Introduction
L-carnitine (LC) plays an important role in the bioenergetic pathways of skeletal muscles. Thus, LC supplementation in the context of exercise metabolism has been studied for decades [, ]. Previous studies have reported that LC consumption increases plasma carnitine concentrations [, ]. However, it does not increase the total carnitine (TC) content in human skeletal muscles, even after 12 weeks of LC intake []. Stephens et al. [] estimated that to increase the muscle TC content by an additional 10%, LC should be consumed for approximately 100 days. Insulin is required for the transport of LC into muscles []. Thus, the muscle carnitine level increases when LC is co-ingested with carbohydrates (CHO) to induce an insulin response []. The effective change in the muscle TC content is associated with improvement in aerobic metabolism and exercise performance [].
Besides its bioenergetic function, LC can also upregulate metabolic pathways involved in maintaining the muscle protein balance [, ]. In animal studies, LC was found to increase the levels of circulating insulin-like growth factor-1 (IGF-1), which consequently led to the activation of protein kinase B (Akt) and the mammalian target of rapamycin (mTOR) []. The activation of mTOR leads to the inhibition of proteolysis and accelerates protein synthesis []. Moreover, an increase in muscle mass and strength was reported in older adults consuming LC mixed with leucine, creatine, and vitamin D for 8 weeks. In addition, these changes were associated with an increase in the total skeletal muscle level of mTOR protein []. By contrast, such an effect was not observed in the group supplemented with LC alone [].
Leucine, a branched-chain amino acid, may play a role as a regulator of intracellular signaling pathways involved in the process of protein synthesis []. However, recent studies have not confirmed the effects of leucine supplementation on muscle strength or hypertrophy []. Nevertheless, leucine has been reported to increase insulin levels []. Therefore, we hypothesized that 24 weeks of LC supplementation in combination with leucine could improve the transport of LC into muscles.
The primary aim of this study was to examine the effects of 24 weeks of LC and leucine supplementation on the skeletal muscle TC content, skeletal muscle mass (SMM), and skeletal muscle strength in active college-aged subjects. The secondary aim was to determine the serum IGF-1 concentration and the activation of the Akt/mTOR signaling pathway in skeletal muscles after supplementation.
Materials and Methods
Ethical Considerations
The study was conducted in accordance with the Declaration of Helsinki. The study protocol was approved by the Independent Bioethics Commission for Scientific Research at the Medical University of Gdansk (approval number: NKBBN/354/2012) and was registered in the ClinicalTrials.gov Registry (NCT05009654). All participants were informed about the procedures, risks, and expected outcomes before starting the experimental procedures, and written informed consent for participation was obtained.
Participants
Participants were included if they were nonsmokers, physically active but not highly trained, aged between 18 and 29 years, and able to swallow capsules. The criteria for exclusion were the presence of cardiovascular disease, liver disease, kidney disease, neuromuscular disease, gastrointestinal disorders (including stomach ulcers and erosions), diabetes, or other severe chronic diseases. A short questionnaire was used to assess the time and intensity of daily exercises, which were converted into metabolic equivalents.
Study Design
The participants were randomly assigned (1:1 ratio) to one of the two supplemented groups using the Random Sequence Generator (RANDOM.ORG, Dublin, Ireland). The supplements were encapsulated in identical gelatin capsules, so that neither the investigators nor the participants were aware of their contents. The participants were instructed to consume the supplements once a day with their main meal. Over the 24 weeks, the participants’ diets were supplemented with either 1 g of LC-L-tartrate and 3 g of leucine per day (LC + L group) or 4 g of leucine per day (L group) as a placebo.
Measurements
The measurements were performed before and 24 weeks after the initiation of the study protocol. Fasting blood samples were taken from the antecubital vein. After collection, the samples were centrifuged at 2000 g at 4°C for 10 min, and aliquots were stored at −80°C for later analyses.
Body mass and composition were measured using a bioelectrical impedance analyzer, InBody720 (Biospace Co., Seoul, South Korea). This device obtains readings from the body using an eight-point tactile electrode method and estimates the body composition using segmental resistances. The SMM and lean mass of arms, legs, and trunk were calculated using the manufacturer’s software. All measurements were performed using the same protocol and instrument. The analysis was carried out with the subjects in a vertical position and wearing only their underwear [].
