Introduction
Chronic limb-threatening ischaemia (CLTI) is a common condition, with an incidence of 220–1000 new cases per million population worldwide. The incidence increases with age and a majority of patients have minor or major tissue loss, corresponding to Rutherford categories 5 and 6. These patients have a high risk of limb amputation or death, and most ultimately need revascularization. However, 20–40% of these patients are considered unsuitable for revascularization owing to anatomical limitations and co-morbidities, and are referred to as ‘no-option’ patients. Previous studies, have demonstrated poor outcomes in such patients, with a mortality rate of 20% and a 40% risk of amputation in the first 6 months after diagnosis, although a recent study reported better than expected 5-year amputation-free survival (AFS) among no-option patients.
Treatment alternatives for no-option patients are limited, and include control of cardiovascular risk factors, pain management, and wound care. Although angio/arteriogenesis to improve limb salvage, using protein or gene transfer of proangiogenic factors, or autologous cell therapy have been suggested, such approaches have failed to demonstrate clinical benefit in placebo-controlled trials. There is thus a crucial need for new medical therapies to improve AFS in patients who are unsuitable for revascularization.
PLX-PAD is an allogeneic, off-the-shelf, cryopreserved cell-based therapy. It is composed of ex vivo expanded placental adherent stromal cells that are derived from human placentas, donated by healthy women undergoing elective caesarean section. PLX-PAD does not require HLA matching. In vitro and in vivo evidence suggests that PLX-PAD cells secrete a variety of proteins that stimulate angio/arteriogenesis,, mitigate overwhelming inflammation, and support muscle regeneration,. Treatment with PLX-PAD has been shown to increase blood flow to ischaemic limbs in a murine hindlimb ischaemia model,.
PLX-PAD was previously assessed in two phase I studies that treated 27 no-option patients with CLTI. The approach demonstrated an acceptable safety profile, with related events generally limited to transient injection-site reactions and higher AFS in patients treated with PLX-PAD compared with rates reported in the literature. In a previous phase I/II study in patients with intermittent claudication using a different PLX-PAD dose, a subpopulation that received two repeated intramuscular injections of a high dose of PLX-PAD cells from two donors into the affected leg showed improved pain-free and maximum walking distance. Moreover, PLX-PAD improved muscle strength in patients after hip arthroplasty associated with immunomodulation in both a phase I/IIa study and a phase III study,.
The aim of the present trial was to confirm the hypothesis that treatment with PLX-PAD would increase AFS in patients with CLTI unsuitable for revascularization.
Methods
PACE was a randomized, placebo-controlled, parallel-group, blinded, multicentre, phase III study conducted in patients who had CLTI with minor tissue loss unsuitable for revascularization. The study protocol was approved by relevant regulatory authorities and institutional review boards, all patients provided informed consent, and the trial was conducted in accordance with the principles of the Helsinki Declaration. An independent data monitoring committee (DMC) periodically reviewed unblinded study data according to a predefined charter. PACE was registered with ClinicalTrials.gov (NCT03006770) and EudraCT (2015-005532-18).
Patients
Enrolled patients were adults with minor tissue loss up to ankle level, owing to atherosclerotic peripheral arterial disease (PAD); an ankle pressure of 70 mmHg or less or toe pressure no higher than 50 mmHg in the index leg; and a maximum ischaemic skin lesion area of 20 cm2. Non-suitability for revascularization was decided by a multidisciplinary team including a vascular surgeon and an endovascular specialist, defined by anatomical or technical considerations, previous failed revascularization or co-morbidities. Patients were excluded if they had non-atherosclerotic PAD (for example Buerger’s disease), major tissue loss, infection, planned amputation (less than 1 month after randomization), insufficient arterial inflow to the index leg (aortoiliac or femoral stenosis 70% or more), a haemoglobin (Hb) A1c level of 10% or higher, or an estimated life expectancy of less than 6 months.
