Introduction
A significant proportion of patients, which can be as much as one-third, who undergo kidney replacement therapy will transfer from one dialysis modality to another []. The patients’ treatment pathway is now considered as “integrated care” and includes a succession of different dialysis modalities rather than a single one. According to the Renal Epidemiology Information Network (REIN) registry, 13% of incident patients on peritoneal dialysis (PD) were transferred to hemodialysis (HD) within the first 2 years, and 21% of patients treated by PD 2 years after RRT initiation had previously been treated by HD [, ].
Only a small proportion of patients switch from HD to PD. However, a report from the USA has shown that economic incentives to perform PD have increased PD uptake []. Interestingly, late PD use increased thanks to an increase in HD-to-PD switches. We can suppose that switch from HD to PD will become more frequent worldwide as payment systems for dialysis are about to change in several developed countries [, ].
There seem to be two different stages in HD to PD transition: one within the first 2 months, reflecting patients’ preferences, and a second one later on due to exhaustion of vascular access or bad tolerance of HD sessions [-]. There is controversy about the outcome of patients transferred from HD to PD in comparison with patients starting with PD as the first RRT []. Technique failure and mortality rates of patients transferred from HD to PD have been found to be higher than those of patients starting RRT directly on PD [, , , ]. However, other studies suggested that the outcomes of these patients did not differ from those of patients starting with PD as the first RRT [, , , ]. Notably, in most of these studies, patients transferred from HD to PD were defined as those who had been treated with HD for at least 3 months before switching to PD, without considering a shorter period of HD treatment before PD start. Only two of these studies were based on data from registries [, ], while others reported monocentric experiences. One could expect that the time spent on HD before transitioning to PD would impact PD outcomes; however, to our knowledge, this has never been described in the literature. Interestingly, when studying PD technique survival, the definition of the outcome of interest can change across the studies. Lan et al. [] proposed a standardized definition, using a composite endpoint of death or transfer to HD. To maximize the amount of information, death (transfer-to-HD censored) and transfer to HD (death censored) should be separately reported []. This registry-based study aimed to describe HD to PD switch in France and to report the effect of transitioning from HD on PD technique survival (death or retransfer to HD), death (retransfer-to-HD censored), and retransfer to HD (death censored), accounting for the effect of time spent on HD before transitioning to PD.
Materials and Methods
Study Population
This was a retrospective study using data from the REIN registry. All patients older than 18 years who began HD between 1 January 2008 and 31 December 2016 were extracted from the database. From those, patients who experienced a transfer from HD to PD during the observation period were included in the study. Of note, patients experiencing kidney recovery or kidney transplantation between HD and PD were not included in the study. The end of the study period was 31 December 2019.
Definition of Variables
The main explanatory variable, time spent on HD before transfer to PD, corresponded to the time between HD start and PD start, in months. Sex, age at HD start, body mass index (BMI), underlying nephropathy, diabetes mellitus, cardiovascular disease (defined as the following conditions: stroke or transient ischemic attack, dysrhythmia, peripheral vascular disease, coronary heart disease), congestive heart failure, chronic respiratory disease, cirrhosis, and active cancer were extracted from the database. Characteristics of HD treatment, including emergency start (defined as first dialysis session performed immediately, due to life-threatening condition: hyperhydration, hyperkalemia, acidosis, confusion, pericarditis, anemia), start on catheter, and erythropoiesis-stimulating agent (ESA) use at HD initiation, were also extracted. Concerning PD treatment, PD modality (continuous ambulatory PD or automated PD) and the use of assistance were obtained from the database. Underlying nephropathy is composed of the following classes: autosomal dominant polycystic kidney disease, diabetes, glomerulonephritis, vascular kidney disease, unknown, and others.
Events of Interest
All patients included in the study were followed until 31 December 2019 or the occurrence of any of the following events: death (including dialysis withdrawal), retransfer to HD, kidney transplantation, or kidney recovery. All HD retransfers longer than 2 months were reported as such.