All strength tests were performed using the Biodex System 4 Pro Dynamometer (Biodex Medical Systems, Inc., Shirley, NY, USA). The Biodex was calibrated according to the manufacturer’s specifications before the initiation of each testing session. The participants warmed up using mechanically braked cycle ergometer (Monark, Vansbro, Sweden) at 50 W for 5 min. They were then stabilized using straps, and the rotational axis of their knee was aligned with the center of the dynamometer shaft. Gravity correction was performed for all trials []. The testing session started with an isometric test at knee angle of 90°. The peak torque was measured by performing maximum voluntary contractions during isometric knee extension/flexion. The test comprised a maximum of 4-s knee isometric contractions, which were repeated three times, separated by a 20-s recovery. For the assessment of muscle isokinetic strength, the participants completed five repetitions of flexion and extension at a speed of 60°/s. Then, for the assessment of muscle endurance, 10 repetitions at a speed of 300°/s were performed to determine the ability of the muscle to maintain work []. The tests were performed for both legs in random order.
Muscle biopsies were performed on separate days. A section of the vastus lateralis muscle was obtained while the participants were under local anesthesia (2% lidocaine) and in the supine position, using a sterile single-use microbiopsy needle (M.D.L. Srl, Delebio, Italy) []. The sample (approximately 10 mg) was immediately frozen in liquid nitrogen and stored at −80°C until analysis.
Analysis
For the determination of free carnitine (FC) content, 5 μL of the sample (plasma, muscle homogenate, and calibration points) was transferred into a 1.5 mL test tube, and then 200 μL of acetonitrile containing the internal standard was added for protein precipitation. For the determination of TC content, after protein precipitation, 100 μL of 1 MKOH in methanol was added to hydrolyze acylcarnitines. The solution was incubated at 50°C for 60 min. Then, 100 μL of 1 M HCl in methanol was added for neutralization []. Samples were centrifuged for 2 min at 14,000 rpm and injected into a liquid chromatography-tandem mass spectrometry system at the Mass Spectrometry Laboratory, Institute of Biochemistry and Biophysics, Polish Academy of Sciences (Warsaw, Poland).
Serum IGF-1 concentration was measured using a commercially available enzyme immunoassay kit (total IGF-1, #DG100; R&D Systems, Minneapolis, MN, USA). The activation levels of specific muscle proteins in the Akt/mTOR pathway were determined using the Bio-Rad Bio-Plex Luminex 200 multiplex assay system and magnetic beads coupled with the following detection antibodies: Akt #171V60001M, p-Akt (Ser473) #171V50001M, mTOR #171V60015M, p-mTOR (Ser 2448) #171V50033M, p70S6K #171V60010M, and p-p70S6K (Thr389) #171V50016M (Bio-Rad, Hercules, CA, USA). The analysis was performed according to the manufacturer’s recommendations.
Statistical Analyses
All calculations were performed using the software Statistica 13.1 (Dell Inc., Tulsa, OK, USA). The analysis of variance for repeated measures was performed to examine the interaction between the treatment and time. In case the analysis of variance yielded a significant effect, Tukey’s test was used for post hoc comparisons. In addition, effect size (Cohen’s d) was calculated as described previously [] and interpreted as follows: small effect (d < 0.5), moderate effect (0.5 ≤ d ≤ 0.8), and large effect (d > 0.8). A probability level of p < 0.05 was considered significant. All data are expressed as mean ± standard deviation.
Results
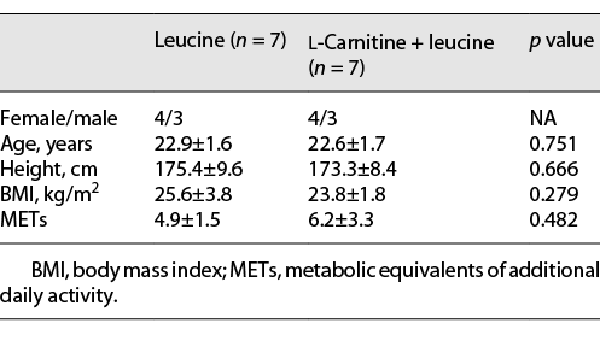
The study protocol was completed by 14 participants (Fig. 1). Their characteristics are presented in Table 1.
