiation but not vice versa. Individuals with claims for both an ADF and non-ADF ER/LA on the index date were excluded.
The ADF and comparator subcohorts included both traditional new users, of opioid analgesics and individuals with a history of prescription opioid use. To be considered a traditional new user, patients had to have ≥ 6 months of continuous enrollment that included a ≥ 6-month washout period with no evidence of immediate-release (IR) or ER/LA opioid claims before ADF or non-ADF ER/LA initiation (Figure S2). If a patient had evidence of opioid use before ADF or non-ADF ER/LA initiation, they had to meet the 6-month continuous enrollment and washout period requirement before initiating IR or non-ADF ER/LA opioids (ADF subcohort) or IR opioids (non-ADF ER/LA subcohort). We limited analyses to the first ADF or non-ADF ER/LA initiation after July 31, 2010, that met these criteria.
Outcomes
Outcomes of interest were (1) first of: fatal opioid overdose (identified using ICD-10 codes for underlying and contributing cause of death in linked death records, Table S3), or nonfatal opioid overdose; (2) diagnosed OUD; and (3) a combined outcome of first of: opioid overdose (fatal or nonfatal) or diagnosed OUD. We treated death from causes other than opioid overdose as a competing risk.,
Follow-up and comparator selection
We defined the index date (baseline) as the date of ADF initiation for patients initiating ADF opioids and the date of the prescription selected as a non-ADF ER/LA comparator (Figures S3-S4).
In defining risk sets and selecting comparators, we included all new ADF prescriptions (ie, all ADF patients) and, for everyone in the ADF cohort, randomly selected 1 non-ADF ER/LA history-based comparator. To account for opioid exposure history and minimize potential selection bias from exclusion criteria in non-ADF ER/LA users with prior opioid exposure (by erroneously excluding incident opioid overdose or OUD outcomes as history events, described in detail by Suissa et al), we arranged ADF index dates by calendar time and then selected 1 non-ADF ER/LA prescription (comparator) with equivalent (1) months since first non-ADF ER/LA (categorized as 0, 1-3, 4-6, 7-9, 10-12, 13-17, 18+ months and matched within ±2 months if category spanned > 4 months; Figure S3), (2) cumulative months of non-ADF ER/LA exposure (±2 months), and (3) initiator type. Initiator type was categorized as new initiator, direct switch (ADF) vs continuation (non-ADF ER/LA), or delayed (> 7-day gap) switch (ADF) vs reinitiation (non-ADF ER/LA), accounting for IR history of no IR use (ie, traditional new user) or prevalent IR use with or without a treatment gap (Figure S4). If a selected control had a history of opioid overdose or OUD before the selected prescription, that individual was excluded from further control selection, and another control was selected in place of the excluded individual. This process was repeated until there was 1 comparator non-ADF ER/LA prescription event for each individual in the ADF subcohort. Comparators were selected without replacement except for stratifications where a small sample size required selection with replacement.
In primary analyses, we used an approach similar to per protocol, where we followed patients from baseline until the first of (1) outcome of interest, (2) death (competing risk), (3) > 30 days without evidence of the treatment of interest (censoring event), (4) treatment switch (censoring event), (5) disenrollment (censoring event), (6) 180 days after the index date (censoring event), or (7) administrative censoring on September 30, 2018.
Patient characteristics
We identified patient characteristics relative to baseline, as defined above. Baseline patient characteristics included demographics (age, sex). We identified comorbid conditions, specifically cancer, any substance use disorder, depression, obesity, and respiratory/metabolic conditions, using Elixhauser Index code groupings with a 6-month lookback. We used a derived clinical indication of acute pain, chronic pain, or surgery, identified using ICD-9-CM and ICD-10-CM codes for pain diagnoses and CPT codes for invasive surgery, with a 30-day lookback period to associate the derived clinical indication with the index prescription. Other prescriptions included benzodiazepines and selective serotonin reuptake inhibitors within 6 months.
In contrast to previous studies, we also identified whether the ER/LA opioid prescription was concurrently prescribed with an IR opioid, classifying episodes where the index prescription overlapped with an IR for ≥ 7 days (or the duration of the opioid prescription of interest if the ADF/non-ADF ER/LA episode was < 7 days) as concurrent. We also identified cumulative morphine milligram equivalent exposure from the first opioid prescription to the index ADF or non-ADF ER/LA prescription of interest (Appendix S1).
Statistical analysis
We used stabilized inverse probability of treatment weighting (IPTW) to account for measured confounding of the relationship between ADF use and opioid overdose or OUD. The propensity score was estimated using logistic regression to model the likelihood of treatment given measured confounders (defined in Patient characteristics, above), stratified by time since initiating non-ADF ER/LAs (in months, categorized as above) and initiator type. Confounders were identified a priori using a directed acyclic graph (Figure S5). We examined balance between treatment groups using standardized mean differences. To account for informative censoring, we used time-varying stabilized inverse probability of censoring weights (IPCW). We multiplied IPTW by IPCW to get IPTCW, truncating weights at 0.1 and 10 to minimize the influence of extreme weights.
We examined the relationship between ADFs and outcomes of interest. First, we used the cumulative incidence function accounting for competing risks to estimate unweighted and weighted cumulative incidence. We used Fine-Gray models to estimate unweighted and weighted hazard ratios (HRws) with the Efron approximation for tied event times. We used Schoenfeld’s residuals to test the proportional hazards assumption and considered stratifications by time if violated. We calculated 95% confidence intervals (CIs) using robust standard errors to account for clustered observations.
