Homozygous familial hypercholesterolemia (HoFH) is a rare genetic disorder characterised by defective clearance of low-density lipoproteins (LDLs). This results in extreme elevations in LDL-cholesterol (LDL-C) from birth, leading to an increased risk of premature atherosclerotic cardiovascular disease (ASCVD) and cardiovascular morbidity and mortality (). The disorder is estimated to affect nearly one in 300,000 individuals globally ().
The genetic variation in HoFH can arise from having mutations in genes with autosomal dominant inheritance; namely the LDL receptor (LDLR) gene, the apolipoprotein B (APOB) gene, or the protein convertase subtilisin/kexin type 9 (PCSK9), or more rarely recessive inheritance mutations of the LDLR adaptor protein 1 (LDRAP1) gene (). True homozygotes carry identical mutations in both alleles of the same gene, while compound heterozygotes have different mutations in the alleles of the same gene. Double heterozygotes, on the other hand, have mutations in two separate genes (,). In up to 90% of cases, the gene mutations involve the LDLR gene, directly affecting LDLR activity, and hence hepatic LDL-C uptake and clearance. Affected patients may either exhibit residual LDLR activity (defective/null or defective/defective variants) or almost no (<2%) LDLR activity (null/null variants) ().
The management of HoFH aims to reduce the cumulative burden of elevated LDL-C exposure, hence early and sustained normalisation of LDL-C with effective lipid-lowering therapy (LLT) is central to the prevention of the major clinical sequelae in patients with HoFH (). As such, patients with HoFH without ASCVD are considered high-risk by the European Society of Cardiology (ESC) and the European Atherosclerosis Society (EAS), with a recommended LDL-C target of <1.8 mmol/L. Patients with HoFH with prior ASCVD are considered very high-risk by the same guidelines, with an even more stringent target of <1.4 mmol/L. The treatment target for children and adolescents with FH is <3.5 mmol/L (). These targets are, however, difficult to achieve in patients with HoFH ().
Recently, Gaudet et al. published findings from their phase 3, single-arm trial investigating the safety and efficacy of the LLT evinacumab in 116 patients with HoFH across 12 countries (). Below, we comment on the findings of this trial in the context of the current landscape of LLTs available to patients with HoFH. Current LLT includes both conventional and emerging treatments, which involve LDLR-dependent and LDLR-independent approaches () (see Figure 1). LDLR-dependent therapies include statins, which inhibit 3-hydroxy-3-methylglutaryl coenzyme A (HMG-CoA) to reduce hepatic cholesterol synthesis and PCSK9 inhibitors, which enhance LDLR recycling (). Ezetimibe, which blocks Niemann Pick C1-like 1 (NPC1L1) protein to inhibit intestinal cholesterol absorption, may secondarily upregulate LDLR activity due to reduced hepatic cholesterol levels (). Statins and PCSK9 inhibitors, therefore, rely to some extent on residual LDLR activity in patients with HoFH for efficacy (). For instance, statins are first-line therapy in all patients in current guidelines; hence, the first recommendation to achieve a ≥50% reduction from baseline LDL-C levels for adults with or without ASCVD effectively means that atorvastatin 40 or 80 mg or rosuvastatin 20 or 40 mg should be used as these doses most closely approximate to 50% LDL-C reduction in clinical trials (,). These therapies are more effective in patients with defective/defective or defective/null variants than in those with null/null variants who experience, at best, minimal LDL-C reduction, hence the need for combination therapies ().
