Introduction
Cardiac-valve calcification increases as kidney function declines [-], and the prevalence of valvular heart disease is double in patients with chronic kidney disease (CKD) compared to an age-matched controls []. One in 7 patients that reach end-stage renal disease (ESRD) requiring hemodialysis (HD) treatment will develop valvular heart disease, and this is associated with increased cardiovascular morbidity [] and overall mortality [, ]. Patients with valvular heart disease on HD often have an indication for warfarin therapy. Best practice guidelines recommend anticoagulation with a vitamin K antagonist (VKA) (e.g., warfarin) in patients with a mechanical prosthetic valve and for a minimum of 3 months in patients with a bioprosthetic mitral or aortic valve replacement []. However, there are patients with ESRD in whom warfarin therapy is relatively or absolutely contraindicated, such as in pregnancy [], warfarin-induced skin necrosis (WSN) [], and calcific uremic arteriolopathy (CUA) [], previously called calciphylaxis. The optimal anticoagulation strategy in such cases remains uncertain.
VKA therapy inhibits vitamin K-dependent carboxylation of matrix-G1a protein, resulting in vascular calcification as demonstrated in murine models [, ]. Rapid increases in vascular and valvular calcification are seen in patients with ESRD [, ]. CUA is an uncommon painful syndrome of calcification of the small blood vessels located within the fatty tissue and deeper layers of the skin that results in chronic nonhealing wounds and death. CUA is a rare complication of VKA therapy occurring predominantly in patients with ESRD [, ], with an incidence of 3.5 cases per 1,000 ESRD patient years []. CUA has a high mortality rate of 30% and 50% at 6 and 12 months, respectively [, ]. CUA is a contraindication to subsequent VKA therapy, but the best alternative anticoagulation strategy is unknown.
Recent randomized control trials comparing VKA therapy to novel oral anticoagulants (NOACs) in patients with ESRD and nonvalvular atrial fibrillation (AF) show efficacy of NOACs [, ]. However, the best alternative anticoagulation strategy for patients with ESRD, a mechanical valve, and contraindication to warfarin remains unclear. The aim of this scoping review was to identify and summarize all cases of alternative oral anticoagulants use in patients with contraindications to VKA therapy and to highlight opportunities for future research.
Methods
Our scoping review was guided using the methodological framework proposed by the Joanna Briggs Institute []. We report this review in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-analyses extension for scoping reviews checklist.
Search Strategies and Study Identification
We developed a search strategy in collaboration with an information specialist. We searched the following online databases (inception to March 2021): MEDLINE (Ovid), Embase (Ovid), Cochrane Central Register of Controlled trials (Ovid), and Web of Science. The search strategy was not limited by study design, language, or year. The final MEDLINE search strategy is presented in online supplementary Table 1 (for all online suppl. material, see http://www.karger.com/doi/10.1159/000519921). We reviewed the gray literature including all published abstracts for the following relevant international conferences using key search terms: American Society of Nephrology (2003–2020), Canadian Society of Nephrology (2012–2020), American Society of Hematology (2007–2020), and European Dialysis and Transplant Society (2010–2020).
Eligible studies included those that reported an anticoagulation strategy for patients with ESRD who developed a contraindication to VKA therapy. Only studies relating to patients with ESRD were included as alternative anticoagulation strategies in this population are less certain.
Study Selection
Search results were imported into Covidence systematic review software (Veritas Health Innovation, Melbourne, VIC, Australia) for screening. Two reviewers (N.G.P. and R.M.H.) independently screened titles and abstracts of articles. Discrepancies were resolved by a third reviewer (B.K.A.T.). Two reviewers (B.K.A.T. and N.G.P.) reviewed full-text articles to determine their eligibility. Disagreements between reviewers at the full-text phase were resolved by a third reviewer (R.M.H.).
Data Extraction and Synthesis
A data extraction form was developed a priori. Study characteristics included the type of study and country of publication. Case characteristics included age, sex, ethnicity, type of dialysis treatment, initial indication for warfarin treatment, type of contraindication developed, and follow-up duration. An alternative anticoagulation strategy was recorded. Any recorded thrombosis or bleeding events were recorded, as well as mortality. When multiple cases were provided in a single case series, we extracted data only from descriptions of cases that met our study eligibility. We categorized and reported data descriptively by counts and frequencies.
Results
Selection of Sources of Evidence
The search of MEDLINE, Ovid/Embase, Cochrane, and Web of Science databases yielded 2,418 references (Fig. 1). Removal of duplicates led to 2,044 total references. Of these, 1,926 references were not relevant to the study question, yielding 118 articles for full-text review. There were 95 studies excluded for wrong patient population (n = 57), outcomes (n = 17), study design (n = 11), intervention (n = 9), or for being a duplicate (n = 1). A search of conference proceedings yielded 40 abstracts, but 100% had already been captured in the database searches. After full-text review, 23 studies were included.