Sensitivity analyses
We conducted several sensitivity analyses to evaluate the impact of analytic decisions. First, we used an approach similar to intent to treat (ITT),, where patients were followed until the first of (1) outcome, (2) death (competing risk), (3) disenrollment (censoring event), (4) 180 days after the index date (censoring event), or (5) administrative censoring.
Next, we examined the relationship between ADFs and the combined outcome of opioid overdose or incident OUD diagnosis among the subset of individuals identified as new initiators of ADFs or non-ADF ER/LAs without prior IR opioid exposure (ie, traditional new users of opioids; Figure S4). By restricting the patient population to new initiators only, this sensitivity analysis is an example of using strict inclusion criteria to examine a single treatment strategy, comparing initiation of ADFs to initiation of non-ADF ER/LAs among individuals without a history of opioid exposure.
Finally, given that OUD is notoriously difficult to accurately measure in claims data, we conducted 2 sensitivity analyses to examine the impact of OUD misclassification. First, we excluded OUD outcomes occurring in the first 30 days of follow-up, assuming that these may not have been incident OUD outcomes. Second, we included medications for OUD (MOUD) in our definition of OUD (Table S4), excluding individuals with a history of OUD using this new definition and including additional OUD events after index based on diagnosed OUD or initiation of MOUD.
Data management was completed in SAS 9.4 (SAS Institute), and analyses were conducted in R 3.6.0 (packages listed in Table S5).
Results
Patient characteristics
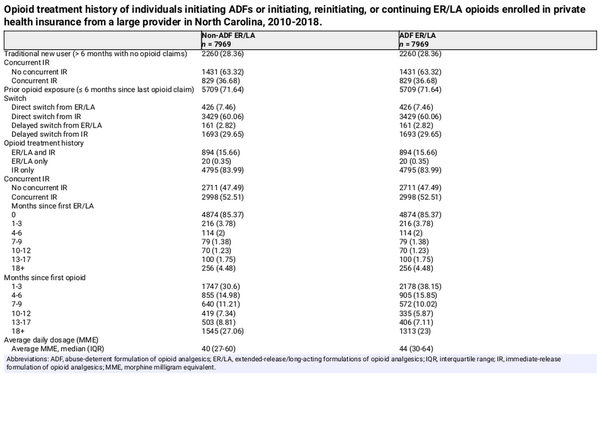
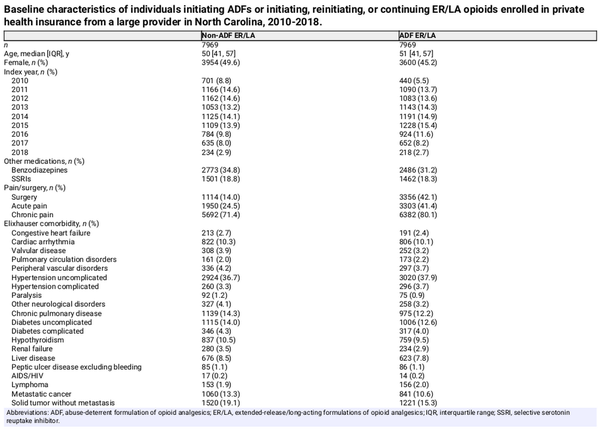
We identified 7969 patients initiating ADFs, of whom 2260 (28.4%) were classified as traditional new users, and 5709 (71.6%) were classified as patients with prior opioid exposure (Table 1). For the group of patients to serve as comparators, we identified 8888 patients initiating non-ADF ER/LAs contributing 48 797 observations used for the selection of 7969 comparator observations to match exposure histories of ADF patients (defined in Comparator selection, above).
The median age at baseline was similar between the ADF (51 years; interquartile range [IQR], 41-57) and non-ADF ER/LA groups (50 years; IQR, 41-57) (Table 2). ADF initiators were less likely to be female (45.2% vs 49.6%) and were less likely to have received benzodiazepines (31.2% vs 34.8%) within 6 months before baseline. However, patients initiating an ADF were more likely to have had recent invasive surgery (42.1% vs 14.0%), an acute pain diagnosis (41.4% vs 24.5%), or a chronic pain diagnosis (80.1% vs 71.4%). Patients initiating an ADF were also less likely to have several baseline comorbidities, including depression (19.8% vs 22.1%), metastatic cancer (10.6% vs 13.3%), or solid tumor without metastasis (15.3% vs 19.1%) but were more likely to have obesity (14.4% vs 12.1%). Covariates were well balanced after implementing IPTW (Figure S6).
Follow-up and events
In our primary analysis, patients were followed for a median of 60 days (IQR, 40-178 days; Table S6), with non-ADF ER/LA patients contributing more follow-up (median 74 days; IQR, 52-182) than ADF initiators (median 50 days; IQR, 39-115 days) due to a higher proportion of ADF patients being censored for treatment switching and/or > 30 days without ADFs (per-protocol censoring). Across all observations, there were 18 fatal or nonfatal opioid overdoses (ADF: n = 8, non-ADF ER/LA: n = 10) and 235 incident OUD diagnoses (ADF: n = 91, non-ADF ER/LA: n = 144). A competing risk of death occurred for 4% of observations in each outcome analysis.
Model results
Cumulative incidence of opioid overdose was consistently higher among patients who initiated, restarted, or continued non-ADF ER/LAs through the first 3 months of follow-up compared to patients who initiated ADFs in both crude (Figure S7a) and IPTCW (Figure 1A) analyses, although with considerable confidence interval overlap. Similarly, cumulative incidence of OUD was consistently higher through the first 3 months of follow-up among patients who initiated, restarted, or continued non-ADF ER/LAs compared to patients who initiated ADFs in both crude (Figure S7b) and IPTCW (Figure 1B) analyses, with the curves converging later in follow-up. Interestingly, the separation between the cumulative incidence curves is much greater in the first 6 weeks of follow-up compared to 7 weeks and later, particularly after weighting to account for confounding and informative censoring.