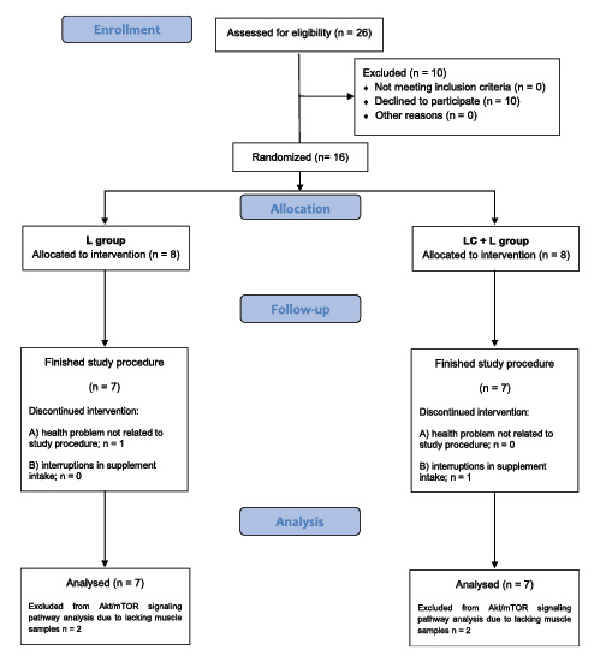
Fig. 1
Flowchart of participant recruitment and participation in the study.
The plasma FC and TC content increased in the LC + L group after 24 weeks of supplementation (p = 0.003 and 0.010, respectively; Fig. 2a). However, the skeletal muscle FC and TC content were not affected by the supplementation protocol (Fig. 2b).
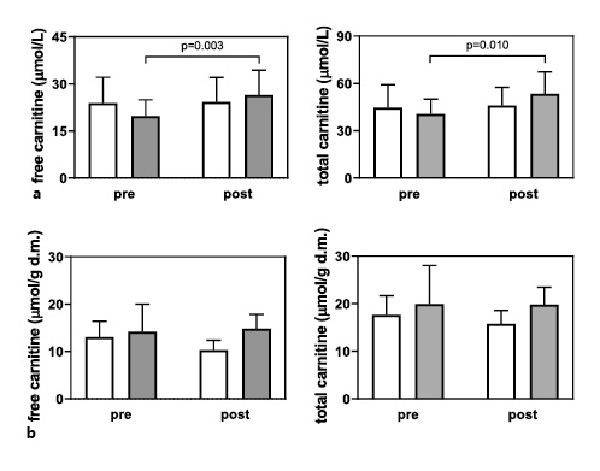
Fig. 2
Plasma (a) and muscle (b) free and total carnitine concentrations in the L group (white bars), and LC + L group (gray bars) before and after 24 weeks of supplementation. Values are presented as means (±SD).
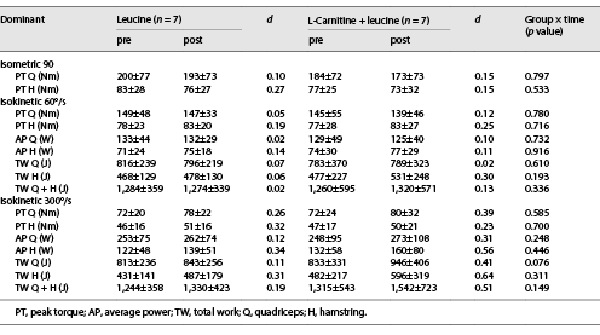
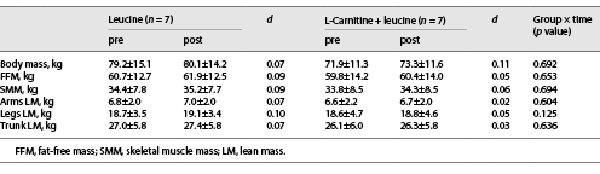
Moderate effect sizes were noted in the LC + L group for the changes in hamstring average power and total work in the dominant (Table 2) and nondominant (online suppl. Table S1; for all online suppl. material, see http://www.karger.com/doi/10.1159/000529333) legs, whereas the effect sizes were small in the L group (Table 2 and online suppl. Table S1). No changes were noted in the body mass and composition (Table 3).