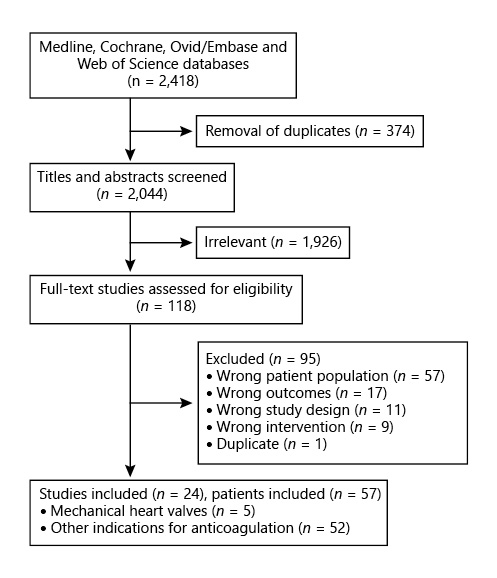
Fig. 1
PRISMA flow diagram for review.
Study Characteristics
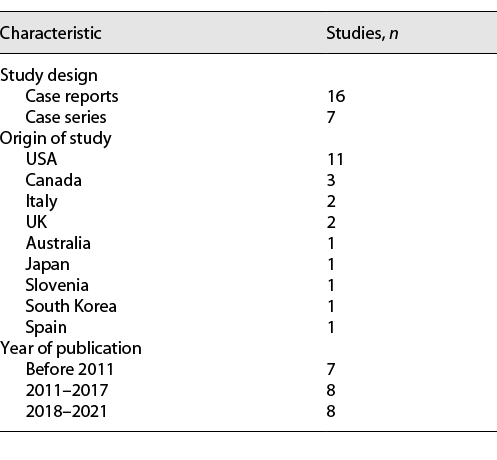
A summary of all 23 included studies is provided in Table 1. All studies were either case reports (16/23) or case series (7/23). No interventional trials were reported. Sixty-one percent of studies (14/23) were either from Canada or the USA. Most studies were reported since 2011 (16/23).
Case Characteristics
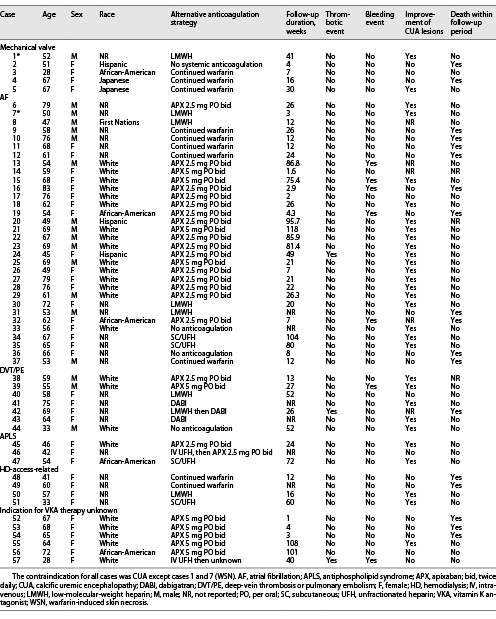
The 23 studies yielded 57 individual patient cases (Table 2). The average patient age was 60 years, and 68% of the cases described females. The ethnicity of patients was as follows: White (42.1%), African-American (9%), Hispanic (5%), Japanese (4%), and Indigenous (2%). The indication for warfarin included mechanical valve (n = 5), AF (n = 32), deep-vein thrombosis or pulmonary embolism (DVT/PE) (n = 7), antiphospholipid syndrome (n = 3), HD-access-related (n = 4), and uncertain indication (n = 6). The most common contraindication to VKA therapy was the development of CUA (55 cases), whereas WSN was the reported contraindication in only 2 cases (case number 1 and 7). There were 10 alternative anticoagulation strategies reported. The most common was apixaban (APX) 2.5 mg PO bid (n = 17) followed by APX 5 mg PO bid (n = 10), continuation of warfarin (n = 10), low-molecular-weight heparin (LMWH) (n = 7), no anticoagulation (n = 4), subcutaneous unfractionated heparin (SC/UFH) (n = 4), dabigatran (DABI) (n = 2), IV UFH then APX (n = 1), IV UFH then unknown (n = 1), and LMWH followed by DABI (n = 1).
In patients with mechanical valves (Table 2), the average follow-up duration was 19.6 weeks, with a range between 4 and 41 weeks. The patient with WSN (case 1) discontinued warfarin and switched to LMWH. The skin lesions improved over the 41-week follow-up period, and no additional adverse outcomes were reported. The remaining 4 cases of patients with mechanical valves developed CUA. Two of these patients died within the follow-up period. One patient (case 2) discontinued VKA therapy, and no alternative anticoagulation was pursued. The patient’s CUA worsened, and she died 4 weeks later from sepsis and acute respiratory distress syndrome. The remaining 3 cases continued warfarin despite the development of CUA. The CUA lesions did not improve in 2 cases, and 1 of these patients died within 16 weeks. In one case, the CUA lesions improved, and no adverse events occurred within 30 weeks of follow-up.