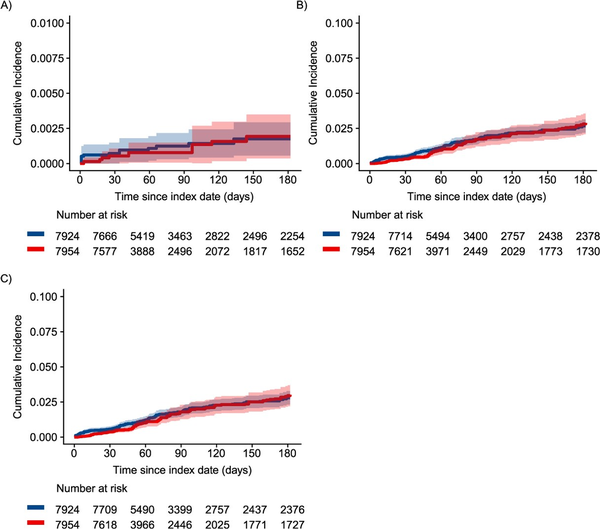
Figure 1
Inverse probability-weighted cumulative incidence of (A) nonfatal or fatal opioid overdose, (B) OUD, and (C) combined outcome of OUD or opioid overdose. Among individuals initiating ADF ER/LAs (red) compared to those initiating, reinitiating, or continuing non-ADF ER/LA (blue) opioids in North Carolina, 2010-2018, using an approach similar to per-protocol. ADF, abuse-deterrent formulation of opioid analgesics; ER/LA, extended-release/long-acting formulations of opioid analgesics; OUD, opioid use disorder.
In Fine-Gray models of opioid overdose outcomes (Figure 2, Table S7), the weighted hazard of nonfatal or fatal opioid overdose among patients initiating ADFs was 0.87 (95% CI, 0.23-3.24) times as high as the hazard among patients who initiated, restarted, or continued non-ADF ER/LAs, respectively. Tests indicated that the proportional hazards assumption for OUD outcome models was not upheld, suggesting the need to report separate estimates for the first 6 weeks of follow-up vs the remainder of follow-up. Among patients initiating ADFs, the weighted hazard of incident OUD was 0.58 (95% CI, 0.35-0.93) times as high as among patients who initiated, restarted, or continued non-ADF ER/LAs in the first 6 weeks of follow-up and 1.30 (95% CI, 0.86-1.95) times as high through the rest of follow-up (Table S8). We observed a similar relationship for the combined outcome of OUD or opioid overdose.
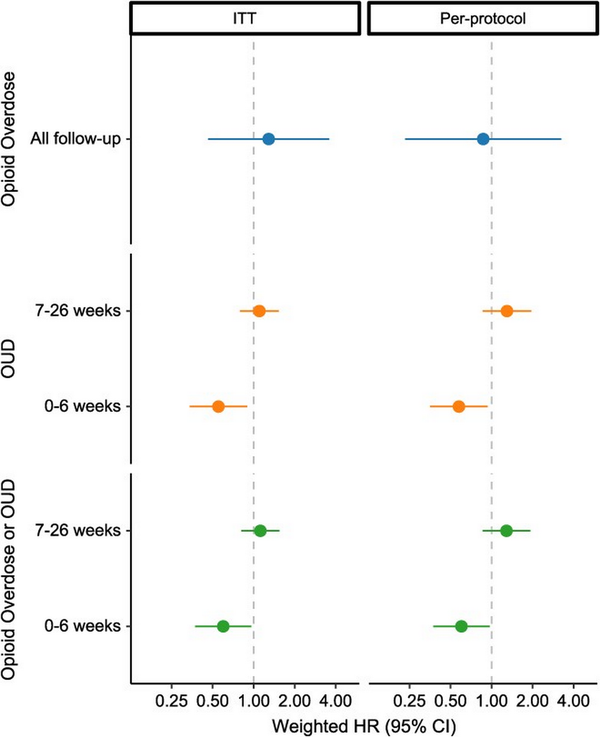
Figure 2
Inverse probability-weighted hazard ratios for nonfatal or fatal opioid overdose (blue), opioid use disorder (orange), and combined outcome of opioid use disorder or opioid overdose (green). Comparing individuals initiating ADF ER/LAs to those initiating, reinitiating, or continuing non-ADF ER/LA opioids in North Carolina, 2010-2018, using ITT (left) and per-protocol (right) approaches. ADF, abuse-deterrent formulation of opioid analgesics; ER/LA, extended-release/long-acting formulations of opioid analgesics; HR, hazard ratio; ITT, intent to treat; OUD, opioid use disorder.
Sensitivity analyses
In the first sensitivity analysis using an ITT approach, patients were followed for a median of 6 months, and there were 29 fatal or nonfatal opioid overdoses and 347 incident OUD diagnoses (Table S9). In this ITT analysis, we again found that the cumulative incidence of opioid overdose was consistently higher among patients who initiated, restarted, or continued non-ADF ER/LAs compared to patients who initiated ADFs through the first 3 months of follow-up (although with considerable CI overlap), with curves converging later in follow-up (Figure 3A, Figure S8). We also observed a similar relationship for OUD outcomes between the per-protocol and ITT analyses, with the greatest separation between cumulative incidence curves occurring early in follow-up (Figure 3B, C). In regression models, the weighted hazard of opioid overdose among patients initiating ADFs was 1.29 (95% CI, 0.46-3.58) times as high as among patients who initiated, restarted, or continued non-ADF ER/LAs (Figure 2, Table S7). Further, the weighted hazard of incident OUD was 0.55 (95% CI, 0.34-0.90) times as high as among patients who initiated, restarted, or continued non-ADF ER/LAs in the first 6 weeks of follow-up and 1.10 (95% CI, 0.79-1.53) times as high through the remainder of follow-up (Figure 2, Table S8).