Serum IGF-1 concentrations did not differ during the study in both groups (LC + L group, pre 156 ± 26 vs. post 152 ± 31 µg/L; L group, pre 178 ± 37 vs. post 168 ± 34 µg/L), and no interaction group per time was noted (p = 0.640). Moreover, no changes were observed in the phosphorylation of the signaling pathway proteins Akt, mTOR, and p70S6K (Fig. 3a–c).
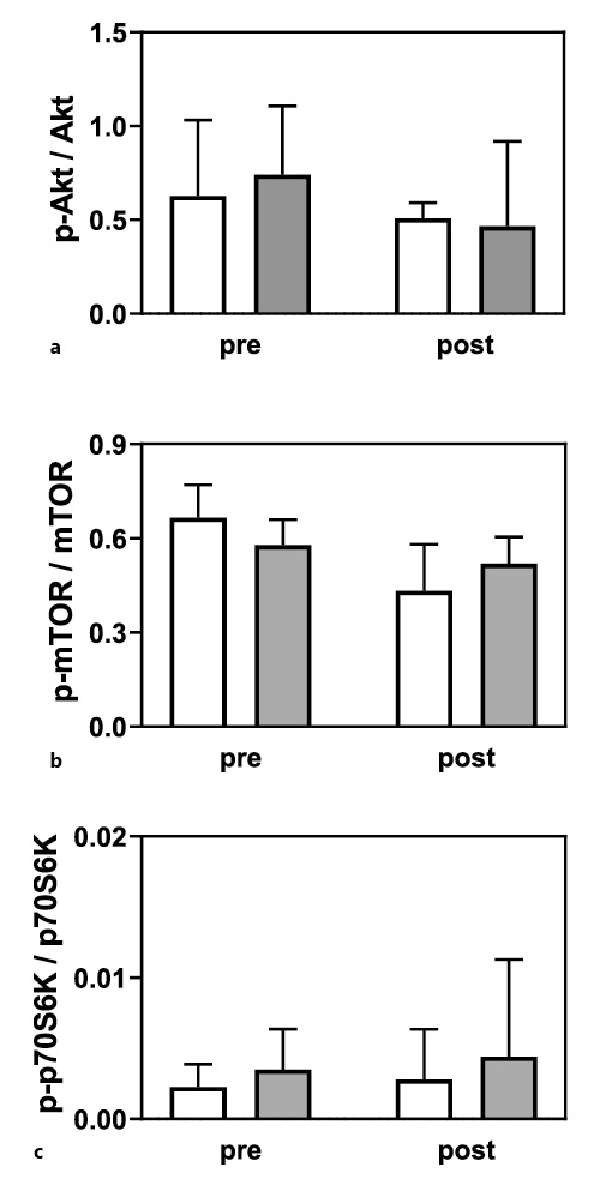
Fig. 3
Phosphorylation of Akt (a), mTOR (b), and p70S6K (c) in the L group (white bars; n = 5), and LC + L group (gray bars; n = 5) before and after 24 weeks of supplementation. Values are presented as means (±SD).
Discussion
The main findings of this study were that 24 weeks of LC and leucine supplementation increased the plasma FC and TC concentrations but did not change the skeletal muscle carnitine content. In addition, no differences were noted in the SMM and signaling pathway activity. Nevertheless, some moderate effects were observed on muscle function.
Circulating carnitine levels increase after 2 weeks of LC supplementation [], but for an increase in the skeletal muscle TC content, LC should be consumed for at least 100 days []. In addition, the muscle carnitine levels increase only when LC is co-ingested with a large amount of CHO to induce an insulin response []. To improve the transport of LC into muscles, we used 24 weeks of LC supplementation in combination with leucine, because of its insulin secretagogue effect []. However, the increase in insulin levels in our study may be rather low compared with those in previous LC supplementation studies using CHO-mediated insulin release []. This may be a reason why we did not observe any changes in the skeletal muscle TC content.