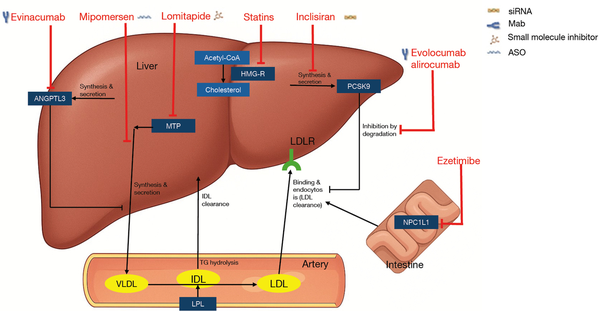
Figure 1
Lipid lowering agents used for homozygous FH treatment and their mode of action (). Redrawn from Kayikcioglu and Tokgozoglu [2022] (). Under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/). ANGPTL3, angiopoietin-like protein 3; ASO, antisense oligonucleotide; CoA, coenzyme A; FH, familial hypercholesterolemia; HMG-R, 3-hydroxy-3-methylglutaryl reductase; IDL, intermediate density lipoprotein; LDL, low-density lipoprotein; LDLR, low-density lipoprotein receptor; LPL, lipoprotein lipase; Mab, monoclonal antibody; MTP, microsomal triglyceride transfer protein; NPC1L1, Niemann Pick C1-like 1; PCSK9, proprotein convertase subtilisin/kexin type 9; siRNA, small interference RNA; TG, triglycerides; VLDL, very low-density lipoprotein.
LDLR-dependent LLTs
Statins, often the initial therapy in patients with HoFH, provide in practice minimal LDL-C reductions in patients with HoFH, typically by 10–25%, even at high doses or potency (). The addition of ezetimibe can decrease LDL-C levels by an additional 10–15%, resulting in an overall LDL-C reduction of 30–40% with combination therapy. The statin-ezetimibe combination is generally well-tolerated in patients with HoFH and is, by and large, cost-effective (). Monoclonal antibodies (Mabs) against PCSK9 approved for HoFH include evolocumab and alirocumab, which have shown LDL-C reductions of 30.9% and 35.6%, respectively, in clinical trials (,). In an evolocumab trial, the LDL-C reduction from baseline was higher for patients with defective/defective variants (31.8%) than for those with defective/null variants (21.0%). The only study patient with null/null status had a 10.3% increase in LDL-C from baseline. Evolocumab also provided a 55.7% reduction from baseline for genotypic double heterozygous (LDLR) patients (). Conversely, adnectins (LIB003) and small interference RNA (inclisiran) against PCSK9, despite being highly efficacious in general dyslipidaemias, demonstrated minimal or no reduction of LDL-C in HoFH (,). It is uncertain why adnectins appear to be less effective than Mabs as, in theory, they should bind all circulating PCSK (irrespective of source). By contrast, inclisiran only reduces hepatic production of PCSK9, which accounts for about 80% of total circulating PCSK; hence the extra 20% lowering of PCSK9 with Mabs may be biologically relevant in those with little or no LDLR activity. There were no severe adverse events or safety concerns in PCSK9 inhibitor trials (). Combination (LLT), including statins, ezetimibe, and PCSK9 inhibitors, has therefore become the bedrock of pharmaceutical HoFH management and is estimated to cause up to 60% reduction in LDL-C (,). Nevertheless, significant delays occur in initiating this therapy at HoFH diagnosis. Given that the average LDL-C level in patients with HoFH is approximately 14 mmol/L, it means that even a 60% lowering in LDL-C leaves the vast majority of these individuals far from recommended goals. As a result, these patients remain at risk of ASCVD, underscoring the need for early and aggressive lipid-lowering management ().
LDLR-independent LLTs
The limitations of LLT combinations involving statins, ezetimibe, and PCSK9 inhibitors have underscored the need for innovative, LDLR-independent therapies to meet essential LDL-C treatment targets in patients with HoFH, particularly in those with null/null variants (). LDLR-independent pathways include extracorporeal removal of LDL-C, reduction of LDL-C production, or increased LDL-C clearance through LDLR-independent pathways (). Lipoprotein apheresis provides extracorporeal extraction of LDL-C from the circulation (). Therapies targeting the reduction of LDL-C production include lomitapide, which inhibits microsomal triglyceride transfer protein (MTP) to suppress hepatic very LDL (VLDL) assembly, and evinacumab, a Mab against angiopoietin-like protein 3 (ANGPTL3), which facilitates non-LDLR-dependent LDL-C clearance by enhancing lipoprotein lipase activity (,). These therapies provide options for patients with severely impaired or virtually no LDLR activity, addressing one of the critical challenges in the management of HoFH ().