Of the 32 patients with AF, 10 died within the follow-up period. The anticoagulation strategy of those who died with AF included continuation of warfarin (5/10), APX 2.5 mg PO bid (3/10), LMWH (1/10), and no anticoagulation (1/10). Of all patients with AF whose anticoagulation strategy was either continuation of warfarin (n = 5) or no anticoagulation (n = 2), the majority (6/7) died. Among the 19 patients who switched to APX, only 3 (16%) died within the follow-up period. There was 1 case of thrombosis occurring in a patient on APX 2.5 mg bid and 5 cases of bleeding occurring in patients who were all on APX (both 2.5 mg PO bid and 5 mg PO bid dosing). In cases where improvement of CUA lesions was noted (n = 18), 13 were switched to APX, 4 were on LMWH or SC/UFH, and 1 was on “no anticoagulation.” In the 9 cases where CUA lesions did not improve, 5 continued with warfarin, 4 were on APX, and 1 was on SC/UFH.
Of the 7 cases of DVT/PE, warfarin was discontinued in all cases, and an alternative anticoagulation strategy was adopted in 6 cases (3 patients switched to DABI, 2 patients switched to APX, and 1 patient switched to LMWH). CUA lesions improved in 5 cases, did not improve in 1 case, and were not reported in 1 case. One patient who was switched to LMWH and then DABI died within the follow-up period. There was one bleeding event reported in a patient receiving APX 5 mg bid. There was one fatal thrombotic event reported in a patient treated with LMWH followed by DABI.
Of 3 cases of antiphospholipid syndrome, warfarin was discontinued in all cases with APX and SC/UFH used as alternatives. CUA lesions improved in 2/3 cases and no deaths were reported. Of 4 cases related to HD access, warfarin was continued in 2 cases, and both patients died. In 6 cases where the indication for warfarin treatment was unknown, 5 patients switched to APX, and 3 of these patients died with no improvement in their CUA lesions.
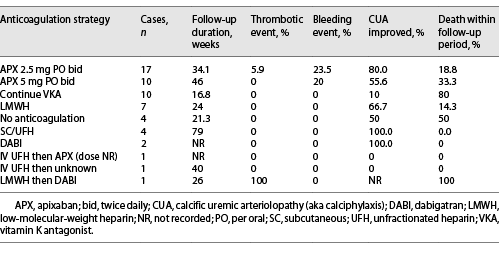
Clinical outcomes were collated per anticoagulation strategy (Table 3). Of all patients who continued warfarin, only 10% experienced an improvement in their CUA lesions, and 80% died within the duration of follow-up. In contrast, of those patients who were switched to APX 2.5 mg bid and 5 mg bid, 80% and 56% of CUA lesions improved, and 20% and 33% of patients died, respectively. Of 7 patients who switched to LMWH, 67% of CUA lesions improved, and only 14.3% of patients died within the time frame of reporting. Of 4 patients who switched to SC/UFH, all 4 survived, and the CUA lesions improved in 100% of patients. The other anticoagulation strategies reported were favorable, but the number of cases described was very low.
Discussion
Scoping Review
This scoping review explored anticoagulation strategies in patients with ESRD who have a contraindication to VKA therapy. Our search strategy yielded 23 published studies describing 57 patients. In these reported cases, we found that continuation of VKA therapy was associated with an increased mortality and decreased improvement in CUA lesions when compared to switching to APX or LMWH. There were few cases where either bleeding or thrombosis was reported. There is controversy about whether warfarin can be continued in patients with CUA with some reports suggesting that continuing VKA is an option for skin necrosis [], while others show that reinitiating warfarin in CUA [] or WSN [] precipitates relapse of skin lesions. This scoping review suggests that when a contraindication for VKA therapy develops (mainly CUA), then continuation of VKA therapy is associated with very high mortality and minimal improvement in CUA lesions. Therefore, in this clinical scenario alternative, non-VKA anticoagulants should be considered.
The optimal anticoagulation strategy in patients with a mechanical valve, ESRD, and a contraindication to VKA therapy is of particular interest. However, the present scoping review only identified 5 such patients. The treatment and outcomes for these 5 patients were quite varied. In case 1 of WSN, switching to LMWH resulted in improvement of skin and survival out to 41 weeks of follow-up. The remaining 4 patients developed CUA; 1 patient discontinued warfarin and died, and 3 patients remained on warfarin with one death at 16 weeks. In one case, the CUA lesions improved, and the patient was alive at the 30-week follow-up interval. Definitive conclusions are not possible with such a small number of patients, and identifying effective non-VKA anticoagulants for patients with ESRD and a mechanical heart valve remains challenging and uncertain.