Previously, 1.5 g of LC supplementation combined with 2 g of leucine, 3 g of creatine, and 10 μg of vitamin D daily for 8 weeks increased muscle mass in older adults. The authors suggested that it was the effect of increased protein anabolism mediated by mTOR []. In the present study, LC and leucine supplementation did not affect the SMM or phosphorylation of mTOR signaling pathway proteins. The observed discrepancies in results may reflect differences between the studies. For example, the age of participants differed between the study by Evans et al. [] and our study. A strong inverse correlation between age and carnitine levels in the skeletal muscle of older adults has been reported []. The intracellular regulation of protein synthesis via mTOR signaling also differs between young and old skeletal muscles []. Deterioration of protein synthesis in muscles of older people may partly reflect the failure in the activation of signals involved in the control of the translation process []. Therefore, our supplementation protocol may have had negligible effects in young adults.
Another difference between studies is the daily amount of LC consumed. A recent meta-analysis indicated that LC supplementation can affect body weight and composition, and that the maximum effect in adults occurs with a dose of 2 g LC per day []. Although the administered daily LC dose was lower in our study than in previous work [], the total amount of LC consumed was higher in the present study because of the prolonged period of supplementation. In our study, no changes in SMM and phosphorylation of mTOR signaling pathway proteins were noted in the placebo group receiving leucine, even though leucine plays an important role in the regulation of protein synthesis [, ]. However, leucine supplementation does not increase muscle mass or strength in untrained [] or resistance-trained [] young adults with an adequate dietary protein intake.
Unfortunately, we did not monitor dietary intake and composition in our study. Nevertheless, the same supplementation protocol in healthy aged women did not improve the efficacy of a resistance exercise training program []. In that study, the changes in muscle function and cross-sectional area in the LC combined with leucine group or leucine-alone group were similar to those observed in the group that performed the same training but without supplementation []. Therefore, it seems likely that the previously reported effects may have resulted from additional components in the supplementation procedure []. For example, vitamin D is involved in various anabolic pathways in skeletal muscle [], and it also increases the stimulatory effect of leucine on protein synthesis through mTOR-mediated signaling in skeletal myotubes []. In addition, creatine supplementation at a dose of 3 g per day for 4 weeks effectively increases the total creatine content in muscles []. Ensuring creatine ingestion at a dose of 3 g daily may provide significant health benefits [].
Animal studies have shown that LC supplementation can regulate the metabolic pathways involved in muscle protein balance [, -]. The mechanism suggested to explain this effect may involve increased circulating IGF-1 level [, ]. However, LC supplementation for 24 weeks in older women [], even during a resistance exercise training program [], did not affect circulating IGF-1 levels. Similarly, we did not observe any changes in blood IGF-1 levels in the present study. A significant increase in fat-free mass has been reported in centenarians supplemented with LC [], and this effect may be linked to the progressive decrease in serum IGF-1 with age [, ]. Taken together, these findings suggest that IGF-1-mediated regulation of protein metabolism in humans cannot be excluded as a possible mechanism, although LC supplementation may affect muscle protein balance in people with low circulating IGF-1 level.
Limitations
A major limitation of the present study was the lack of dietary control. Consumption of adequate amounts of dietary protein may explain the lack of an anabolic signaling effect observed in the group receiving leucine. Second, the small amount of muscle samples obtained limited the number of skeletal muscle markers that could be evaluated. Determination of muscle IGF-1 or myostatin protein levels would have provided more information about any effects of LC supplementation. A third limitation pertains to the small sample size, which may have limited the statistical power and the ability to identify intergroup differences, especially those related to the ability of the muscle to maintain work in the tests of muscle function.
Conclusion
No changes were noted in the skeletal muscle TC content after 24 weeks of LC and leucine supplementation. In addition, no changes were observed in the SMM, function, and phosphorylation of the Akt/mTOR signaling pathway proteins. Nevertheless, LC supplementation may have the potential to exert beneficial effects in muscle atrophy. Therefore, additional research is necessary to investigate the effect of various LC supplementation protocols.
Acknowledgment
The equipment used was sponsored in part by the Centre for Preclinical Research and Technology (CePT), a project co-sponsored by European Regional Development Fund and Innovative Economy, The National Cohesion Strategy of Poland. The practical contribution of Dr Ryszard Zaleski, Angelika Sawicka, and Damian Sadowski is greatly acknowledged.
Statement of Ethics
The study was conducted in accordance with the Declaration of Helsinki. This study protocol was reviewed and approved by the Independent Bioethics Commission for Scientific Research at the Medical University of Gdansk, approval number [NKBBN/354/2012]. The written informed consent was obtained from participants before starting the experimental procedures.