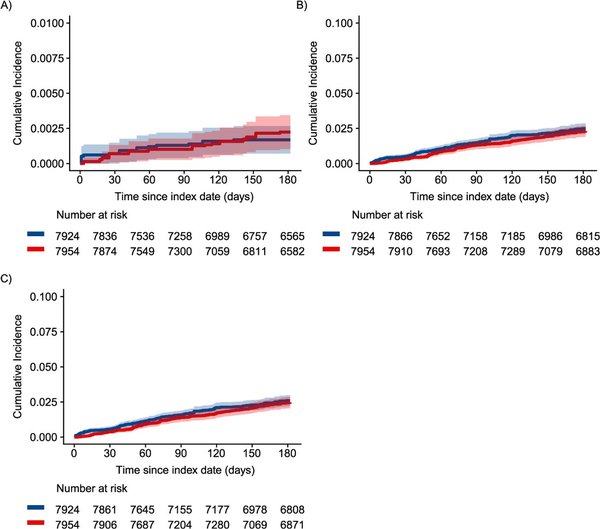
Figure 3
Inverse probability-weighted cumulative incidence of (A) nonfatal or fatal opioid overdose, (B) OUD, and (C) combined outcome of OUD or opioid overdose. Among individuals initiating ADF ER/LAs (red) compared to those initiating, reinitiating, or continuing non-ADF ER/LA (blue) opioids in North Carolina, 2010-2018, using an approach similar to intent to treat. ADF, abuse-deterrent formulation of opioid analgesics; ER/LA, extended-release/long-acting formulations of opioid analgesics; OUD, opioid use disorder.
Second, we restricted our analysis to patients who met the criteria of traditional new users (n = 4520) and examined the combined outcome of opioid overdose or OUD. In this subcohort, there were 39 and 45 events within 6 months of follow-up using per-protocol and ITT approaches, respectively. We found that ADFs were associated with a lower risk of opioid overdose or OUD through 6 months of follow-up using both per-protocol (HRw = 0.37; 95% CI, 0.14-1.01; Figures S9-S10) and ITT approaches (HRw = 0.44; 95% CI, 0.19-1.01; Figures S11-S12).
Third, there were 62 OUD events (ADF subcohort: n = 28) occurring in the first 30 days of follow-up and 58 OUD events (ADF subcohort: n = 27) occurring in the first 30 days when considering the combined outcome of OUD and opioid overdose, which were excluded from the analytic cohort for this sensitivity analysis. In this restricted subcohort, there were 173 incident OUD diagnoses (Tables S10-S11).
In the final sensitivity analysis including MOUD in the definition of OUD, there were 23 individuals who had evidence of MOUD (ADF: n = 6, non-ADF ER/LA: n = 17) before cohort entry and were thus excluded from the analytic cohort for this sensitivity analysis. In this subcohort restricted to individuals without evidence of MOUD before cohort entry, there were 237 incident OUD diagnoses or initiation of MOUD (Tables S10-S11). Results from these sensitivity analyses were consistent with results from primary analyses (Figures S13-S15).
Discussion
In this large cohort of patients exposed to ER/LA opioids from 2010 through 2018 in NC, we examined the incidence of fatal or nonfatal opioid overdoses and diagnosed OUD, comparing patients who initiated ADFs to patients initiating, restarting, or continuing non-ADF ER/LA opioids. While the cumulative incidence of opioid overdose was lower in the ADF subcohort through the first 3 months of follow-up, there was considerable confidence interval overlap. Further, there was no notable relationship between ADF use and opioid overdose through 6 months of follow-up in regression models. We additionally found that ADF use was associated with a lower risk of OUD early in follow-up. Interestingly, while the hazard of OUD was significantly lower for patients prescribed ADFs compared to patients prescribed non-ADF ER/LAs for the first 6 weeks of follow-up, the direction of this relationship changed later in follow-up, with the hazard of OUD higher 7 weeks through 6 months among patients exposed to ADFs compared to non-ADF ER/LAs.
Several studies have sought to address whether the introduction of ADFs, which tend to be more expensive than non-ADF ER/LA opioids, was effective in reducing opioid-related harms using varied study designs, but this growing body of evidence has yielded mixed results.,,,,- Our findings are consistent with 1 large study on OxyContin reformulation commissioned by the manufacturer using Medicaid and commercial claims data. That study found no meaningful difference in overdose rates in general but noted somewhat lower overdose among patients not taking other opioid medications concurrently. However, 78% of patients receiving ER/LA opioids are also prescribed IR opioids, limiting interpretation. The other large study on this topic, which used electronic health records, found increased overdose and OUD diagnoses in the ADF oxycodone cohort but only used IR oxycodone as the comparison group. A study of an ADF morphine product in Medicaid found higher overdose and OUD diagnoses in the non-ADF ER/LA group but had no adjustment for confounding. Given the complexity of evaluating ADFs in a postmarketing setting, there have been multiple calls to critically evaluate and improve upon the methods used to conduct postmarketing surveillance studies of ADFs.,,,
When designing observational postmarketing studies, key considerations include the choice of study design, appropriate comparators, accounting for confounding by “indication,” and selection bias.,,, Many of these studies examined only a single ADF formulation instead of all ADFs as a class, thereby limiting generalizability. These choices influence the reliability of conclusions that can be drawn from observational studies. Our goal in this study was to implement a methodologically rigorous approach to estimate the relationship between ADFs and opioid-related harms in a population of patients we believed to be representative of the general clinical population of patients who are prescribed ADF opioids while accounting for confounding by “indication” through comparator selection and propensity score–based methods. In primary analyses, our finding of no clear relationship between ADF use and opioid overdose and a short-term reduction in risk of OUD compared to non-ADF ER/LAs was supported by multiple sensitivity analyses and is consistent with much of the growing evidence base.