A number of trials have evaluated NOACs compared to warfarin in patients without severe CKD with mechanical heart valves. In the RE-ALIGN trial, patients with mechanical aortic or mitral valves randomized to DABI had higher rates of bleeding with no improvement in death or thromboembolic disease, compared to warfarin []. A subsequent US Food and Drug Administration (FDA) [] black box warning was issued for DABI in patients with mechanical valves. In the ENGAGE AF-TIMI 48 trial evaluating edoxaban (low-dose and high-dose) versus warfarin, there were a small number of patients included with bioprosthetic (nonmechanical) heart valves. Rates of bleeding were lower in low-dose edoxaban versus warfarin, but a trend toward more strokes or systemic embolizations in the low-dose edoxaban arm was observed. The high-dose edoxaban arm had lower rates of the composite outcome of stroke, myocardial infarction, or cardiac death than those in the warfarin arm and had lower rates of major bleeding []. In the RIVER trial, patients with a bioprosthetic (nonmechanical) mitral valve were randomized to rivaroxaban versus warfarin, and those receiving rivaroxaban had the same rates of death from cardiovascular or thromboembolic events, and major bleeding, with lower rates of stroke compared with warfarin []. It is important to note that the RE-ALIGN, ENGAGE AF-TIMI 48, and RIVER trials excluded patients with CrCl <40 mL/min/m2, 30 mL/min/m2, and 30 mL/min/m2, respectively. Furthermore, only the RE-ALIGN trial included patients with mechanical, as opposed to bioprosthetic valves. Taken together, the American Heart Association still recommends long-term anticoagulation with VKA in patients with mechanical heart valves [], and “NOACs are not recommended” [].
NOAC Use in ESRD
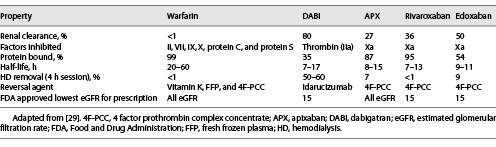
The intrinsic properties of NOACs are important to consider when evaluating anticoagulation strategies for patients with ESRD and contraindication to VKA therapy (Table 4). DABI’s clearance is mostly through renal mechanisms (80%); this likely explains DABI’s association with increased major bleeding risk, when compared to warfarin, in HD patients []. Similarly, achieving stable DABI levels would be challenging in HD as there is significant HD removal. These properties make DABI a poor choice for anticoagulation in patients with ESRD and especially for patients with ESRD with mechanical heart valves, considering the RE-ALIGN trial [].
Renal elimination of edoxaban is approximately 50%, and edoxaban plasma levels increase as renal function declines []. The ENGAGE AF-TIMI 48 trial demonstrated that plasma edoxaban levels correlated well with clinical outcomes; patients with low plasma edoxaban levels, due to high renal clearance, had higher rates of ischemic stroke than those with warfarin []. Conversely, there are concerns that high edoxaban levels in patients with ESRD may be associated with increased bleeding risk. While there are no randomized controlled data on edoxaban use in patients with ESRD, there is sufficient concern from existing studies to avoid its use in this clinical population.
The desirable attributes of low renal clearance and minimal HD clearance are both met with APX and rivaroxaban. While there are no randomized controlled trials of APX or rivaroxaban in the ESRD population with mechanical heart valves, there are studies in patients with AF that may be relevant. In the RENAL-AF trial, patients with AF and ESRD on HD had similar rates of bleeding and stroke in both the APX 5 mg PO bid and warfarin arms []. Unfortunately, this trial was stopped early due to loss of funding when only 154 of the required 760 patients had been enrolled. Many (40%) of the patients enrolled in this trial also received aspirin, which has since been removed from both the European [] and the American Heart Association [] guidelines for management of AF due to an increase in bleeding risk without a change in stroke risk. While this trial and one limited pharmacokinetic study [] led to the FDA granting approval for APX use in patients with ESRD [], APX 5 mg PO bid bioaccumulates in ESRD [, ], with cases of bioaccumulation also reported at the lower dose of 2.5 mg PO bid []. The lower dose APX dose of 2.5 mg PO bid is recommended in patients with ESRD based on past pharmacokinetic trials []. There were insufficient data in this scoping review to identify different trends in bleeding or thrombosis risk between low- and high-dose APX. However, APX was used as a VKA alternative in 27 reported cases of patients with a nonmechanical valve indication for anticoagulation. Compared to continuation of warfarin, CUA lesions improved, and substantially fewer patients died. Although few bleeding events were reported, all cases involving bleeding did occur in patients who had switched to APX. These data suggest that NOACs could be considered in a patient with ESRD with a contraindication to VKA therapy, but further study on the use of APX in patients with ESRD and mechanical valves is warranted before its use can be recommended.