Events of interest were PD cessation, studied by the occurrence of events of death on PD or retransfer to HD (composite endpoint), death on PD (retransfer-to-HD censored), and retransfer to HD (death censored). Competing events were transplantation and kidney function recovery. For each patient, survival time was defined by the length of time between PD initiation and any of the events of interest, competing events, or end of follow-up. Patients lost to follow-up were censored at the latest available date.
Statistical Analysis
Continuous variables were described by medians (first and third quartiles), while categorical variables were described by frequencies and percentages. Based on spline visualization from Cox model regression, age did not meet the assumption of log linearity and was divided into classes of clinical importance: 18–40 years old; 40–60 years old; 60–80 years old; and >80 years old.
Time spent on HD before transfer to PD was our main explanatory variable of interest. Regression splines were used to explore the possibility that it did not respect the log linearity assumption. Transformation of this continuous variable to a linear or categorical variable was performed based on the aspect of the graph of the functional form of the predictor [], and fractional polynomial transformation was explored [].
To explore the association between the time spent on HD and the events of interest, cause-specific hazard ratios (cs-HRs) were estimated using a Cox regression model. For competing events, subdistribution hazard ratios were obtained by performing a Fine and Gray competing risks regression model. The uncertainty of the results was expressed with 95% confidence intervals (CIs). For the cs-HR, the risk set decreases at each time point at which there is a PD cessation for a cause other than death or retransfer to HD (events that are not the events of interest are censored), measuring the specific association between the variable and the event of interest. For the sd-HR, patients experiencing PD cessation for an event other than the one under consideration remain in the risk set, assessing the net association between the variable and all possible events [, ]. All variables considered relevant were included a priori in the multivariate analyses. Interactions between age-sex, time in HD-age, and time in HD-cardiovascular disease were tested in the multivariate models.
Data were missing for several variables, with more than 10% missing values for three variables (BMI, ESA use at HD start, and nurse assistance). A complete case analysis would have excluded 836 patients (42%) from the dataset. Multiple imputation by chained equation was performed, imputing 50 sets of missing values []. A Cox model regression analysis on complete cases and then a pooling analysis of the estimate effect were conducted on the 50 imputed datasets. Complete case analysis was used for the Fine and Gray regression models. BMI, with 20% missing values, was excluded from this part of the analysis so that complete case analyses excluded 632 patients (32%). Analyses were performed with R software, version 3.6.3 (R Foundation for Statistical Computing, Vienna, Austria, including the survival and cmrpsk packages).
Results
Patient Characteristics
Of the 77,587 patients who started HD between 2008 and 2016, 1,985 (3%) were transferred to PD. We excluded 469 patients under 18 years of age.
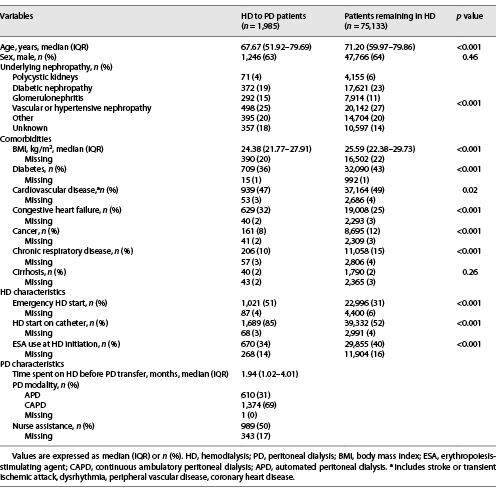
Patient characteristics at baseline are presented in Table 1. HD to PD patients, compared to patients who remained on HD, seemed to be younger (median age 67.67 vs. 71.2 years, respectively) and less comorbid. They also started HD in emergency situations (51 vs. 31%) and on catheters (85 vs. 52%) more frequently and had less ESA at HD initiation (34 vs. 40%). Other characteristics were comparable, including the frequency of cardiovascular disease.