Study treatment
Patients were randomized in a 2 : 1 ratio to receive PLX-PAD or placebo using an interactive web-based response system. Patients, study staff, investigators, contract research organization staff, and sponsor personnel involved in data review and site management were blinded to treatment assignments. PLX-PAD was provided in cryogenic vials containing placental expanded mesenchymal stromal cell-like cells, 10% dimethyl sulphoxide (DMSO), 5% human serum albumin, and Plasma-Lyte. Placebo vials were identical to those for PLX-PAD, and contained 10% DMSO, 5% human serum albumin, and Plasma-LyteTM (Baxter Healthcare Ltd., Thetford, Norfolk, UK). The study treatment was administered via 30 intramuscular injections (0.5 ml each) into the index leg (Fig. S1). Overall, 60 intramuscular doses were administered over 2 treatment sessions (days 0 and 60). The protocol required control of cardiovascular risk factors according to applicable guidelines, and all patients continued to receive standard medical care (such as wound care and analgesia) according to local practices. All patients received recommendations on lifestyle changes, including smoking cessation, before randomization.
Study schedule and assessments
After screening, eligible patients were randomized and received the study treatment or placebo. Patients were followed up until the end of the study (12 months after first study treatment of the last randomized patient, or the time at which 82 primary endpoint events occurred, whichever was later) or until completion of 36 months of follow-up, the earliest of the two. Assessments included: examination of lesion size and status, pain and quality-of-life assessments (the latter to be reported in a future paper), ankle : brachial pressure index and toe : brachial pressure index, vital signs, and ECG. Data on adverse events and treatment emergency adverse events (TEAEs, defined as adverse events with an onset time later than the first study treatment administration), concomitant procedures and medications, and laboratory tests were collected. Complete eligibility criteria and the schedule of assessments can be found in the Supplementary material.
Endpoints, randomization, and statistical methods
Randomization was done using permuted blocks stratified initially by region (North America, Western Europe, Eastern/Central Europe), extent of ischaemic lesions, and diabetes mellitus (DM, yes or no). Stratification by risk of ischaemic lesions (high versus low) was added during the study (Supplementary material).
The primary endpoint was AFS, measured as the interval in days from randomization to occurrence of major amputation of the index leg or death from any cause. The principal analysis involved a baseline adjusted Cox proportional hazards model (SAS® PROC PHREG; SAS Institute, Cary, NC, USA). Model co-variates included the three stratification factors and lesion number (fewer than 2 versus 2 or more lesions). Patients who were lost to follow-up were censored at the time of early discontinuation. Results are presented as adjusted HR (PLX-PAD versus placebo) with 95% confidence interval.
Assessment of the primary endpoint was undertaken in predefined subgroups and by stratification factors, including age (less than 70 versus at least 70 years), sex, smoking status, region, DM, number of lesions (fewer than 2 versus 2 or more), wound risk (low versus high), total wound area (less than 10 cm2versus 10 cm2 or more; above/below median area).
Post hoc efficacy analyses were carried out using a statistical methodology similar to that used for the primary endpoint and included: analysis of all amputation and death events occurring in the first 12 months after randomization; and AFS in a subpopulation of patients who had an HbA1c level below 6.5% at baseline.
The sample size was calculated based on the assumption of placebo AFS probabilities of 65% in year 1, 54% in year 2, and 49% in year 3. The expected PLX-PAD survival curve was a piecewise linear function defined by the following three time points: 0.82, 0.75, and 0.71 at 1, 2, and 3 years respectively. The 50% risk reduction assumption in the first year was based on pooled data from the phase I studies, which showed an AFS rate of 85% at 1 year for patients treated with PLX-PAD. For sample-size calculation purposes, an α level of 0.0452 was used. This was done to maintain the overall type I error probability (α = 0.05), using the O’Brien–Fleming α-spending function, which distributes the α to 0.01 at interim, and to 0.0452 at the end of study. Based on these assumptions, the required number of primary endpoint events needed to achieve 90% power was 82, and the expected number of patients 264. By the end of the study, 246–300 patients were expected.