Rivaroxaban’s elimination is mostly hepatic, and with minimal clearance with dialysis, it has a favorable pharmacokinetic and pharmacodynamic profile for use in patients with ESRD. A retrospective cohort study in patients with CKD stage 4 and 5 (including some with ESRD) compared rivaroxaban and warfarin in nonvalvular AF. Rivaroxaban use was associated with no change in stroke or systemic embolism, ischemic stroke, but lower rates of major bleeding []. This result came after two published systematic reviews that showed rivaroxaban use associated with increased major bleeding risk compared to warfarin use, in HD patients with AF [, ]. The differing results with respect to rivaroxaban-associated bleeding may be due to the lack of standardization of the rivaroxaban dose. A 10 mg dose of rivaroxaban in a patient with ESRD is equivalent to a 20 mg dose in a population without CKD, with no bioaccumulation []. Using the 10 mg dose, the Valkyrie multicenter randomized controlled trial evaluated rivaroxaban with or without vitamin K2 (compared to warfarin) in HD patients with AF []. After 18 months of follow-up, there was no difference in death from any cause, stroke, or systemic embolism, but a significant reduction in life-threatening or major bleeding. However, the Valkyrie study also evaluated coronary artery, thoracic aorta, and mitral and aortic valve calcification, and no differences were reported between rivaroxaban or warfarin groups at 6, 12, or 18 months of follow-up. This result differed from a multicenter observational retrospective study in CKD stage 3B and 4 patients with nonvalvular AF, in whom rivaroxaban use was associated with significant reductions in mitral and aortic valve calcifications compared to warfarin []. Unfortunately, this scoping review did not identify any case of rivaroxaban use in patients with ESRD when VKA therapy was contraindicated. Further study of rivaroxaban use in mechanical heart valves in CKD will therefore be needed before it can be recommended in this clinical context.
LMWH and UFH in ESRD
UFH is cleared through hepatic and renal mechanisms, whereas LMWH is dependent mostly on renal clearance. Thus, patients with kidney disease are at increased risk of bleeding due to reduced clearance and prolonged anticoagulation effect of LMWH [-]. UFH inhibits factor II (thrombin) and Xa equally, whereas LMWH inhibits primarily factor Xa. In the scoping review, there were 3 patients who switched to LMWH anticoagulation after developing a contraindication to VKA therapy (Table 2). The outcomes of these 3 patients were excellent, with all 3 surviving the follow-up period without thrombotic or bleeding events. Only one of these patients had a mechanical valve, but he had a long follow-up period of 41 weeks without any clinical complications.
Given the impracticality of outpatient intravenous UFH administration, SC administration was evaluated in patients with newly diagnosed DVT/PE in the FIDO trial []. The trial found that SC twice daily UFH and once daily LMWH had equivalent outcomes including recurrent thromboembolism, major bleeding, and death. While the FIDO trial excluded patients with serum creatinine of 200 µmol/L (2.3 mg/dL) or higher, SC/UFH use in thromboembolic disease has been described in 3 patients with ESRD [], where it was found to be a safe alternative option. Similarly, in a nonrandomized study in patients with mechanical heart valves anticoagulated subcutaneously with UFH or LMWH for a duration of about 14 days, there were low rates of major bleeding and thrombosis []. While the study excluded patients with ESRD, it provides some reassurance that both UFH and LMWH may be reasonable options in those patients in whom VKA therapy is contraindicated. In this scoping review, there were 4 patients with CUA whose warfarin was stopped and started SC/UFH. While none of these patients had mechanical heart valves, all of these patients survived follow-up with improvement of CUA lesions.
The strengths of the present scoping review include our comprehensive search strategy and novel research question. It is the first study to summarize all reported cases of VKA therapy contraindication in patients with ESRD. The lack of study types other than case reports and case series highlights an important literature gap for patients with ESRD. Case reports and case series are subject to information bias such as recall bias and may include nonrepresentative population samples, often with uncontrolled observations and limited quantitative data. Further, we acknowledge an inherent reporting bias that published cases are more likely to reflect treatment successes rather than failures. Also, relatively newer anticoagulants, such as a NOAC or LMWH, may have led to selective reporting over older agents such as warfarin. The number of cases of warfarin-associated CUA that we found is relatively low in comparison with the number of cases that occur [], suggesting that many cases are unreported.
Given the complete lack of high-quality studies in this area, significant conclusions regarding anticoagulation strategies cannot be made with confidence. Only 5 reported cases included patients with a mechanical valve, and thus, we also had to identify cases with a similar context but with different indications for anticoagulation. In these reported cases, continuing warfarin was associated with high mortality and failure of CUA lesions to resolve. Whether this finding can be extrapolated to patients with mechanical valves is unknown, and we caution against overinterpretation and the generalizability of these findings. At best, we can provide a summary of different options that have been attempted and their associated outcomes. This review highlights the need for the inclusion of patients with ESRD in clinical trials, especially for patients with ESRD and either bioprosthetic or mechanical heart valves. Additional consideration may be given to an open-access online case reporting database for clinicians’ collaboration.
Acknowledgments
Ms. Sandra McKeown collaborated on strategy for literature search.