The 75,133 patients not experiencing transfer to PD were removed for the subsequent analysis (online suppl. Fig. 1; see http://www.karger.com/doi/10.1159/000524960 for all online suppl. material). For the 1,985 patients included in our study, the median time spent on HD before transfer to PD was 1.94 months (interquartile range [IQR] 1.02–4.01). Continuous ambulatory PD was the modality used by 1,374 (69%) patients, and 989 (50%) were nurse-assisted. Among these HD to PD transitions, 1,344 (68%) occurred within the first 3 months on HD. The distribution of time spent on HD is shown in Figure 1.
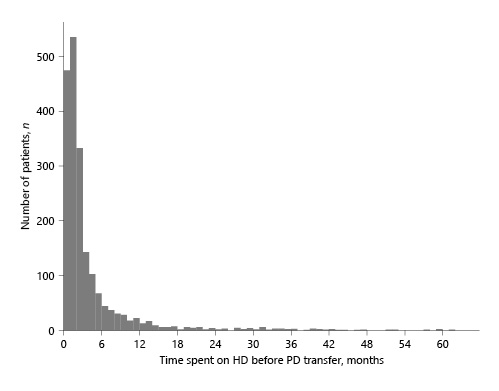
Fig. 1
Distribution of time spent on HD before transfer to PD (<60 months). HD, hemodialysis; PD, peritoneal dialysis.
Patients’ characteristics according to the time spent on HD are described in online supplementary Table 1. Patients who stayed in HD for less than 3 months were more often suffering from heart failure (35 vs. 25%), started HD as an emergency (55 vs. 43%) and were more frequently assisted once in PD (52 vs. 45%).
Causes of PD Cessation
During the study period, there were 732 (37%) deaths, 732 (37%) retransfers to HD, 313 (16%) transplants, and 62 (3%) kidney function recoveries. At the end of the observation period, 140 (7%) patients were still on PD. Only 6 patients were lost to follow-up. The cumulative incidental functions of these events are presented in Figure 2.
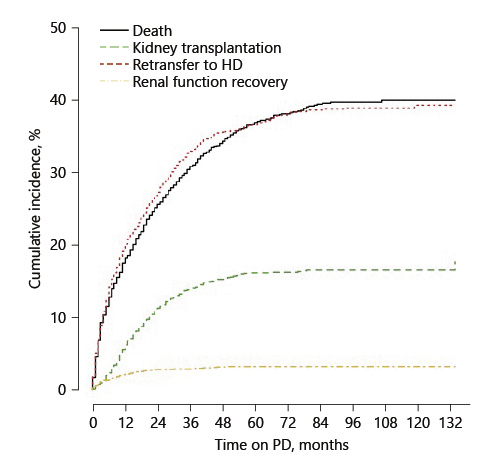
Fig. 2
Cumulative incidence functions. HD, hemodialysis; PD, peritoneal dialysis.
PD Cessation for Death or Retransfer to HD (Composite Endpoint)
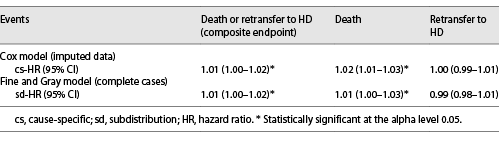
The median time until death or retransfer to HD was 20 (IQR 18–21) months (online suppl. Fig. 2). In the multivariable analysis (Fig. 3a), time spent on HD before transfer to PD was significantly associated with a higher occurrence of death or retransfer to HD (cs-HR 1.01, 95% CI: 1.00–1.02 for 1-month increase). It was linearly modeled, without polynomial transformation being required for the variable time spent on HD. When considering competing events, time spent on HD before transfer to PD remained associated with the composite endpoint (sd-HR 1.01, 95% CI: 1.00–1.02).