Our primary analysis combined patient populations with heterogeneous treatment histories, including those who had no history of exposure to opioid analgesics, patients who had prior IR opioid exposure but no non-ADF ER/LA exposure, and patients with prior non-ADF ER/LA use. This approach allows us to examine the relationship between ADF exposure and opioid-related harms in varying treatment strategies that attempt to more closely capture real-world prescribing trajectories: (1) directly switch from the comparator (ie, non-ADF ER/LA) to the drug of interest (ie, ADF), (2) switch from the comparator to the drug of interest after a treatment gap (ie, delayed switch), or (3) initiate the drug of interest without prior exposure to the comparator (ie, new initiator). However, combining these different patient populations adds analytic complexity due to different anticipated confounding structures given heterogeneous treatment histories. We attempt to address this complexity in our weighting strategy by modeling the probability of initiating ADFs stratified by treatment history. Our sensitivity analysis restricting the population to patients without prior opioid exposure is an example of an analysis that utilizes a narrow, explicit set of inclusion criteria to examine a single treatment comparison: initiation of ADFs vs non-ADF ER/LAs among patients without prior opioid exposure. This sensitivity analysis yielded similar results to our primary analysis. Further, in our main analyses, we present average effect estimates of ADF initiation on opioid-related harm. Effect measure modification by treatment history is possible, and future studies that explicitly examine the question of modification of the relationship between ADF initiation and opioid-related harms by treatment history would be valuable contributions to the evidence base.
There are several additional limitations to consider when interpreting the findings from this study. First, opioid overdose and OUD are notoriously difficult to measure in claims data., Linking death records allowed us to examine fatal opioid overdoses in our study population. Additionally, we conducted 2 sensitivity analyses of OUD outcomes, incorporating MOUD into our outcome definition and excluding OUD outcomes in the first 30 days in the event that these represented prevalent OUD diagnoses rather than incident events, with results consistent with findings from primary analyses. Second, while enrollment requirements and washout periods can exclude eligible patients and claims data are an imperfect source of historical information about a patient’s treatment history, we chose to impose specific enrollment and washout requirements because it was important to ensure that we could capture a patient’s full treatment history with opioid analgesics (since their most recent washout period) when implementing the study design outlined in this article. We believe that treatment history is an important factor in the relationship between ADF use and opioid-related harm, and by ensuring that we were capturing a more complete treatment history, we sought to minimize the risk of measurement error of prior prescribed opioid analgesic exposure. Third, while we used IPTW to control for measured confounding, there remained the potential for unmeasured confounding. Fourth, many patients disenrolled before the end of follow-up or were censored after 30 days without evidence of an ADF or non-ADF ER/LA prescription in the per-protocol analysis. That extended-release opioids are being used for such short durations has not been previously documented. We used IPTCW to account for informative censoring and included a secondary analysis with an ITT approach to evaluate the impact of the per-protocol assumption on our findings, which produced similar results. Fifth, we caution against drawing firm conclusions about the relationship between ADF use and opioid overdose, given that these estimates had low precision. Finally, our data source included privately insured patients in NC and may not be representative of patients with Medicaid, Medicare, or those without insurance or in other regions of the country.
Even though the current problems with overdose in the United States are more closely linked to illicitly manufactured opioids (eg, street fentanyl), ADFs of novel opioid analgesics, stimulants, and cannabinoids are currently under development by pharmaceutical companies. We humbly suggest that the methods described in this study can provide a starting point for observational studies of real-world impacts of ADFs on the intended patient population.
Conclusions
Our study aimed to answer the growing call to improve the methodological approaches in opioid safety research. In this sample of privately insured patients prescribed ER/LA opioids in NC, we used a methodologically rigorous study design to evaluate the relationship between ADFs and opioid-related harms among patients with variable opioid treatment histories and found no relationship between ADF use and opioid overdose. Interestingly, we found that ADF use was associated with a lower risk of OUD early in follow-up, but this relationship did not hold after 6 weeks, suggesting a possible limited short-term benefit from ADFs. These findings add to the expanding body of evidence that there is not a clear long-term reduction in harm from ADFs. Future work will continue to evaluate the implications of design and analytic choices in opioid safety research.
Acknowledgments
We are grateful to colleagues at the FDA Office of Surveillance and Epidemiology for conceptual discussions that shaped our thinking on this analysis. We also thank research support staff at UNC who helped make the science possible, specifically Maryalice Nocera and LaMonda Sykes.
Supplementary material
Supplementary material is available at the American Journal of Epidemiology online.