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
This research was supported by the National Science Centre in Poland (2014/15/B/NZ7/00893) and the Foundation of Polish Science TEAM TECH CORE FACILITY/2016-2/2 Mass Spectrometry of Biopharmaceuticals – improved methodologies for qualitative, quantitative, and structural characterization of drugs, proteinaceous drug targets, and diagnostic molecules.
Author Contributions
Emilia Samborowska: funding acquisition; sample analysis; critical revision of the manuscript for important intellectual content; and final approval of the version to be published. Robert A. Olek: conception of the work; funding acquisition; sample collection; sample analysis; statistical analysis; drafting manuscript; and final approval of the version to be published.
Data Availability Statement
Data are not publicly available due to ethical reasons. Further inquiries can be directed to the corresponding author.
References
- 1. Brass EP. Supplemental carnitine and exercise. <X00_Journal>Am J Clin Nutr</X00_Journal>. 2000;72(2 Suppl):618S–23S.
- 2. Oliveira C, Sousa M. The effects of L-carnitine supplementation in athletic performance. <X00_Journal>Sci Sports</X00_Journal>. 2019;34(2):63–72.
- 3. Barnett C, Costill DL, Vukovich MD, Cole KJ, Goodpaster BH, Trappe SW, et al. Effect of L-carnitine supplementation on muscle and blood carnitine content and lactate accumulation during high-intensity sprint cycling. <X00_Journal>Int J Sport Nutr</X00_Journal>. 1994;4(3):280–8.
- 4. Lee JK, Lee JS, Park H, Cha YS, Yoon CS, Kim CK. Effect of L-carnitine supplementation and aerobic training on FABPc content and beta-HAD activity in human skeletal muscle. <X00_Journal>Eur J Appl Physiol</X00_Journal>. 2007;99(2):193–9.
- 5. Novakova K, Kummer O, Bouitbir J, Stoffel SD, Hoerler-Koerner U, Bodmer M, et al. Effect of L-carnitine supplementation on the body carnitine pool, skeletal muscle energy metabolism and physical performance in male vegetarians. <X00_Journal>Eur J Nutr</X00_Journal>. 2016;55(1):207–17.
- 6. Stephens FB, Evans CE, Constantin-Teodosiu D, Greenhaff PL. Carbohydrate ingestion augments L-carnitine retention in humans. <X00_Journal>J Appl Physiol</X00_Journal>. 2007;102(3):1065–70.
- 7. Stephens FB, Constantin-Teodosiu D, Laithwaite D, Simpson EJ, Greenhaff PL. Insulin stimulates L-carnitine accumulation in human skeletal muscle. <X00_Journal>FASEB J</X00_Journal>. 2006;20(2):377–9.
- 8. Wall BT, Stephens FB, Constantin-Teodosiu D, Marimuthu K, Macdonald IA, Greenhaff PL. Chronic oral ingestion of L-carnitine and carbohydrate increases muscle carnitine content and alters muscle fuel metabolism during exercise in humans. <X00_Journal>J Physiol</X00_Journal>. 2011;589(Pt 4):963–73.
- 9. Ringseis R, Keller J, Eder K. Mechanisms underlying the anti-wasting effect of L-carnitine supplementation under pathologic conditions: evidence from experimental and clinical studies. <X00_Journal>Eur J Nutr</X00_Journal>. 2013;52(5):1421–42.
- 10. Sawicka AK, Renzi G, Olek RA. The bright and the dark sides of L-carnitine supplementation: a systematic review. <X00_Journal>J Int Soc Sports Nutr</X00_Journal>. 2020;17(1):49.
- 11. Keller J, Couturier A, Haferkamp M, Most E, Eder K. Supplementation of carnitine leads to an activation of the IGF-1/PI3K/Akt signalling pathway and down regulates the E3 ligase MuRF1 in skeletal muscle of rats. <X00_Journal>Nutr Metab</X00_Journal>. 2013;10(1):28.
- 12. Schiaffino S, Dyar KA, Ciciliot S, Blaauw B, Sandri M. Mechanisms regulating skeletal muscle growth and atrophy. <X00_Journal>FEBS J</X00_Journal>. 2013;280(17):4294–314.