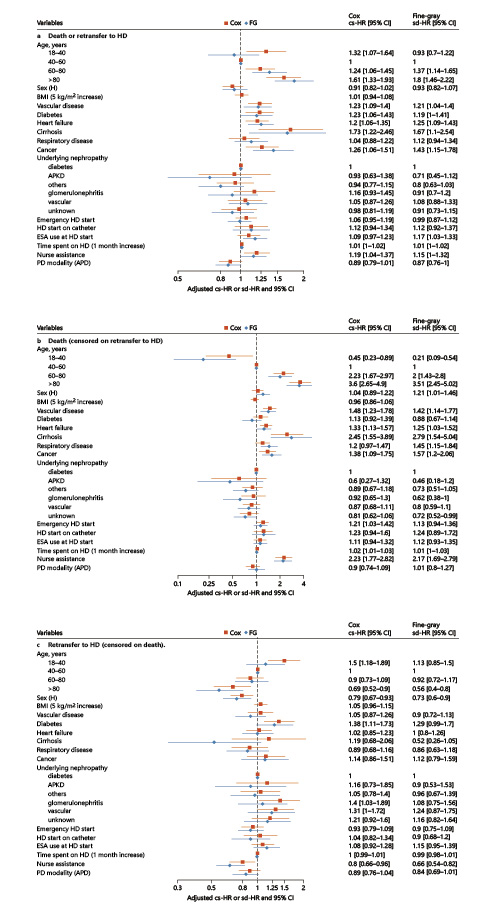
Fig. 3
Forest plots representing multivariate Cox and Fine-Gray models for the 3 events of interest. a Death or retransfer to HD (composite endpoint). b Death. c Retransfer to HD. HD, hemodialysis; PD, peritoneal dialysis; FG, Fine and Gray; HR, hazard ratio; CI, confidence interval; cs, cause specific; sd, subdistribution; BMI, body mass index; APKD, autosomal dominant polycystic kidney disease; ESA, erythropoiesis-stimulating agent; APD, automated peritoneal dialysis.
PD Cessation for Death (Censored on Retransfer to HD)
Time spent on HD before transfer to PD was significantly associated with death censored on retransfer to HD (cs-HR 1.02, 95% CI: 1.01–1.03 for 1-month increase) after adjustment for other variables (Fig. 3b). Again, it was modeled linearly across all imputed datasets.
Age was strongly associated with death as well as comorbidities. Nurse assistance was associated with death even after adjustment for comorbidities (cs-HR 2.23, 95% CI: 1.77–2.82).
Unplanned HD start markers seemed to be associated with death and included the following: emergency HD start (cs-HR 1.21, 95% CI: 1.03–1.42) and HD start on catheter (cs-HR 1.23, 95% CI: 0.94–1.6). Cox and Fine and Gray regression models provided similar results.
PD Cessation for HD Retransfer (Censored on Death)
Time spent on HD before PD was not associated with retransfer to HD censored on death (cs-HR 1.00, 95% CI: 0.99–1.01 for 1-month increase) in the multivariate analysis. Once again, no polynomial transformation was retained. Table 2 summarizes the HR of time spent on HD on the events of interest.
Diabetes was a risk factor for retransfer to HD (cs-HR 1.38, 95% CI: 1.11–1.73). Age, nurse assistance (cs-HR 0.80, 95% CI: 0.66–0.96), and female sex (cs-HR 0.79 95% CI: 0.67–0.93) were protective factors for retransfer to HD. Considering competing events did not change these associations. The results are shown in Figure 3c.
All interactions tested on multivariate models across the 3 events of interest were not significant. Notably, the results of Cox model regressions on complete cases and imputed analyses were similar (online suppl. Fig. 3).
Discussion
The transition from HD to PD remains an understudied event in the integrated management of kidney failure. We have shown that the time spent on HD before transfer to PD is associated with an increased risk of PD cessation due to death or retransfer to HD (composite endpoint) and death (retransfer-to-HD censored).
In the few studies describing this transition, time spent on HD before PD is usually considered as a categorical variable divided into early and late transfers. The threshold used to distinguish them is often 3 months. The motivation for the transfer seems to be drastically different depending on its timing. Early transfers may be motivated by the patient’s choice in a context of unplanned arrivals, whereas late transfers may result from exhaustion of vascular access, bad tolerance of HD sessions, or by choice [-]. Our study, by investigating several statistical possibilities of modeling the time spent on HD before transfer to PD, questioned this concept. It appears that linear modeling best described the association between the time spent on HD before transfer and PD outcomes. We can thus assume that the effect of the time spent on HD before transfer on the risk of PD cessation appears from the initiation of HD and increases gradually over time. If a transfer to PD is considered for a patient on HD, it should be prepared and performed as soon as possible after HD initiation to limit the time spent on HD.