References
- 1. Dart RC, Surratt HL, Cicero TJ, et al Trends in opioid analgesic abuse and mortality in the United States. N Engl J Med. 2015;372(3):241–248. 10.1056/NEJMsa1406143
- 2. Katz NP, Adams EH, Chilcoat H, et al Challenges in the development of prescription opioid abuse-deterrent formulations. Clin J Pain. 2007;23(8):648–660. 10.1097/AJP.0b013e318125c5e8
- 3. Gasior M, Bond M, Malamut R. Routes of abuse of prescription opioid analgesics: a review and assessment of the potential impact of abuse-deterrent formulations. Postgrad Med. 2016;128(1):85–96. 10.1080/00325481.2016.1120642
- 4. Mastropietro DJ, Omidian H. Current approaches in tamper-resistant and abuse-deterrent formulations. Drug Dev Ind Pharm. 2013;39(5):611–624. 10.3109/03639045.2012.680468
- 5. Paljarvi T, Strang J, Quinn PD, et al Abuse-deterrent extended-release oxycodone and risk of opioid-related harm. Addiction. 2021;116(9):2409–2415. 10.1111/add.15392
- 6. Baum C, Hsu JP, Nelson RC. The impact of the addition of naloxone on the use and abuse of pentazocine. Public Health Rep. 1987;102(4):426–429.
- 7. Reed DA, Schnoll SH. Abuse of pentazocine-naloxone combination. JAMA. 1986;256(18):2562–2564. 10.1001/jama.1986.03380180124033
- 8. Poklis A. Decline in abuse of pentazocine/tripelennamine (T's and blues) associated with the addition of naloxone to pentazocine tablets. Drug Alcohol Depend. 1984;14(2):135–140. 10.1016/0376-8716(84)90039-5
- 9. Fudala PJ, Johnson RE. Development of opioid formulations with limited diversion and abuse potential. Drug Alcohol Depend. 2006;83(suppl 1):S40–S47. 10.1016/j.drugalcdep.2006.01.016
- 10. Katz N. Abuse-deterrent opioid formulations: are they a pipe dream? Curr Rheumatol Rep. 2008;10(1):11–18. 10.1007/s11926-008-0003-z
- 11. Broz D, Zibbell J, Foote C, et al Multiple injections per injection episode: high-risk injection practice among people who injected pills during the 2015 HIV outbreak in Indiana. Int J Drug Policy. 2018;52:97–101. 10.1016/j.drugpo.2017.12.003
- 12. Hunt R, Yalamanoglu A, Tumlin J, et al A mechanistic investigation of thrombotic microangiopathy associated with IV abuse of Opana ER. Blood. 2017;129(7):896–905. 10.1182/blood-2016-08-736579
- 13. Mateu-Gelabert P, Guarino H. The opioid epidemic and injection drug use: MIPIE and health harms related to the injection of prescription opioids. Int J Drug Policy. 2018;57:130–132. 10.1016/j.drugpo.2018.03.019
- 14. Katz N, Dart RC, Bailey E, et al Tampering with prescription opioids: nature and extent of the problem, health consequences, and solutions. Am J Drug Alcohol Abuse. 2011;37(4):205–217. 10.3109/00952990.2011.569623
- 15. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). Abuse-Deterrent Opioids—Evaluation and Labeling. Guidance for Industry; 2015.
- 16. U.S. Department of Health and Human Services, Food and Drug Administration, Center for Drug Evaluation and Research (CDER). General Principles for Evaluating the Abuse Deterrence of Generic Solid Oral Opioid Drug Products. Guidance for Industry; 2017.
- 17. Severtson SG, Ellis MS, Kurtz SP, et al Sustained reduction of diversion and abuse after introduction of an abuse deterrent formulation of extended release oxycodone. Drug Alcohol Depend. 2016;168:219–229. 10.1016/j.drugalcdep.2016.09.018
- 18. Coplan PM, Chilcoat HD, Butler SF, et al The effect of an abuse-deterrent opioid formulation (OxyContin) on opioid abuse-related outcomes in the postmarketing setting. Clin Pharmacol Ther. 2016;100(3):275–286. 10.1002/cpt.390
- 19. Jewell J, Black J, Ellis M, et al A cross-sectional study of tampering in Xtampza ER, an abuse-deterrent formulation of an extended-release opioid, in a treatment center population. Clin Drug Investig. 2023;43(3):197–203. 10.1007/s40261-023-01248-9
- 20. Cassidy TA, DasMahapatra P, Black RA, et al Changes in prevalence of prescription opioid abuse after introduction of an abuse-deterrent opioid formulation. Pain Med. 2014;15(3):440–451. 10.1111/pme.12295
- 21. Wolff C, Dowd WN, Ali MM, et al The impact of the abuse-deterrent reformulation of extended-release OxyContin on prescription pain reliever misuse and heroin initiation. Addict Behav. 2020;105:106268. 10.1016/j.addbeh.2019.106268
- 22. Alpert A, Powell D, Pacula RL. Supply-side drug policy in the presence of substitutes: evidence from the introduction of abuse-deterrent opioids. Am Econ J Econ Policy. 2018;10(4):1–35. 10.1257/pol.20170082
- 23. Nolan ML, Harocopos A, Allen B, et al Reformulation of oxycodone 80 mg to prevent misuse: a cohort study assessing the impact of a supply-side intervention. Int J Drug Policy. 2020;83:102848. 10.1016/j.drugpo.2020.102848
- 24. DiNardi M. The release of abuse-deterrent OxyContin and adolescent heroin use. Drug Alcohol Depend. 2021;229(pt B):109114. 10.1016/j.drugalcdep.2021.109114