- 13. Evans M, Guthrie N, Pezzullo J, Sanli T, Fielding RA, Bellamine A. Efficacy of a novel formulation of L-Carnitine, creatine, and leucine on lean body mass and functional muscle strength in healthy older adults: a randomized, double-blind placebo-controlled study. <X00_Journal>Nutr Metab</X00_Journal>. 2017;14:7.
- 14. Anthony JC, Anthony TG, Kimball SR, Jefferson LS. Signaling pathways involved in translational control of protein synthesis in skeletal muscle by leucine. <X00_Journal>J Nutr</X00_Journal>. 2001;131(3):856S–60S.
- 15. Plotkin DL, Delcastillo K, Van Every DW, Tipton KD, Aragon AA, Schoenfeld BJ. Isolated leucine and branched-chain amino acid supplementation for enhancing muscular strength and hypertrophy: a narrative review. <X00_Journal>Int J Sport Nutr Exerc Metab</X00_Journal>. 2021;31(3):292–301.
- 16. van Loon LJC. Leucine as a pharmaconutrient in health and disease. <X00_Journal>Curr Opin Clin Nutr Metab Care</X00_Journal>. 2012;15(1):71–7.
- 17. Sawicka AK, Hartmane D, Lipinska P, Wojtowicz E, Lysiak-Szydlowska W, Olek RA. l-Carnitine supplementation in older women. A pilot study on aging skeletal muscle mass and function. <X00_Journal>Nutrients</X00_Journal>. 2018;10(2):255.
- 18. Symons TB, Vandervoort AA, Rice CL, Overend TJ, Marsh GD. Reliability of a single-session isokinetic and isometric strength measurement protocol in older men. <X00_Journal>J Gerontol A Biol Sci Med Sci</X00_Journal>. 2005;60(1):114–9.
- 19. Devan MR, Pescatello LS, Faghri P, Anderson J. A prospective study of overuse knee injuries among female athletes with muscle imbalances and structural abnormalities. <X00_Journal>J Athl Train</X00_Journal>. 2004;39(3):263–7.
- 20. Olek RA, Kujach S, Ziemann E, Ziolkowski W, Waz P, Laskowski R. Adaptive changes after 2 Weeks of 10-s sprint interval training with various recovery times. <X00_Journal>Front Physiol</X00_Journal>. 2018;9:392.
- 21. Sowell J, Fuqua M, Wood T. Quantification of total and free carnitine in human plasma by hydrophilic interaction liquid chromatography tandem mass spectrometry. <X00_Journal>J Chromatogr Sci</X00_Journal>. 2011;49(6):463–8.
- 22. Thalheimer W, Cook SR. <X00_Journal>How to calculate effect sizes from published research:</X00_Journal><X00_Journal> a simplified methodology</X00_Journal>. Work-Learning Research; 2002.
- 23. Costell M, O’Connor JE, Grisolia S. Age-dependent decrease of carnitine content in muscle of mice and humans. <X00_Journal>Biochem Biophys Res Commun</X00_Journal>. 1989;161(3):1135–43.
- 24. D’Antona G, Nisoli E. mTOR signaling as a target of amino acid treatment of the age-related sarcopenia. <X00_Journal>Interdiscip Top Gerontol</X00_Journal>. 2010;37:115–41.
- 25. Talenezhad N, Mohammadi M, Ramezani-Jolfaie N, Mozaffari-Khosravi H, Salehi-Abargouei A. Effects of l-carnitine supplementation on weight loss and body composition: a systematic review and meta-analysis of 37 randomized controlled clinical trials with dose-response analysis. <X00_Journal>Clin Nutr ESPEN</X00_Journal>. 2020;37:9–23.
- 26. Borack MS, Volpi E. Efficacy and safety of leucine supplementation in the elderly. <X00_Journal>J Nutr</X00_Journal>. 2016;146(12):2625S–2629S.
- 27. Aguiar AF, Grala AP, da Silva RA, Soares-Caldeira LF, Pacagnelli FL, Ribeiro AS, et al. Free leucine supplementation during an 8-week resistance training program does not increase muscle mass and strength in untrained young adult subjects. <X00_Journal>Amino Acids</X00_Journal>. 2017;49(7):1255–62.