Another particularity of our study lies in the fact that all patients transferred from HD to PD were included in our study, regardless of the time spent on HD, while the majority of the previous studies excluded patients staying less than 3 months on HD [, , , , ]. The majority (68%) of our transfers occurred early, within the first 3 months after HD initiation. This result is consistent with the results described by Nessim et al. [], where the median time on HD before transfer to PD was 83 days. It could reflect a lack of preparation for RRT since compared to patients remaining on HD, our HD to PD population started HD more frequently in emergency (51 vs. 31%) and catheter (85 vs. 52%), with a lower use of ESA at HD initiation (34 vs. 40%). Excluding early transfers could have led to a significant underestimation of the incidence of HD-to-PD transfer and its underlying effects. In our opinion, future research should include HD-to-PD transfers regardless of the time spent on HD.
We have shown that the time spent on HD before transfer to PD was associated with an increased risk of death, which could be explained by several reasons. First, one can argue that the transition between dialysis modalities is a period at risk and could have a direct impact on patient survival. In a previous study from our team, unplanned transitions from PD to HD were marked by 100% inpatient admissions and 24% deaths, highlighting the risk of transition periods []. Although our study did not compare the outcome of PD-first patients versus HD-to-PD patients, the fact that time spent on HD before transfer to PD was linearly associated with PD outcomes suggests not only an effect of the transition but also an effect of the time spent on HD. Second, the fact that the time spent on HD before transfer to PD is associated with an increased risk of death (cs-HR of 1.02, 95% CI: 1.01–1.03 for each 1 month spent on HD) could reflect a specific effect of an increased total exposure to RRT. It is acknowledged that time spent on HD is a risk factor for mortality, with a relative risk of death between 1.02 and 1.07 for 1 year spent on HD [-]. The survival on HD and PD is considered similar []. It can therefore be assumed that PD vintage has the same effect on mortality as HD vintage. When we consider our results on the same scale, we get an increased relative risk of death of 1.24 (95% CI: 1.11–1.38) for each 1 year spent on HD before PD transfer. Indeed, a vintage effect cannot be ruled out, but we believe that the time spent on HD before transfer has its own effect on mortality.
Surprisingly, time spent on HD was not associated with retransfer to HD (cs-HR 1.00, 95% CI: 0.99–1.01) in our study. When considering retransfer, Nessim et al. [] described a higher risk of PD technique failure for patients transferred from HD to PD compared to patients starting RRT with PD (HR 1.37, 95% CI: 1.26–1.49). Similar results have been described in a previous study from our team []. As previous studies have mostly investigated the impact of early transfer that is driven by unplanned arrival, this may explain the difference with our study. Residual kidney function is modified by time spent on HD and would be an important factor to study in this context [-]. Unfortunately, we were not able to capture this information in the present study. One could expect that access to kidney transplantation impacts PD survival. We accounted for this hypothesis by considering transplantation as a competing event and performed a Fine and Gray model, which did not change the lack of association observed between time spent on HD before PD and retransfer to HD (sd-HR 0.99, 95% CI: 0.98–1.01).
In our study, nurse assistance was significantly associated with a higher risk of mortality but a decreased risk of retransfer to HD, particularly in the population aged over 80 years. Similar results have been previously described [-] and are likely a reflection of increased comorbidity and frailty. One could also expect clinicians to be less likely to suggest a retransfer to HD for frail and dependent patients to favor quality of life on home dialysis.
We also reported a trend toward mortality in cases of unplanned arrival in HD, with cs-HRs 1.21 (95% CI: 1.03–1.42) and 1.23 (95% CI: 0.94–1.6) for an emergency start and HD start on catheter, respectively. These elements have previously been described [] and underline the importance of predialysis care.