Lipoprotein apheresis has long been used alongside conventional LLT and can reduce LDL-C by 50–70% post apheresis session with average interval reductions of 20–36% depending on treatment frequency and baseline values. However, it is invasive, typically required twice a month, costly, and its use is limited in many countries, especially low-income countries (). Lomitapide has been shown to reduce LDL-C by about 50% relative to baseline. However, since it acts by suppressing hepatic VLDL assembly, its use is often limited by hepatic fat accumulation, gastrointestinal side effects, and the necessity for adopting a low-fat diet. As a result, dosing is often reduced in real-world settings, with observed LDL-C reduction of approximately 33% ().
Evinacumab is a fully human Mab that targets ANGPTL3, thereby enhancing lipoprotein lipase and endothelial lipase activity, leading to significant reductions in LDL-C and other atherogenic lipoproteins independent of LDLR function (). The past 5 years have seen several studies of evinacumab of various durations in adults and children (Table 1).
Table 1
Summary of key evinacumab studies in HoFH patients
| Author | Publication year | Study title | Study design | Sample size, n | Age (years) | Duration (weeks) | Overall LDL-C reduction from baseline | LDL-C reduction in null/null | LDL-C reduction in non-null | Reported AEs |
|---|---|---|---|---|---|---|---|---|---|---|
| Raal et al. () | 2020 | Evinacumab for homozygous familial hypercholesterolemia (ELIPSE HoFH) | Double-blind, placebo-controlled phase 3 trial | 64 | ≥12 | 24 | 47.1% | 43.4% | 49.1% | Nasopharyngitis, influenza-like illness, headache, rhinorrhoea, gastroenteritis |
| Stefanutti et al. () | 2022 | The long-term efficacy and safety of evinacumab in patients with homozygous familial hypercholesterolemia: real-world clinical experience | Real-world study | 7 | ≥14 | 104 | 46.8% | NA | NA | NA |
| Raal et al. () | 2023 | The long-term efficacy and safety of evinacumab in patients with homozygous familial hypercholesterolemia | Open-label Phase 3 trial (ELIPSE extension) | 64 | ≥12 | 24 | 46.3% | 47.2% | 45.9% | Headache, nasopharyngitis, back pain, nausea, asthenia, influenza-like illness |
| Béliard et al. () | 2024 | Evinacumab and cardiovascular outcome in patients with homozygous familial hypercholesterolemia (OLE ELIPSE HoFH) | Open-label phase 3 trial (ELIPSE extension) | 12 | ≥12 | 24 | 54% | 52% | 56% | Face swelling, paraesthesia of the hand and feet, headache, fatigue |
| Wiegman et al. () | 2024 | Evinacumab for pediatric patients with homozygous familial hypercholesterolemia | Open-label, phase 3 trial | 14 | 5–11 | 24 | 48.3% | 45.5% | 32.3% defective/defective | Oropharyngeal pain, upper abdominal pain, diarrhoea, nausea, vomiting, nasopharyngitis |
| 66.7% defective/null | ||||||||||
| Gaudet et al. () | 2024 | Evinacumab in homozygous familial hypercholesterolemia: long-term safety and efficacy | Open-label, single-arm, phase 3 trial | 116 | ≥12 | 104 | 43.6% | 49.0% | 41.4% | Nasopharyngitis, coronavirus disease 2019, headache, influenza-like illness, nausea, diarrhoea, asthenia, face oedema |
In the pivotal phase 3 ELIPSE HoFH trial, evinacumab resulted in a 49% reduction in LDL-C levels compared to placebo at week 24, with no significant difference in adverse events between the two groups (). Substantial LDL-C reduction with evinacumab occurred both in patients with null/null variants (43.1%) and those with non-null variants (49.1%). LDL-C reduction in the evinacumab group was reported by individual genotypic status. For individuals who were true homozygotes for LDLR or compound heterozygotes for LDLR, LDL-C reduction ranged from approximately 20% to 80%. A single patient who was a homozygote for LDLRAP1 had an LDL-C reduction of about 40%, while reductions ranged from 30% to 80% in patients who were double heterozygotes for LDLR and APOB (). Another phase 3 open-label, single-arm clinical trial conducted in children aged 5 to 11 years showed an LDL-C reduction of 48% with a safety profile similar to that in adults from the ELIPSE trial. In this trial, 71.4% of the patients were compound heterozygotes, and 28.6% were true homozygotes (LDLR). LDL-C reduction was 32.3% in the defective/defective group, 66.6% in the defective/null group, and 45.5% in the null/null group ().