- 28. DE Andrade IT, Gualano B, Hevia-Larrain V, Neves-Junior J, Cajueiro M, Jardim F, et al. Leucine supplementation has No further effect on training-induced muscle adaptations. <X00_Journal>Med Sci Sports Exerc</X00_Journal>. 2020;52(8):1809–14.
- 29. Sawicka AK, Jaworska J, Brzeska B, Sabisz A, Samborowska E, Radkiewicz M, et al. L-carnitine combined with leucine supplementation does not improve the effectiveness of progressive resistance training in healthy aged women. <X00_Journal>J Nutr Health Aging</X00_Journal>. 2022;26(10):945–53.
- 30. Dzik KP, Kaczor JJ. Mechanisms of vitamin D on skeletal muscle function: oxidative stress, energy metabolism and anabolic state. <X00_Journal>Eur J Appl Physiol</X00_Journal>. 2019;119(4):825–39.
- 31. Salles J, Chanet A, Giraudet C, Patrac V, Pierre P, Jourdan M, et al. 1,25 (OH)2-vitamin D3 enhances the stimulating effect of leucine and insulin on protein synthesis rate through Akt/PKB and mTOR mediated pathways in murine C2C12 skeletal myotubes. <X00_Journal>Mol Nutr Food Res</X00_Journal>. 2013;57(12):2137–46.
- 32. Hultman E, Soderlund K, Timmons JA, Cederblad G, Greenhaff PL. Muscle creatine loading in men. <X00_Journal>J Appl Physiol</X00_Journal>. 1996;81(1):232–7.
- 33. Kreider RB, Kalman DS, Antonio J, Ziegenfuss TN, Wildman R, Collins R, et al. International Society of Sports Nutrition position stand: safety and efficacy of creatine supplementation in exercise, sport, and medicine. <X00_Journal>J Int Soc Sports Nutr</X00_Journal>. 2017;14:18.
- 34. Kita K, Kato S, Amanyaman M, Okumura J, Yokota H. Dietary L-carnitine increases plasma insulin-like growth factor-I concentration in chicks fed a diet with adequate dietary protein level. <X00_Journal>Br Poult Sci</X00_Journal>. 2002;43(1):117–21.
- 35. Busquets S, Serpe R, Toledo M, Betancourt A, Marmonti E, Orpi M, et al. L-Carnitine: an adequate supplement for a multi-targeted anti-wasting therapy in cancer. <X00_Journal>Clin Nutr</X00_Journal>. 2012;31(6):889–95.
- 36. Keller J, Ringseis R, Priebe S, Guthke R, Kluge H, Eder K. Dietary L-carnitine alters gene expression in skeletal muscle of piglets. <X00_Journal>Mol Nutr Food Res</X00_Journal>. 2011;55(3):419–29.
- 37. Jang J, Park J, Chang H, Lim K. l-Carnitine supplement reduces skeletal muscle atrophy induced by prolonged hindlimb suspension in rats. <X00_Journal>Appl Physiol Nutr Metab</X00_Journal>. 2016;41(12):1240–7.
- 38. Malaguarnera M, Cammalleri L, Gargante MP, Vacante M, Colonna V, Motta M. L-Carnitine treatment reduces severity of physical and mental fatigue and increases cognitive functions in centenarians: a randomized and controlled clinical trial. <X00_Journal>Am J Clin Nutr</X00_Journal>. 2007;86(6):1738–44.
- 39. Juul A, Bang P, Hertel NT, Main K, Dalgaard P, Jorgensen K, et al. Serum insulin-like growth factor-I in 1030 healthy children, adolescents, and adults: relation to age, sex, stage of puberty, testicular size, and body mass index. <X00_Journal>J Clin Endocrinol Metab</X00_Journal>. 1994;78(3):744–52.
- 40. Bidlingmaier M, Friedrich N, Emeny RT, Spranger J, Wolthers OD, Roswall J, et al. Reference intervals for insulin-like growth factor-1 (igf-i) from birth to senescence: results from a multicenter study using a new automated chemiluminescence IGF-I immunoassay conforming to recent international recommendations. <X00_Journal>J Clin Endocrinol Metab</X00_Journal>. 2014;99(5):1712–21.