Statement of Ethics
This study is consistent with the principles outlined by the Committee on Publication Ethics (COPE). Health Sciences Research Ethics Board approval was not required since all information used in this manuscript was already freely available in the public domain, and the dataset analyzed was properly anonymized with informed consent obtained at the time of original data collection.
Conflict of Interest Statement
None of the authors have conflicts of interest to declare.
Funding Sources
The authors did not receive any funding for this study.
Author Contributions
All the authors modified each draft version, including the final draft. Authors R.M.H. and B.K.A.T. created the study concept and developed the methods. All the authors provided critical intellectual input for the development of the methods.
Data Availability Statement
Data is available on request, by contacting the corresponding author.
References
- 1. Guerraty MA, Chai B, Hsu JY, Ojo AO, Gao Y, Yang W, et al. Relation of aortic valve calcium to chronic kidney disease (from the Chronic Renal Insufficiency Cohort Study). Am J Cardiol. 2015;115:1281–6. http://dx.doi.org/10.1016/j.amjcard.2015.02.011.
- 2. Ix JH, Shlipak MG, Katz R, Budoff MJ, Shavelle DM, Probstfield JL, et al. Kidney function and aortic valve and mitral annular calcification in the Multi-Ethnic Study of Atherosclerosis (MESA). Am J Kidney Dis. 2007;50:412–20. http://dx.doi.org/10.1053/j.ajkd.2007.05.020.
- 3. Reilly RF, Jain N. Warfarin in nonvalvular atrial fibrillation-time for a change?Semin Dial. 2019;32:520–6. http://dx.doi.org/10.1111/sdi.12829.
- 4. Saran R, Robinson B, Abbott KC, Bragg-Gresham J, Chen X, Gipson D, et al. US renal data system 2019 annual data report: epidemiology of kidney disease in the USA. Am J Kidney Dis. 2020;75:A6–7. http://dx.doi.org/10.1053/j.ajkd.2019.09.003.
- 5. Raggi P, Bellasi A, Gamboa C, Ferramosca E, Ratti C, Block GA, et al. All-cause mortality in hemodialysis patients with heart valve calcification. Clin J Am Soc Nephrol. 2011;6:1990–5. http://dx.doi.org/10.2215/CJN.01140211.
- 6. Samad Z, Sivak JA, Phelan M, Schulte PJ, Patel U, Velazquez EJ. Prevalence and outcomes of left-sided valvular heart disease associated with chronic kidney disease. J Am Heart Assoc. 2017;6:e006044. http://dx.doi.org/10.1161/JAHA.117.006044.
- 7. Nishimura RA, Otto CM, Bonow RO, Carabello BA, Erwin JP 3rd, Fleisher LA, et al. 2017 AHA/ACC focused update of the 2014 AHA/ACC guideline for the management of patients with valvular heart disease: a report of the American college of cardiology/American heart association task force on clinical practice guidelines. Circulation. 2017;135:e1159–95. http://dx.doi.org/10.1161/CIR.0000000000000503.
- 8. Pieper PG. Use of medication for cardiovascular disease during pregnancy. Nat Rev Cardiol. 2015;12:718–29. http://dx.doi.org/10.1038/nrcardio.2015.172.
- 9. Inayatullah S, Phadke G, Vilenski L, Pasya SK, Albrecht W. Warfarin-induced skin necrosis. South Med J. 2010;103:74–5. http://dx.doi.org/10.1097/SMJ.0b013e3181c47dcf.
- 10. Hasegawa H. Clinical assessment of warfarin therapy in patients with maintenance dialysis-clinical efficacy, risks and development of calciphylaxis. Ann Vasc Dis. 2017;10(3):170–77. http://dx.doi.org/10.3400/avd.ra.17-00062.
- 11. Wallin R, Cain D, Sane DC. Matrix Gla protein synthesis and gamma-carboxylation in the aortic vessel wall and proliferating vascular smooth muscle cells: a cell system which resembles the system in bone cells. Thromb Haemost. 1999;82:1764–7.
- 12. Howe AM, Webster WS. Warfarin exposure and calcification of the arterial system in the rat. Int J Exp Pathol. 2000;81:51–6. http://dx.doi.org/10.1046/j.1365-2613.2000.00140.x.
- 13. Griffin TP, Islam MN, Wall D, Ferguson J, Griffin DG, Griffin MD, et al. Plasma dephosphorylated-uncarboxylated Matrix Gla-protein (dp-ucMGP): reference intervals in Caucasian adults and diabetic kidney disease biomarker potential. Sci Rep. 2019;9:18452. http://dx.doi.org/10.1038/s41598-019-54762-2.
- 14. Brandenburg VM, Kramann R, Rothe H, Kaesler N, Korbiel J, Specht P, et al. Calcific uraemic arteriolopathy (calciphylaxis): data from a large nationwide registry. Nephrol Dial Transplant. 2017;32:126–32. http://dx.doi.org/10.1093/ndt/gfv438.