Our study has some limitations. In the REIN registry, the collection of changes in dialysis modalities of less than 2 months is not exhaustive, which may lead to an underestimation of the number of patients transferred from HD to PD and of the different HRs. Residual kidney function, peritonitis, and ultrafiltration failure were not available in the registry.
In conclusion, this study shows that the transition from HD to PD is a rare event in France since only 3% of patients beginning on HD were transferred to PD in our study. It happens early during RRT, mostly within the first 3 months. Our results appear reassuring in that the median survival time on PD after transitioning was 20 months (IQR 18–21) when considering the composite endpoint (death or retransfer to HD). We found that time spent on HD before transfer to PD impacts patient survival but does not impact retransfer to HD and that the effect of the time spent on HD on the risk of PD cessation seems to increase gradually over time. We therefore think that it will be interesting to further study the causes of transitioning from HD to PD and the factors associated with better outcomes to identify the patients who would benefit from this strategy.
Acknowledgments
The authors thank all REIN registry participants, especially the nephrologists and professionals in charge of data collection and quality control. Dialysis units participating in the registry are listed in the REIN annual report (https://www.agence-biomedecine.fr/Les-chiffres-du-R-E-I-N).
Statement of Ethics
Not concerned. All participants provided consent for the collection of their data by the REIN registry.
Conflict of Interest Statement
C.B. is member of the ISPD International Liaison Committee. The results presented in this paper have not been published previously in whole or part.
Funding Sources
None declared.
Author Contributions
B.L. participated in design, analysis and interpretation of data, and drafting the article. C.B. and A.B. participated in design, interpretation of data, and revising the article. T.L. participated in providing intellectual content of critical importance and revising the article. A.L. participated in providing intellectual content of critical importance and drafting the article. C.C. and M.L. participated in conception, design, interpretation of data, and providing intellectual content of critical importance. I.K. participated in conception of data.
Data Availability Statement
All data used for this research were extracted from the REIN registry, coordinated and supported by the French Biomedecine Agency. The access to national data is regulated by a scientific committee of the French Biomedecine Agency which analyzes each request, and so cannot be made publicly available due to legal restrictions. Data are available upon request.
References
- 1. Chan C, Combes G, Davies S, Finkelstein F, Firanek C, et alINTEGRATED Group consists of (in alphabetical order). Transition between different renal replacement modalities: gaps in knowledge and care-the integrated research initiative. Perit Dial Int. 2019 Feb;39(1):4–12.
- 2.
- 3. Sloan CE, Coffman CJ, Sanders LL, Maciejewski ML, Lee SYD, Hirth RA, et al. Trends in peritoneal dialysis use in the United States after medicare payment reform. Clin J Am Soc Nephrol. 2019 Dec;14(12):1763–72.
- 4. Blake PG, McCormick BB, Taji L, Jung JK, Ip J, Gingras J, et al. Growing home dialysis: the Ontario renal network home dialysis initiative 2012–2019. Perit Dial Int. 2021 Sep;41(5):441–52.
- 5. Van Biesen W, Vanholder RC, Veys N, Dhondt A, Lameire NH. An evaluation of an integrative care approach for end-stage renal disease patients. J Am Soc Nephrol. 2000 Jan;11(1):116–25. http://dx.doi.org/10.1681/asn.v111116.
- 6. Zhang L, Cao T, Li Z, Wen Q, Lin J, Zhang X, et al. Clinical outcomes of peritoneal dialysis patients transferred from hemodialysis: a matched case-control study. Perit Dial Int. 2013 Jun;33(3):259–66.
- 7. Nguyen ANL, Prasad Kafle M, Sud K, Lee VW. Predictors and outcomes of patients switching from maintenance haemodialysis to peritoneal dialysis in Australia and New Zealand: strengthening the argument for “peritoneal dialysis first” policy. Nephrology. 2019 Sep;24(9):958–66.
- 8. Barone RJ, Cámpora MI, Gimenez NS, Ramirez L, Panese SA, Santopietro M. Peritoneal dialysis as a first versus second option after previous haemodialysis: a very long-term assessment. Int J Nephrol. 2014;2014:693670. http://dx.doi.org/10.1155/2014/693670.