The planned sequence of analysis encompassed an interim analysis, performed at a fixed α level of 0.01 and including a scenario of overwhelming efficacy or a scenario of futility (conditional power less than 30%). The interim analysis was conducted when approximately half of the planned primary endpoint events (major amputation or death) had occurred. The overall α level for the study was 0.05, using two-tailed tests. At the end of the study, the hierarchical method for multiple endpoint testing for the primary and secondary endpoints was planned, using the gatekeeping approach, ensuring, altogether, that the overall experiment-wise type I error of 0.05 was preserved, with an α of 0.0452 for each endpoint in the following order: the primary endpoint was analysed first, followed by the five predefined secondary endpoints in the order listed below. If an endpoint was not met, significance testing for the next endpoint was carried out for exploratory purposes only.
Secondary endpoints were: interval in days from randomization to first occurrence of any of the events major amputation of the index leg, revascularization owing to worsening of CLTI in the index leg, or all-cause mortality; interval in days from randomization to major amputation of the index leg; change in worst ischaemic pain from baseline to 6 months, measured on a numerical rating scale; proportion of patients with complete healing of all ischaemic lesions at 12 months; and interval in days from randomization to death. Exploratory endpoints are described in the Supplementary material.
All analyses were conducted using SAS® version 9.4. The interim analysis was conducted as planned after 45 primary endpoint events had been observed. At this point the DMC indicated that the study was unlikely to demonstrate a statistically significant difference in AFS between the PLX-PAD and placebo arms by the time of the final analysis, and the sponsor ended the study early owing to futility.
Results
Demographics and baseline characteristics
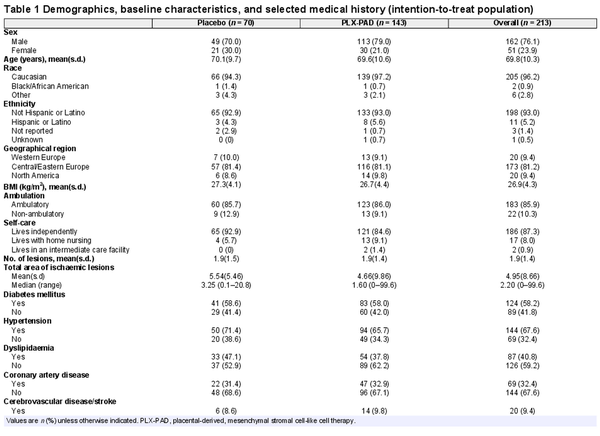
From September 2017 to November 2020, 377 patients were screened, 213 were enrolled, and 205 were treated, at 42 sites in the USA and Europe (Supplementary material). Overall, 143 patients were randomized to PLX-PAD and 70 to placebo (Fig. 1). Baseline demographics and characteristics were well balanced (Table 1). The overall mean age was 69.8 years, 76% of the patients were men, and 96.2% Caucasian. Most patients were ambulatory (85.9%) and lived independently (87.3%); 58% had DM, with a mean HbA1c level of 7.2%.
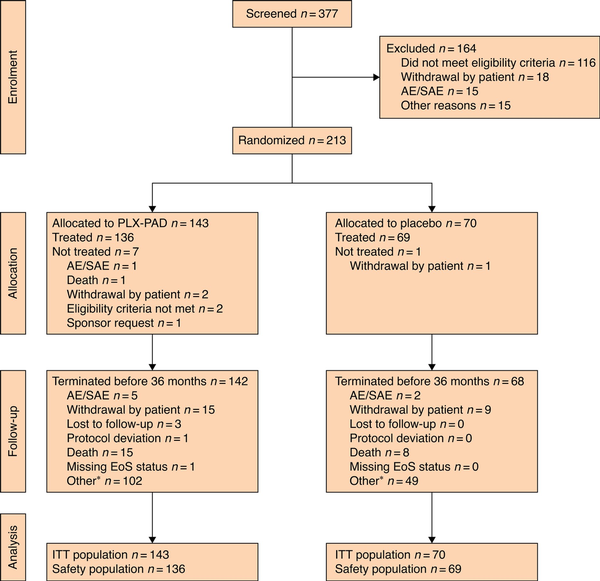
Fig. 1
CONSORT diagram for the trial
*Includes all patients who did not complete 36 months owing to early termination of the study. AE, adverse event; SAE, serious adverse event; PLX-PAD, placental-derived, mesenchymal stromal cell-like cell therapy; EoS, end of study; ITT, intention to treat. Nine patients had an outcome of death after receiving treatment with placebo, for one of these patients the reason for early termination was recorded as "other".