- 25. Rodriguez RD, Dailey Govoni T, Rajagopal V, et al Evaluating the effectiveness of reformulated extended-release oxycodone with abuse-deterrent properties on reducing non-oral abuse among individuals assessed for substance abuse treatment with the Addiction Severity Index-Multimedia Version (ASI-MV). Curr Med Res Opin. 2023;39(4):579–587. 10.1080/03007995.2023.2178080
- 26. Havens JR, Leukefeld CG, DeVeaugh-Geiss AM, et al The impact of a reformulation of extended-release oxycodone designed to deter abuse in a sample of prescription opioid abusers. Drug Alcohol Depend. 2014;139:9–17. 10.1016/j.drugalcdep.2014.02.018
- 27. Hwang CS, Chang HY, Alexander GC. Impact of abuse-deterrent OxyContin on prescription opioid utilization. Pharmacoepidemiol Drug Saf. 2015;24(2):197–204. 10.1002/pds.3723
- 28. Buer LM, Havens JR, Leukefeld C. Does the new formulation of OxyContin(R) deter misuse? A qualitative analysis. Subst Use Misuse. 2014;49(6):770–774. 10.3109/10826084.2013.866963
- 29. Chilcoat HD, Coplan PM, Harikrishnan V, et al Decreased diversion by doctor-shopping for a reformulated extended release oxycodone product (OxyContin). Drug Alcohol Depend. 2016;165:221–228. 10.1016/j.drugalcdep.2016.06.009
- 30. Coplan PM, Kale H, Sandstrom L, et al Changes in oxycodone and heroin exposures in the National Poison Data System after introduction of extended-release oxycodone with abuse-deterrent characteristics. Pharmacoepidemiol Drug Saf. 2013;22(12):1274–1282. 10.1002/pds.3522
- 31. Dasgupta N, Brown JR, Nocera M, et al Abuse-deterrent opioids: a survey of physician beliefs, behaviors, and psychology. Pain Ther. 2022;11(1):133–151. 10.1007/s40122-021-00343-z
- 32. Brown JR, Oh G, Wang Y, et al Variation in abuse-deterrent formulation opioid prescribing in California, Florida, and Kentucky in 2018. J Rural Health. 2021;37(1):23–28. 10.1111/jrh.12496
- 33. Oyler DR, Slavova S, Brown JR, et al Kentucky pharmacists' experiences in dispensing abuse-deterrent opioid analgesics. J Am Pharm Assoc (2003). 2022;62(6):1836–1842. 10.1016/j.japh.2022.07.017
- 34. Degenhardt L, Larance B, Bruno R, et al Evaluating the potential impact of a reformulated version of oxycodone upon tampering, non-adherence and diversion of opioids: the National Opioid Medications Abuse Deterrence (NOMAD) study protocol. Addiction. 2015;110(2):226–237. 10.1111/add.12746
- 35. Larance B, Dobbins T, Peacock A, et al The effect of a potentially tamper-resistant oxycodone formulation on opioid use and harm: main findings of the National Opioid Medications Abuse Deterrence (NOMAD) study. Lancet Psychiatry. 2018;5(2):155–166. 10.1016/S2215-0366(18)30003-8
- 36. Degenhardt L, Bruno R, Ali R, et al The introduction of a potentially abuse deterrent oxycodone formulation: early findings from the Australian National Opioid Medications Abuse Deterrence (NOMAD) study. Drug Alcohol Depend. 2015;151:56–67. 10.1016/j.drugalcdep.2015.02.038
- 37. Campbell G, Bruno R, Lintzeris N, et al Defining problematic pharmaceutical opioid use among people prescribed opioids for chronic noncancer pain: do different measures identify the same patients? Pain. 2016;157(7):1489–1498. 10.1097/j.pain.0000000000000548
- 38. Cicero TJ, Mendoza M, Cattaneo M, et al Real-world misuse, abuse, and dependence of abuse-deterrent versus non-abuse-deterrent extended-release morphine in Medicaid non-cancer patients. Postgrad Med. 2019;131(3):225–229. 10.1080/00325481.2019.1585688
- 39. Taber L, Bond TC, Wang X, et al Real-world utilization of once-daily extended-release abuse deterrent formulation of hydrocodone: a comparison with the pre-approval randomized clinical trials. J Pain Res. 2017;10:1741–1746. 10.2147/JPR.S140990
- 40. Beachler DC, Hall K, Garg R, et al An evaluation of the effect of the OxyContin reformulation on unintentional fatal and nonfatal overdose. Clin J Pain. 2022;38(6):396–404. 10.1097/AJP.0000000000001034
- 41. Turk DC, O'Connor AB, Dworkin RH, et al Research design considerations for clinical studies of abuse-deterrent opioid analgesics: IMMPACT recommendations. Pain. 2012;153(10):1997–2008. 10.1016/j.pain.2012.05.029
- 42. Peacock A, Larance B, Bruno R, et al Post-marketing studies of pharmaceutical opioid abuse-deterrent formulations: a framework for research design and reporting. Addiction. 2019;114(3):389–399. 10.1111/add.14380
- 43. Walker AM. Confounding by indication. Epidemiology. 1996;7(4):335–336.
- 44. US Food and Drug Administration. Data and Methods for Evaluating the Impact of Opioid Formulations with Properties Designed to Deter Abuse in the Postmarket Setting: A Scientific Discussion of Present and Future Capabilities. FDA; 2017.