- 15. McCarthy JT, El-Azhary RA, Patzelt MT, Weaver AL, Albright RC, Bridges AD, et al. Survival, risk factors, and effect of treatment in 101 patients with calciphylaxis. Mayo Clin Proc. 2016;91:1384–94. http://dx.doi.org/10.1016/j.mayocp.2016.06.025.
- 16. Nigwekar SU, Zhao S, Wenger J, Hymes JL, Maddux FW, Thadhani RI, et al. A nationally representative study of calcific uremic arteriolopathy risk factors. J Am Soc Nephrol. 2016;27:3421–9. http://dx.doi.org/10.1681/ASN.2015091065.
- 17. De Vriese AS, Caluwé R, Pyfferoen L, De Bacquer D, De Boeck K, Delanote J, et al. Multicenter randomized controlled trial of vitamin K antagonist replacement by rivaroxaban with or without vitamin K2 in hemodialysis patients with atrial fibrillation: the Valkyrie Study. J Am Soc Nephrol. 2020;31:186–96. http://dx.doi.org/10.1681/ASN.2019060579.
- 18. Pokorney SD. Presentation slides for the RENAL-AF trial. J Am Coll Cardiol. 2020;75(11):1299–1308.
- 19.
- 20. Zauber NP, Stark MW. Successful warfarin anticoagulation despite protein C deficiency and a history of warfarin necrosis. Ann Intern Med. 1986;104:659–60. http://dx.doi.org/10.7326/0003-4819-104-5-659.
- 21. Russ P, Russwurm M, Kortus-Goetze B, Hoyer J, Kamalanabhaiah S. Phenprocoumon based anticoagulation is an underestimated factor in the pathogenesis of calciphylaxis. BMC Nephrol. 2019;20:114. http://dx.doi.org/10.1186/s12882-019-1301-6.
- 22. Park JE, Byeon S, Kim HK, Moon SM, Moon JH, Jang KT, et al. Warfarin skin necrosis mimicking calciphylaxis in a patient with secondary hyperparathyroidism undergoing peritoneal dialysis. Kidney Res Clin Pract. 2016;35:55–8. http://dx.doi.org/10.1016/j.krcp.2015.07.003.
- 23. Eikelboom JW, Connolly SJ, Brueckmann M, Granger CB, Kappetein AP, Mack MJ, et al. Dabigatran versus warfarin in patients with mechanical heart valves. N Engl J Med. 2013;369:1206–14. http://dx.doi.org/10.1056/NEJMoa1300615.
- 24.
- 25. Giugliano RP, Ruff CT, Braunwald E, Murphy SA, Wiviott SD, Halperin JL, et al. Edoxaban versus warfarin in patients with atrial fibrillation. N Engl J Med. 2013;369:2093–104. http://dx.doi.org/10.1056/NEJMoa1310907.
- 26. Guimaraes HP, Lopes RD, de Barros E Silva PGM, Liporace IL, Sampaio RO, Tarasoutchi F, et al. Rivaroxaban in patients with atrial fibrillation and a bioprosthetic mitral valve. N Engl J Med. 2020;383:2117–26.
- 27. Otto CM, Nishimura RA, Bonow RO, Carabello BA, Erwin JP 3rd, Gentile F, et al. 2020 ACC/AHA guideline for the management of patients with valvular heart disease: executive summary: a report of the American college of cardiology/American heart association joint committee on clinical practice guidelines. Circulation. 2021;143(5):e35–71. http://dx.doi.org/10.1161/CIR.0000000000000932.
- 28. Feldberg J, Patel P, Farrell A, Sivarajahkumar S, Cameron K, Ma J, et al. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol Dial Transplant. 2019;34:265–77. http://dx.doi.org/10.1093/ndt/gfy031.
- 29. Di Lullo L, Tripepi G, Ronco C, D’Arrigo G, Barbera V, Russo D, et al. Cardiac valve calcification and use of anticoagulants: preliminary observation of a potentially modifiable risk factor. Int J Cardiol. 2019;278:243–9. http://dx.doi.org/10.1016/j.ijcard.2018.11.119.
- 30. Parasrampuria DA, Truitt KE. Pharmacokinetics and pharmacodynamics of edoxaban, a non-vitamin K antagonist oral anticoagulant that inhibits clotting factor Xa. Clin Pharmacokinet. 2016;55:641–55. http://dx.doi.org/10.1007/s40262-015-0342-7.
- 31. Hindricks G, Potpara T, Dagres N, Arbelo E, Bax JJ, Blomstrom-Lundqvist C, et al. 2020 ESC guidelines for the diagnosis and management of atrial fibrillation developed in collaboration with the European association of cardio-thoracic surgery (EACTS). Eur Heart J. 2021;42(5):373–498.