Based on these findings, evinacumab received authorization in 2021 from the European Medicines Agency (EMA) and Food and Drug Administration (FDA) for use in adolescents aged ≥12 years and adults; in 2023, the FDA approval was extended for use in children aged 5–11 years (). However, the clinical trials that resulted in these approvals comprised relatively small sample sizes (under 80 participants across both the adult and children’s trials) and only lasted 24 weeks. Larger studies on the safety and longer-term efficacy of evinacumab were the next step to provide information on repeat exposure and any potential rare adverse effects which may only emerge over time.
Consequently, three studies investigated the long-term efficacy and safety of evinacumab in patients with HoFH (). One was a phase 3 open-label single-arm continuation of the ELIPSE study, which included 64 patients treated for 24 weeks (). The second study was also an open-label extension of the ELIPSE trial but included both newly diagnosed patients with HoFH and those who completed the ELIPSE trial, with a sample size of 12 patients over a 6-month period (). The third was a real-world study comprising seven patients followed up for 36 months (). All these studies’ efficacy and safety findings were largely similar to the ELIPSE trial () findings.
Evinacumab in homozygous familial hypercholesterolaemia: long-term safety and efficacy (Gaudet et al., 2024) ()
This was an open-label, single-arm, phase 3 trial () with a larger sample size (n=116) than the preceding three long-term studies combined (), lending it a higher power and greater generalisability of findings on evinacumab efficacy and long-term safety. The study included both patients naïve to evinacumab and those who had prior evinacumab exposure in previous clinical trials (labelled evinacumab-continue). The genotypic distribution of both groups was 45.7% homozygous (LDLR), 1.7% homozygous (LDLRAP1), 35.3% compound heterozygotes (LDLR), and 0.9% double heterozygotes (LDLR and APOB). All participants received monthly intravenous injections of evinacumab at 15 mg/kg, and the median treatment duration was 104 weeks ().
LDL-C reduction was 43.6% across all participants at week 24, and this was maintained at week 48. A higher reduction was noted in the evinacumab-naïve group (47.8%) vs. the evinacumab-continue group (41.3%) (). The LDL-C reduction in the evinacumab-naïve group (47.8% at week 24) was similar to that observed in the ELIPSE trial, where LDL-C reduction was 47.1% in the evinacumab group (,).
Null/null patients experienced similar LDL-C reductions (49.0%) as non-null patients (41.4%). While the numerically higher LDL-C reduction in the null/null patients is an interesting finding, the reasons for this should be further investigated. It is also important to note that adherence to background therapy at baseline and during follow-up affects the interpretation of the LDL-C change from baseline since such analyses assume constant background adherence. It is possible that in the open-label Gaudet et al. trial, as news of earlier evinacumab trial results filtered through to the HoFH patient population, compliance with other LLTs was reduced especially in the non-null group who might have been aware of their less severe phenotype and anticipated substantial LDL-C lowering with evinacumab alone (). In the ELIPSE trial, patients with non-null variants achieved greater LDL-C reduction (49.1%) than those with null/null variants (43.4%) (). In the 24-week open-label treatment period following the ELIPSE trial, the null/null (47.2%) and the non-null (45.9%) groups had similar LDL-C reduction ().