- 45. Suissa S, Moodie E, Dell'Aniello S. Prevalent new-user cohort designs for comparative drug effect studies by time-conditional propensity scores. Pharmacoepidemiol Drug Saf. 2017;26(4):459–468. 10.1002/pds.4107
- 46. Webster-Clark M, Ross RK, Lund JL. Initiator types and the causal question of the prevalent new-user design: a simulation study. Am J Epidemiol. 2021;190(7):1341–1348. 10.1093/aje/kwaa283
- 47. Lund JL, Richardson DB, Stürmer T. The active comparator, new user study design in pharmacoepidemiology: historical foundations and contemporary application. Curr Epidemiol Rep. 2015;2(4):221–228. 10.1007/s40471-015-0053-5
- 48. Ray WA. Evaluating medication effects outside of clinical trials: new-user designs. Am J Epidemiol. 2003;158(9):915–920. 10.1093/aje/kwg231
- 49. Cole SR, Hudgens MG, Brookhart MA, Westreich D. Risk. Am J Epidemiol 2015;181(4):246–250. 10.1093/aje/kwv001
- 50. Cole SR, Lau B, Eron JJ, et al Estimation of the standardized risk difference and ratio in a competing risks framework: application to injection drug use and progression to AIDS after initiation of antiretroviral therapy. Am J Epidemiol. 2015;181(4):238–245. 10.1093/aje/kwu122
- 51. Hernan MA, Hernandez-Diaz S. Beyond the intention-to-treat in comparative effectiveness research. Clin Trials. 2012;9(1):48–55. 10.1177/1740774511420743
- 52. Moore BJJ, White S, Washington R, et al Identifying increased risk of readmission and in-hospital mortality using hospital administrative data: The AHRQ Elixhauser Comorbidity Index. Med Care. 2017;55(7):698–705. 10.1097/MLR.0000000000000735
- 53. Dasgupta N, Wang Y, Bae J, et al Inches, centimeters, and yards: overlooked definition choices inhibit interpretation of morphine equivalence. Clin J Pain. 2021;37(8):565–574. 10.1097/AJP.0000000000000948
- 54. Austin PC. Balance diagnostics for comparing the distribution of baseline covariates between treatment groups in propensity-score matched samples. Stat Med. 2009;28(25):3083–3107. 10.1002/sim.3697
- 55. Cole SR, Hernán MA. Constructing inverse probability weights for marginal structural models. Am J Epidemiol. 2008;168(6):656–664. 10.1093/aje/kwn164
- 56. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999;94(446):496–509. 10.1080/01621459.1999.10474144
- 57. Mumford SL, Schisterman EF, Cole SR, et al Time at risk and intention-to-treat analyses: parallels and implications for inference. Epidemiology. 2015;26(1):112–118. 10.1097/EDE.0000000000000188
- 58. Ranapurwala SI, Naumann RB, Austin AE, et al Methodologic limitations of prescription opioid safety research and recommendations for improving the evidence base. Pharmacoepidemiol Drug Saf. 2019;28(1):4–12. 10.1002/pds.4564
- 59. R Core Team. R: A Language and Environment for Statistical Computing. R Foundation for Statistical Computing; 2019. Accessed May 2019.
- 60. US Food and Drug Administration. FDA analysis of long-term trends in prescription opioid analgesic products: quantity, sales, and Price trends. March 1, 2018. https://www.fda.gov/media/111695/download
- 61. Litman RS, Pagan OH, Cicero TJ. Abuse-deterrent opioid formulations. Anesthesiology. 2018;128(5):1015–1026. 10.1097/ALN.0000000000002031
- 62. Cicero TJ, Ellis MS. Abuse-deterrent formulations and the prescription opioid abuse epidemic in the United States: lessons learned from OxyContin. JAMA Psychiatry. 2015;72(5):424–430. 10.1001/jamapsychiatry.2014.3043
- 63. Severtson SG, Bartelson BB, Davis JM, et al Reduced abuse, therapeutic errors, and diversion following reformulation of extended-release oxycodone in 2010. J Pain. 2013;14(10):1122–1130. 10.1016/j.jpain.2013.04.011
- 64. Sessler NE, Downing JM, Kale H, et al Reductions in reported deaths following the introduction of extended-release oxycodone (OxyContin) with an abuse-deterrent formulation. Pharmacoepidemiol Drug Saf. 2014;23(12):1238–1246. 10.1002/pds.3658
- 65. Butler SF, Cassidy TA, Chilcoat H, et al Abuse rates and routes of administration of reformulated extended-release oxycodone: initial findings from a sentinel surveillance sample of individuals assessed for substance abuse treatment. J Pain. 2013;14(4):351–358. 10.1016/j.jpain.2012.08.008
- 66. Dasgupta N. Commentary on Paljarvi et al: can harder-to-crush oxycodone prevent overdose? Addiction. 2021;116(9):2416–2417. 10.1111/add.15617
- 67. By K, McAninch JK, Keeton SL, et al Important statistical considerations in the evaluation of post-market studies to assess whether opioids with abuse-deterrent properties result in reduced abuse in the community. Pharmacoepidemiol Drug Saf. 2018;27(5):473–478. 10.1002/pds.4287
- 68. Rowe C, Vittinghoff E, Santos GM, et al Performance measures of diagnostic codes for detecting opioid overdose in the emergency department. Acad Emerg Med. 2017;24(4):475–483. 10.1111/acem.13121
- 69. Ranapurwala SI, Alam IZ, Pence BW, et al Development and validation of an electronic health records-based opioid use disorder algorithm by expert clinical adjudication among patients with prescribed opioids. Pharmacoepidemiol Drug Saf. 2023;32(5):577–585. 10.1002/pds.5591
- 70. Conover MM, Sturmer T, Poole C, et al Classifying medical histories in US Medicare beneficiaries using fixed vs all-available look-back approaches. Pharmacoepidemiol Drug Saf. 2018;27(7):771–780. 10.1002/pds.4435
- 71. Connolly JG, Schneeweiss S, Glynn RJ, et al Quantifying bias reduction with fixed-duration versus all-available covariate assessment periods. Pharmacoepidemiol Drug Saf. 2019;28(5):665–670. 10.1002/pds.4729
- 72. Brunelli SM, Gagne JJ, Huybrechts KF, et al Estimation using all available covariate information versus a fixed look-back window for dichotomous covariates. Pharmacoepidemiol Drug Saf. 2013;22(5):542–550. 10.1002/pds.3434