- 32. January CT, Wann LS, Calkins H, Chen LY, Cigarroa JE, Cleveland JC Jr, et al. 2019 AHA/ACC/HRS focused update of the 2014 AHA/ACC/HRS guideline for the management of patients with atrial fibrillation: a report of the American college of cardiology/American heart association task force on clinical practice guidelines and the heart rhythm society in collaboration with the society of thoracic surgeons. Circulation. 2019;140:e125–51. http://dx.doi.org/10.1161/CIR.0000000000000665.
- 33. Wang X, Tirucherai G, Marbury TC, Wang J, Chang M, Zhang D, et al. Pharmacokinetics, pharmacodynamics, and safety of apixaban in subjects with end-stage renal disease on hemodialysis. J Clin Pharmacol. 2016;56:628–36. http://dx.doi.org/10.1002/jcph.628.
- 34. Bristol-Myers Squibb Company.. ELIQUIS (Apixaban) package insert. Reference ID 3961165. 2018.
- 35. Mavrakanas TA, Samer CF, Nessim SJ, Frisch G, Lipman ML. Apixaban pharmacokinetics at steady state in hemodialysis patients. J Am Soc Nephrol. 2017;28:2241–8. http://dx.doi.org/10.1681/ASN.2016090980.
- 36. Pokorney SD, Garonzik S, Chertow GM, Washam JB, Mussina K, Bansal N, et al. Pharmacokinetics of apixaban in patients with end stage renal disease on hemodialysis and atrial fibrillation: results from the RENAL-AF trial. Eur Heart J. 2020;41:1299–1308.
- 37. Kufel WD, Zayac AS, Lehmann DF, Miller CD. Clinical application and pharmacodynamic monitoring of apixaban in a patient with end-stage renal disease requiring chronic hemodialysis. Pharmacotherapy. 2016;36:e166–71. http://dx.doi.org/10.1002/phar.1836.
- 38. Coleman CI, Kreutz R, Sood NA, Bunz TJ, Eriksson D, Meinecke AK, et al. Rivaroxaban versus warfarin in patients with nonvalvular atrial fibrillation and severe kidney disease or undergoing hemodialysis. Am J Med. 2019;132:1078–83. http://dx.doi.org/10.1016/j.amjmed.2019.04.013.
- 39. Kuno T, Takagi H, Ando T, Sugiyama T, Miyashita S, Valentin N, et al. Oral anticoagulation for patients with atrial fibrillation on long-term hemodialysis. J Am Coll Cardiol. 2020;75:273–85. http://dx.doi.org/10.1016/j.jacc.2019.10.059.
- 40. De Vriese AS, Caluwé R, Bailleul E, De Bacquer D, Borrey D, Van Vlem B, et al. Dose-finding study of rivaroxaban in hemodialysis patients. Am J Kidney Dis. 2015;66:91–8. http://dx.doi.org/10.1053/j.ajkd.2015.01.022.
- 41. Becker RC, Spencer FA, Gibson M, Rush JE, Sanderink G, Murphy SA, et al. Influence of patient characteristics and renal function on factor Xa inhibition pharmacokinetics and pharmacodynamics after enoxaparin administration in non-ST-segment elevation acute coronary syndromes. Am Heart J. 2002;143:753–9. http://dx.doi.org/10.1067/mhj.2002.120774.
- 42. Gerlach AT, Pickworth KK, Seth SK, Tanna SB, Barnes JF. Enoxaparin and bleeding complications: a review in patients with and without renal insufficiency. Pharmacotherapy. 2000;20:771–5. http://dx.doi.org/10.1592/phco.20.9.771.35210.
- 43. Lim W, Crowther M, Wang L, Douketis J, Schnurr T, Moreau C, et al. Serial trough anti-Xa levels to assess low molecular weight heparin accumulation in patients with chronic kidney disease: analysis of CrCl <30 mL/min from the Trivel Study. Blood. 2016;128:90.
- 44. Kearon C, Ginsberg JS, Julian JA, Douketis J, Solymoss S, Ockelford P, et al. Comparison of fixed-dose weight-adjusted unfractionated heparin and low-molecular-weight heparin for acute treatment of venous thromboembolism. JAMA. 2006;296:935–42. http://dx.doi.org/10.1001/jama.296.8.935.
- 45. Metzger NL, Chesson MM. Subcutaneous unfractionated heparin for treatment of venous thromboembolism in end-stage renal disease. Ann Pharmacother. 2010;44:2023–7. http://dx.doi.org/10.1345/aph.1P403.
- 46. Montalescot G, Polle V, Collet JP, Leprince P, Bellanger A, Gandjbakhch I, et al. Low molecular weight heparin after mechanical heart valve replacement. Circulation. 2000;101:1083–6. http://dx.doi.org/10.1161/01.cir.101.10.1083.
- 47. Budisavljevic MN, Cheek D, Ploth DW. Calciphylaxis in chronic renal failure. J Am Soc Nephrol. 1996;7:978–82. http://dx.doi.org/10.1681/ASN.V77978.