- 9. Najafi I, Hosseini M, Atabac S, Sanadgol H, Majelan NN, Seirafian S, et al. Patient outcome in primary peritoneal dialysis patients versus those transferred from hemodialysis and transplantation. Int Urol Nephrol. 2012 Aug;44(4):1237–42.
- 10. Koc Y, Unsal A, Basturk T, Sakaci T, Ahbap-Dal E, Sinangil-Arar A, et al. Is there impact of mortality prior hemodialysis therapy in peritoneal dialysis patients?Nefrologia. 2012 May;32(3):335–42.
- 11. Liberek T, Renke M, Skonieczny B, Kotewicz K, Kowalewska J, Chmielewski M, et al. Therapy outcome in peritoneal dialysis patients transferred from haemodialysis. Nephrol Dial Transplant. 2009 Sep;24(9):2889–94.
- 12. Wang J, Zeng J, Liu B, Cai B, Li Y, Dong L. Outcomes after transfer from hemodialysis to peritoneal dialysis vs peritoneal dialysis as initial therapy: a systematic review and meta-analysis. Semin Dial. 2020 Jul;33(4):299–308. http://dx.doi.org/10.1111/sdi.12896.
- 13. Nessim SJ, Bargman JM, Jassal SV, Oliver MJ, Na Y, Perl J. The impact of transfer from hemodialysis on peritoneal dialysis technique survival. Perit Dial Int. 2015 Jun;35(3):297–305. http://dx.doi.org/10.3747/pdi.2013.00147.
- 14. Béchade C, Guittet L, Evans D, Verger C, Ryckelynck JP, Lobbedez T. Early failure in patients starting peritoneal dialysis: a competing risks approach. Nephrol Dial Transplant. 2014 Nov;29(11):2127–35. http://dx.doi.org/10.1093/ndt/gft055.
- 15. Lan PG, Clayton PA, Johnson DW, McDonald SP, Borlace M, Badve SV, et al. Duration of hemodialysis following peritoneal dialysis cessation in Australia and New Zealand: proposal for a standardized definition of technique failure. Perit Dial Int. 2016 12;36(6):623–30.
- 16. Royston P, Parmar MK. Flexible parametric proportional-hazards and proportional-odds models for censored survival data, with application to prognostic modelling and estimation of treatment effects. Stat Med. 2002 Aug;21(15):2175–97. http://dx.doi.org/10.1002/sim.1203.
- 17. Jansen JP. Network meta-analysis of survival data with fractional polynomials. BMC Med Res Methodol. 2011 May;11:61. http://dx.doi.org/10.1186/1471-2288-11-61.
- 18. Lau B, Cole SR, Gange SJ. Competing risk regression models for epidemiologic data. Am J Epidemiol. 2009 Jul;170(2):244–56. http://dx.doi.org/10.1093/aje/kwp107.
- 19. Fine JP, Gray RJ. A proportional hazards model for the subdistribution of a competing risk. J Am Stat Assoc. 1999 Jun;94(446):496–509. http://dx.doi.org/10.1080/01621459.1999.10474144.
- 20. White IR, Royston P, Wood AM. Multiple imputation using chained equations: issues and guidance for practice. Stat Med. 2011 Feb;30(4):377–99. http://dx.doi.org/10.1002/sim.4067.
- 21. Boissinot L, Landru I, Cardineau E, Zagdoun E, Ryckelycnk JP, Lobbedez T. Is transition between peritoneal dialysis and hemodialysis really a gradual process?Perit Dial Int. 2013 Aug;33(4):391–7. http://dx.doi.org/10.3747/pdi.2011.00134.
- 22. Sumida K, Yamagata K, Iseki K, Tsubakihara Y. Different impact of hemodialysis vintage on cause-specific mortality in long-term hemodialysis patients. Nephrol Dial Transplant. 2016 Feb;31(2):298–305. http://dx.doi.org/10.1093/ndt/gfv402.