Safety and tolerability
A total of 100 patients (73.5%) treated with PLX-PAD and 57 (82.6%) who received the placebo experienced at least one TEAE. The most frequent TEAEs were expected for the study population, and included skin ulcer, extremity necrosis, gangrene, and ischaemic limb pain (Table 2). The intramuscular injections were associated with similar rates of injection-site reactions for both PLX-PAD (12.5%) and placebo (10.1%) (Table S1).
Twenty-nine patients died during the study (Table S2), 4 after screening and before randomization, 1 after randomization but before dosing, 15 (11%) after PLX-PAD administration (deemed unrelated to treatment) and 9 (13%) after placebo administration. Cardiovascular events as cause of death occurred in 66.7% in the PLX-PAD versus 56% in the placebo arm.
Five patients had a myocardial infarction after study treatment (2.2% in PLX-PAD arm versus 2.9% in placebo arm), deemed unrelated to the study treatment. Five patients experienced stroke, ischaemic stroke, or cerebral infarction, all in the PLX-PAD arm (stroke rate 2.44 per 100 patient-years) (Supplementary material), deemed unrelated to the study treatment.
Prespecified efficacy endpoints
The rate of major amputation of the index leg or death was similar in the placebo and PLX-PAD arms, at 33 and 28.6% respectively (HR 0.93, 95% c.i. 0.53 to 1.63; P = 0.788) with a median time to event of 110 versus 128 days (Table S3 and Fig. 2). This was unchanged both in the modified intention-to-treat population and when analysed without co-variates. Among patients without DM, the AFS rate was slightly higher for those treated with PLX-PAD versus patients who received the placebo (HR 0.59, 0.26 to 1.34; P = 0.150). Regarding secondary endpoints, a similar risk of death, major amputation, and revascularization was observed in the PLX-PAD and placebo arms. Although median time to death was threefold longer for patients treated with PLX-PAD (365 versus 104 days respectively), the median time to amputation was similar (113.5 versus 95 days) (Table S3). Ischaemic pain and rate of complete healing were similar in the two treatment arms.
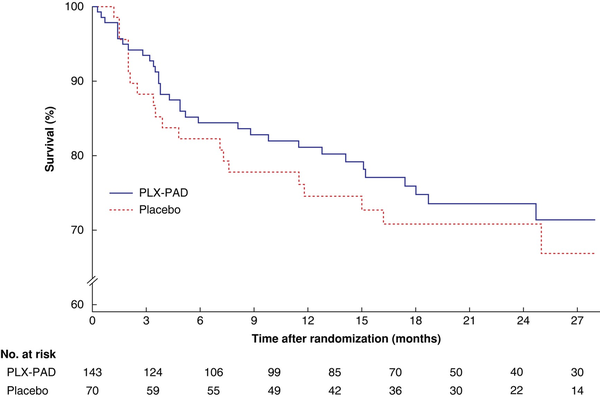
Fig. 2
Time to occurrence of major amputation of index leg or death in intention-to-treat analysis by cohort
PLX-PAD, placental-derived, mesenchymal stromal cell-like cell therapy. P = 0.489 (log rank test).
Post hoc efficacy analyses
In analysis of the primary endpoint using data accrued during the first 12 months of follow-up for each patient, the HR changed from 0.93 to 0.80 (95% c.i. 0.43 to 1.49; P = 0.483), in favour of PLX-PAD.
Given the higher AFS rate after treatment with PLX-PAD in patients without DM, a subpopulation comprising patients without DM or those with well controlled DM was defined based on the Centers for Disease Control and Prevention cut-off for DM (HbA1c 6.5% or higher). The subpopulation with an HbA1c level below 6.5% represented 57% of randomized patients (121 of 213) and included 60% of the death or amputation events (32 of 53). In this subpopulation, a positive treatment effect was observed for AFS at 12 months (HR 0.46, 0.21 to 0.99; P = 0.048) yet there was no difference in AFS at 36 months between the treatment groups (HR 0.60, 0.30 to 1.21; P = 0.151) (Fig. 3). The treatment effect for PLX-PAD decreased as the baseline HbA1c level increased (Fig. 4).