Among all participants, 80.2% experienced treatment-emergent adverse events (TEAEs), 23.3% had serious TEAEs, and three patients discontinued due to TEAEs. Two TEAEs led to death, but both of these were attributed to complications from ASCVD, not to evinacumab. Compared to the evinacumab-naïve group, the evinacumab-continue group experienced more TEAEs (85.7% vs. 71.7%) and serious TEAEs (31.4% vs. 10.9%) (). Except for TEAEs that led to death and study discontinuation, no new TEAEs were reported in this study that differed from those observed in the pivotal phase 3 ELIPSE trial and previous long-term studies (,). In a 5-year, longer-term safety and efficacy lomitapide study, adverse events () were present in 75.7% of patients, and 22.2% were serious AEs. In the same study, 15.1% of the patients had hepatic-related AEs that led to treatment discontinuation in 7% ().
Regarding LDL-C goal attainment, only a minor proportion of patients (14.7%) achieved a treatment target of <1.4 mmol/L, and 19.8% achieved that of <1.8 mmol/L with evinacumab. However, the fact that 42.2% of the study population achieved ≥50% LDL-C reduction is encouraging. Those who achieved ≥50% reduction in LDL-C were mostly adolescents (64.3%), suggesting that this young patient population stands to gain substantial benefit from reducing the cumulative burden of high LDL-C and associated ASCVD risk later in life (). These findings are in line with the phase 3 trial in 14 children and adolescents, where 78.6% of patients achieved >50% LDL-C reduction (), and suggest that evinacumab use in this population may completely change their trajectory with regard to ASCVD risk.
The percent change from baseline for other atherogenic lipids such as APOB, non-HDL-C, total cholesterol, triglycerides, and lipoprotein(a) [LP(a)] in this study was 41.2%, 49.6%, 47.5%, 50.8%, and 18.2%, respectively, in the evinacumab naïve group and 34.6%, 44.2%, 42.3%, 51.5%, and 12.4%, respectively in the evinacumab-continue group. While the results were generally consistent across most atherogenic parameters observed in the ELIPSE trial and other studies, greater LP(a) reductions (≥32%) were previously reported in the paediatric phase 3 trial and another long-term evinacumab study (,,). Given the rarity of HoFH, future evaluation of evinacumab effects should focus on the durability of these effects on atherogenic lipoproteins and longer-term safety. This is being evaluated in a post-authorisation safety study (PASS), reference number (EMA/404060/2015 Rev. 6).
Strengths and limitations of the Gaudet et al.’s trial
The Gaudet et al.’s study demonstrated high power and generalisability due to its large, multinational study size with diverse HoFH genotypes. Its extended follow-up period allowed for robust long-term efficacy and safety evaluation. However, its single-arm design inherently limited comparability to placebo or alternative therapies. Additionally, additive effects from combination therapy were difficult to interpret due to limited reporting. Lastly, the possibility of inconsistent background LLT adherence may have influenced outcomes.
Conclusions
The evidence from relatively large, long-term safety evinacumab trials underscores its role as a pivotal advance in the management of HoFH, particularly for null/null patients who derive limited benefit from other LLTs and remain at high risk due to persistently elevated LDL-C levels despite first-line therapies. While the efficacy of evinacumab in reducing LDL-C is well demonstrated, further studies are needed to assess its longer-term safety, impact on ASCVD, and optimization as part of combination therapy regimens.
Acknowledgments
None.
Footnote
Provenance and Peer Review: This article was commissioned by the editorial office, AME Clinical Trials Review. The article has undergone external peer review.
Peer Review File: Available at https://actr.amegroups.com/article/view/10.21037/actr-24-266/prf
Funding: None.
Conflicts of Interest: All authors have completed the ICMJE uniform disclosure form (available at https://actr.amegroups.com/article/view/10.21037/actr-24-266/coif). I.K. reports participation in research grant to Imperial College from Ultragenyx. K.K.R. reports receiving funding and support from Amgen, Sanofi-Regeneron, Pfizer, Merck Sharp & Dohme, Daiichi Sankyo, and Ultragenyx; consulting fees from AstraZeneca, Kowa, Novartis, Sanofi, Amgen, Eli Lilly, Algorithm, Boehringer Ingelheim, Novo Nordisk, Silence Therapeutics, Bayer, Esperion, Daiichi Sankyo, Abbott, New Amsterdam, SCRIBE, CRISPR, VAXXINITY, EMENDOBIO, Cargene, Viatris, Amarin, Nodthera, and Resverlogix; honoraria for lectures and presentations from AstraZeneca, Novartis, Sanofi, Amgen, Algorithm, Boehringer Ingelheim, Novo Nordisk, Esperion, Daiichi Sankyo, and Amarin; and serves a leadership role as President of the EAS and holds stock options in New Amsterdam Pharma. A.E. reports participation in research grant to Imperial College from Amgen, Regeneron, Daiichi Sankyo, and Ultragenyx. The authors have no other conflicts of interest to declare.