- 23. Iseki K, Tozawa M, Takishita S. Effect of the duration of dialysis on survival in a cohort of chronic haemodialysis patients. Nephrol Dial Transplant. 2003 Apr;18(4):782–7. http://dx.doi.org/10.1093/ndt/gfg145.
- 24. Chertow GM, Johansen KL, Lew N, Lazarus JM, Lowrie EG. Vintage, nutritional status, and survival in hemodialysis patients. Kidney Int. 2000 Mar;57(3):1176–81. http://dx.doi.org/10.1046/j.1523-1755.2000.00945.x.
- 25. Yeates K, Zhu N, Vonesh E, Trpeski L, Blake P, Fenton S. Hemodialysis and peritoneal dialysis are associated with similar outcomes for end-stage renal disease treatment in Canada. Nephrol Dial Transplant. 2012 Sep;27(9):3568–75. http://dx.doi.org/10.1093/ndt/gfr674.
- 26. Imbeault B, Nadeau-Fredette AC. Optimization of dialysis modality transitions for improved patient care. Can J Kidney Health Dis. 2019;6:2054358119882664. http://dx.doi.org/10.1177/2054358119882664.
- 27. Bargman JM, Thorpe KE, Churchill DN. Relative contribution of residual renal function and peritoneal clearance to adequacy of dialysis: a reanalysis of the CANUSA study. J Am Soc Nephrol. 2001 Oct;12(10):2158–62. http://dx.doi.org/10.1681/ASN.V12102158.
- 28. Perl J, Bargman JM. The importance of residual kidney function for patients on dialysis: a critical review. Am J Kidney Dis. 2009 Jun;53(6):1068–81. http://dx.doi.org/10.1053/j.ajkd.2009.02.012.
- 29. Misra M, Vonesh E, Van Stone JC, Moore HL, Prowant B, Nolph KD. Effect of cause and time of dropout on the residual GFR: a comparative analysis of the decline of GFR on dialysis. Kidney Int. 2001 Feb;59(2):754–63. http://dx.doi.org/10.1046/j.1523-1755.2001.059002754.x.
- 30. Jansen MAM, Hart AAM, Korevaar JC, Dekker FW, Boeschoten EW, Krediet RT, et al. Predictors of the rate of decline of residual renal function in incident dialysis patients. Kidney Int. 2002 Sep;62(3):1046–53.
- 31. Termorshuizen F, Korevaar JC, Dekker FW, van Manen JG, Boeschoten EW, Krediet RT, et al. The relative importance of residual renal function compared with peritoneal clearance for patient survival and quality of life: an analysis of the Netherlands Cooperative Study on the Adequacy of Dialysis (NECOSAD )-2. Am J Kidney Dis. 2003 Jun;41(6):1293–302.
- 32. Lobbedez T, Verger C, Ryckelynck JP, Fabre E, Evans D. Is assisted peritoneal dialysis associated with technique survival when competing events are considered?Clin J Am Soc Nephrol. 2012 Apr;7(4):612–8. http://dx.doi.org/10.2215/CJN.10161011.
- 33. Béchade C, Lobbedez T, Ivarsen P, Povlsen JV. Assisted peritoneal dialysis for older people with end-stage renal disease: the French and Danish experience. Perit Dial Int. 2015 Nov;35(6):663–6. http://dx.doi.org/10.3747/pdi.2014.00344.
- 34. Lanot A, Bechade C, Boyer A, Ficheux M, Lobbedez T. Assisted peritoneal dialysis and transfer to haemodialysis: a cause-specific analysis with data from the RDPLF. Nephrol Dial Transplant. 2021 Jan;36(2):330–9. http://dx.doi.org/10.1093/ndt/gfaa289.
- 35. Ravani P, Palmer SC, Oliver MJ, Quinn RR, MacRae JM, Tai DJ, et al. Associations between hemodialysis access type and clinical outcomes: a systematic review. J Am Soc Nephrol. 2013 Feb;24(3):465–73.
Dr. Annabel Boyer and Dr. Clémence Béchade have contributed equally to this work.