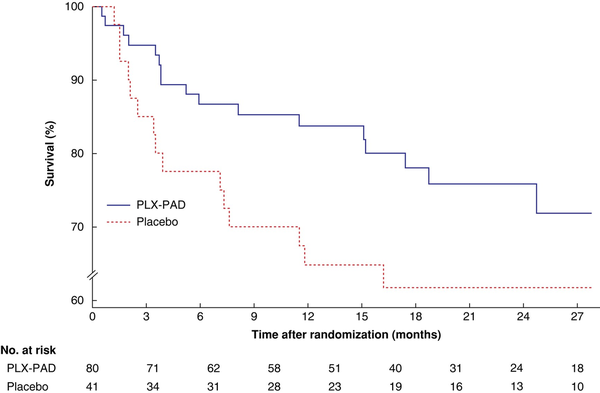
Fig. 3
Time to occurrence of major amputation of the index leg or death in the subpopulation of patients with haemoglobin A1c level below 6.5% by cohort
PLX-PAD, placental-derived, mesenchymal stromal cell-like cell therapy. P = 0.114 (log rank test).
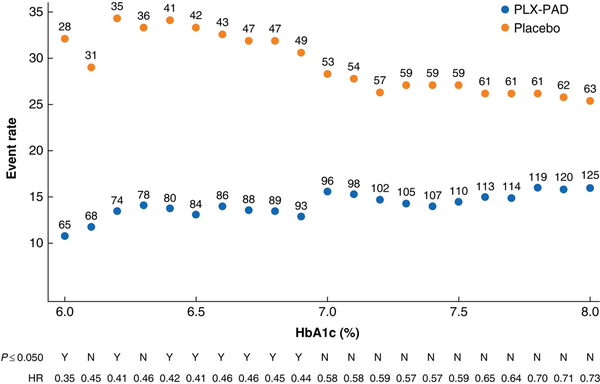
Fig. 4
Occurrence of major amputation of the index leg or death in relation to haemoglobin A1c cut-off values
Event rates and HRs for the occurence of major amputation or death are presented by baseline haemoglobin (Hb) A1c cut-off levels from 6 to 8%. The number next to each dot represents the number of patients included in a specific HbA1c cut-off. HRs are shown above above the x-axis, with statistical significance depicted as yes (Y) or no (N) for P ≤ 0.050. The event rates for PLX-PAD and placebo are further apart in low HbA1c cut-offs.
Demographics, baseline characteristics and HbA1c levels were similar for the two treatment arms in the subpopulation of patients with an HbA1c level below 6.5% (Tables S4 and S5, Fig. S3). However, this subpopulation included a higher proportion of women (31 versus 15%), a higher proportion of non-ambulatory patients (16 versus 5%), and a higher proportion of patients from Western Europe (13 versus 4%) than the subpopulation with an HbA1c level of 6.5% or greater.
Discussion
PACE was a large, randomized, controlled, multicentre, cell therapy study conducted in patients with CLTI. AFS in this patient population managed with optimal conservative treatment was higher than expected, consistent with recent reports. Crucially, there was no difference in AFS between patients treated with PLX-PAD and those who received the placebo in this study. Failure to demonstrate clinical benefit may reflect the overall better prognosis observed, the high rate of ambulatory patients at baseline, or geographical distribution introducing variability in standard of care.
Although the primary endpoint was not met in the overall population, the results suggested that, among PLX-PAD-treated patients without DM and those with well controlled DM, defined by a baseline HbA1c level below 6.5%, AFS was improved compared with that among placebo-treated patients. In an analysis of the impact of baseline HbA1c on AFS in the entire population, a relationship between baseline glycaemic control and treatment response was apparent.