Ethical Statement: The authors are accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Open Access Statement: This is an Open Access article distributed in accordance with the Creative Commons Attribution-NonCommercial-NoDerivs 4.0 International License (CC BY-NC-ND 4.0), which permits the non-commercial replication and distribution of the article with the strict proviso that no changes or edits are made and the original work is properly cited (including links to both the formal publication through the relevant DOI and the license). See: https://creativecommons.org/licenses/by-nc-nd/4.0/.
References
1
Cuchel M, Bruckert E, Ginsberg HN, et al. Homozygous familial hypercholesterolaemia: new insights and guidance for clinicians to improve detection and clinical management. A position paper from the Consensus Panel on Familial Hypercholesterolaemia of the European Atherosclerosis Society. Eur Heart J 2014;35:2146-57. 10.1093/eurheartj/ehu274250536602
Hu P, Dharmayat KI, Stevens CAT, et al. Prevalence of Familial Hypercholesterolemia Among the General Population and Patients With Atherosclerotic Cardiovascular Disease: A Systematic Review and Meta-Analysis. Circulation 2020;141:1742-59. 10.1161/CIRCULATIONAHA.119.044795324688333
Nohara A, Tada H, Ogura M, et al. Homozygous Familial Hypercholesterolemia. J Atheroscler Thromb 2021;28:665-78. 10.5551/jat.RV17050338674214
Gu J, Gupta RN, Cheng HK, et al. Current treatments for the management of homozygous familial hypercholesterolaemia: a systematic review and commentary. Eur J Prev Cardiol 2024;31:1833-49. 10.1093/eurjpc/zwae144386404335
Mach F, Baigent C, Catapano AL, et al. 2019 ESC/EAS Guidelines for the management of dyslipidaemias: lipid modification to reduce cardiovascular risk. Eur Heart J 2020;41:111-88. 10.1093/eurheartj/ehz455315044186
Gaudet D, Greber-Platzer S, Reeskamp LF, et al. Evinacumab in homozygous familial hypercholesterolaemia: long-term safety and efficacy. Eur Heart J 2024;45:2422-34. Erratum in: Eur Heart J 2024;45:4314.10.1093/eurheartj/ehae325388566787
Brandts J, Ray KK. Familial Hypercholesterolemia: JACC Focus Seminar 4/4. J Am Coll Cardiol 2021;78:1831-43. 10.1016/j.jacc.2021.09.004347113428
Kayikcioglu M, Tokgozoglu L. Current Treatment Options in Homozygous Familial Hypercholesterolemia. Pharmaceuticals (Basel) 2022;16:64. 10.3390/ph16010064366785639
Phan BA, Dayspring TD, Toth PP. Ezetimibe therapy: mechanism of action and clinical update. Vasc Health Risk Manag 2012;8:415-27. 10.2147/VHRM.S336642291063310
Karlson BW, Palmer MK, Nicholls SJ, et al. To what extent do high-intensity statins reduce low-density lipoprotein cholesterol in each of the four statin benefit groups identified by the 2013 American College of Cardiology/American Heart Association guidelines? A VOYAGER meta-analysis. Atherosclerosis 2015;241:450-4. 10.1016/j.atherosclerosis.2015.05.0292607431911
Hovingh GK, Davidson MH, Kastelein JJ, et al. Diagnosis and treatment of familial hypercholesterolaemia. Eur Heart J 2013;34:962-71. 10.1093/eurheartj/eht0152341679112
Gagné C, Gaudet D, Bruckert E, et al. Efficacy and safety of ezetimibe coadministered with atorvastatin or simvastatin in patients with homozygous familial hypercholesterolemia. Circulation 2002;105:2469-75. 10.1161/01.cir.0000018744.58460.621203465113
Raal FJ, Honarpour N, Blom DJ, et al. Inhibition of PCSK9 with evolocumab in homozygous familial hypercholesterolaemia (TESLA Part B): a randomised, double-blind, placebo-controlled trial. Lancet 2015;385:341-50. 10.1016/S0140-6736(14)61374-X2528252014
Blom DJ, Harada-Shiba M, Rubba P, et al. Efficacy and Safety of Alirocumab in Adults With Homozygous Familial Hypercholesterolemia: The ODYSSEY HoFH Trial. J Am Coll Cardiol 2020;76:131-42. 10.1016/j.jacc.2020.05.0273264656115
Raal F, Durst R, Bi R, et al. Efficacy, Safety, and Tolerability of Inclisiran in Patients With Homozygous Familial Hypercholesterolemia: Results From the ORION-5 Randomized Clinical Trial. Circulation 2024;149:354-62. 10.1161/CIRCULATIONAHA.122.0634603785037916
Raal F, Mehta V, Kayikcioglu M, et al. Randomized, open-label, cross-over, phase-3 study to evaluate efficacy and safety of LIB003 compared with evolocumab in homozygous familial hypercholesterolaemia patients on stable lipid-lowering therapy (liberate-HOFH). Atherosclerosis 2023;379:S24-5.17
Agnello F, Ingala S, Laterra G, et al. Novel and Emerging LDL-C Lowering Strategies: A New Era of Dyslipidemia Management. J Clin Med 2024;13:1251. 10.3390/jcm130512513859209118
Tromp TR, Hartgers ML, Hovingh GK, et al. Worldwide experience of homozygous familial hypercholesterolaemia: retrospective cohort study. Lancet 2022;399:719-28. 10.1016/S0140-6736(21)02001-83510117519
Waldmann E, Parhofer KG. Apheresis for severe hypercholesterolaemia and elevated lipoprotein(a). Pathology 2019;51:227-32. 10.1016/j.pathol.2018.10.0163061154320
Underberg JA, Cannon CP, Larrey D, et al. Long-term safety and efficacy of lomitapide in patients with homozygous familial hypercholesterolemia: Five-year data from the Lomitapide Observational Worldwide Evaluation Registry (LOWER). J Clin Lipidol 2020;14:807-17. 10.1016/j.jacl.2020.08.0063302385921
Raal FJ, Rosenson RS, Reeskamp LF, et al. Evinacumab for Homozygous Familial Hypercholesterolemia. N Engl J Med 2020;383:711-20. 10.1056/NEJMoa20042153281394722
Stefanutti C, Chan DC, Di Giacomo S, et al. Long-Term Efficacy and Safety of Evinacumab in Patients with Homozygous Familial Hypercholesterolemia: Real-World Clinical Experience. Pharmaceuticals (Basel) 2022;15:1389. 10.3390/ph151113893642251923
Raal FJ, Rosenson RS, Reeskamp LF, et al. The Long-Term Efficacy and Safety of Evinacumab in Patients With Homozygous Familial Hypercholesterolemia. JACC Adv 2023;2:100648. 10.1016/j.jacadv.2023.1006483893872324
Béliard S, Saheb S, Litzler-Renault S, et al. Evinacumab and Cardiovascular Outcome in Patients With Homozygous Familial Hypercholesterolemia. Arterioscler Thromb Vasc Biol 2024;44:1447-54. 10.1161/ATVBAHA.123.3206093869516925
Wiegman A, Greber-Platzer S, Ali S, et al. Evinacumab for Pediatric Patients With Homozygous Familial Hypercholesterolemia. Circulation 2024;149:343-53. 10.1161/CIRCULATIONAHA.123.0655293786086326
Yusuf S, Bosch J, Dagenais G, et al. Cholesterol Lowering in Intermediate-Risk Persons without Cardiovascular Disease. N Engl J Med 2016;374:2021-31. 10.1056/NEJMoa160017627040132