This observation may reflect the proposed role of PLX-PAD in supporting angiogenesis and the impact of DM on angiogenesis, which per se may contribute to increased rates of amputation, via vascular endothelial growth factor and placenta-derived growth factor resistance. Chronic inflammation that is stronger in patients with uncontrolled DM may influence the response to PLX-PAD. Meanwhile, in vitro results indicated that the ability of PLX-PAD to reduce reactive oxygen species and increase tube formation is present but attenuated in hyperglycaemia, and in vivo studies (unpublished data, Pluri-Biotech) showed improved blood flow in normoglycaemic versus hyperglycaemic mice after administration of PLX-PAD. These preclinical findings support the clinical observation of a favourable treatment effect for PLX-PAD in the subpopulation of patients with an HbA1c level below 6.5%.
The subpopulation with a baseline HbA1c below 6.5% included more women, supporting previous findings. Additionally, a higher proportion of patients in this subpopulation were non-ambulatory, a condition that has previously been linked to poorer AFS,. The high rate of ambulatory patients in this study was unexpected and may indicate that inclusion parameters were imprecise. Finally, differences in geographical distribution were observed, which may influence CLTI outcome because of potential variation in standard of care. Lesion parameters (for example total area and number of lesions) were similar in the two subpopulations, indicating that the differential response was not likely driven by different wound characteristics.
Two previously published studies using autologous cell therapy in CLTI had results similar to those of PACE. Sun et al., using autologous granulocyte colony-stimulating factor-mobilized peripheral blood mononuclear cells, showed a poorer response in patients with high blood glucose levels (over 180 mg/dl). The MOBILE study, using autologous concentrated bone marrow aspirate (cBMA), showed improved AFS for patients without DM treated with cBMA versus placebo (HR 0.31, 95% c.i. 0.12 to 0.81; P = 0.016) but not for those with DM (HR 1.45, 0.42 to 5.05; P = 0.555). These findings contrast with those of a meta-analysis of autologous cell therapy studies, which showed a greater freedom from amputation in trials with a high prevalence of patients with DM. Patients with DM have atypical leucocyte responses, poorly functioning inflammatory responses, and a more complex local tissue response.
This study has provided an updated prospective outcome for the time to index event for patients with CLTI who are unsuitable for revascularization. Previously reported values include a mortality rate of 22% at 12 months, an amputation rate of 22% at 12 months, and worsening of tissue loss in 35% of patients. The present prospectively collected data set includes an 11.4% mortality rate and a 14.3% amputation rate at 12 months for patients treated with placebo. Improved risk factor management in this motivated, recruited population may explain the difference. Nevertheless, the amputation and mortality rates observed in the placebo arm should inform study power calculations in future clinical studies.
The safety and tolerability profile of PLX-PAD in this study population was as expected. The incidence of ischaemic stroke was in line with published rates for CLTI, with all stroke events considered unrelated to the study treatment. Nevertheless, the reason for the difference between treatment arms should be elucidated in future studies with PLX-PAD.
The key limitation of this study is the post hoc nature of the subpopulation in which a treatment effect on AFS was observed. Furthermore, pain and lesion size are patient- and assessor-dependent respectively, making them prone to bias. Given the need to include a large number of sites, variation in standard of care may have affected protocol outcomes and limited the interpretability of the results. The generalizability of the results should also be considered given that patients with very large lesions, severe co-morbidity, planned amputation or active local infection were excluded from the study. These limitations notwithstanding, the study design as a global, multicentre, double-blinded, randomized, placebo-controlled trial remains a key strength which should be considered when interpreting the results.
Although the AFS rate was similar in the two treatment arms (HR 0.93, 95% c.i. 0.53 to 1.63; P = 0.79), the favourable treatment effect observed for PLX-PAD in patients without DM or those with well controlled DM (HR 0.46, 0.21 to 0.99; P = 0.048) is encouraging and of clinical relevance. Further studies are urgently needed to confirm this finding.
Acknowledgements
This study would not have been possible without the PACE consortium (coordinated by Charité, speaker: H. D. Volk) and all its members. The authors acknowledge the contribution of patients who participated in this study and their caregivers, as well as the clinical-site staff who conducted research visits and participated in the collection of clinical data. Additionally, the authors acknowledge past and current Pluri-Biotech and BeCAT employees who were instrumental in the day-to-day management of study-related activities.