Introduction
Peptide receptor radionuclide therapy (PRRT) improves outcomes of patients with non-functioning pancreatic neuroendocrine tumours (NF-PanNETs). This therapeutic approach uses radiolabelled somatostatin analogues, including 90Y-labelled DOTA0-Tyr3-octreotide (90Y-DOTATOC), 177Lu-labelled DOTA0-octreotate (177Lu-DOTATATE), and 177Lu-labelled DOTA0-Tyr3-octreotide, and has garnered substantial achievements in objective response rates (ORRs) (15–35%). 177Lu-DOTATATE has been demonstrated to provide superior progression-free survival and superior response rates in comparison to octreotide long-acting release for patients with advanced midgut neuroendocrine tumours.
Although the efficacy of PRRT is well established in the context of advanced disease, surgical intervention remains the cornerstone for resectable NF-PanNETs. Even though radical surgery is deemed curative in most patients, a recurrence rate of 20–30% remains a significant challenge. Preoperative factors such as tumour size, tumour grade, distant and nodal metastases, and vascular or adjacent organ infiltration, are pivotal in recurrence risk assessment. These factors increase the recurrence risk and complicate surgical efforts, sometimes hindering the possibility of radical resection. Given these challenges, neoadjuvant therapy could play a key role in reducing the risk of relapse and in making surgery more feasible for NF-PanNETs exhibiting invasive traits. Owing to its high response rates, PRRT emerges as a leading option for neoadjuvant treatment.
Preliminary findings have suggested favourable outcomes when PRRT is administered as neoadjuvant therapy. Nevertheless, it is important to acknowledge that these studies were mostly retrospective and carried important limitations, such as selection bias and limited sample sizes. Prospective studies are needed to better define the value of neoadjuvant PRRT in this context. The aim of this prospective study was to evaluate the safety and efficacy of neoadjuvant treatment with 177Lu-DOTATATE in patients with resectable or potentially resectable NF-PanNETs at high risk of recurrence.
Methods
Study design and participants
NEOLUPANET was designed as a multicentre, open-label, prospective phase II single-arm trial (NCT04385992). The study was conducted between March 2020 and February 2023 at eight Italian institutions, including San Raffaele Hospital (Milan), Verona University Trust Hospital (Verona), Humanitas Research Hospital (Rozzano), Pisa University Hospital Trust (Pisa), Bologna University Hospital Trust (Bologna), Pederzoli Hospital (Peschiera del Garda), University Hospital of Ferrara (Ferrara), and European Institute of Oncology (Milan). All surgical centres were high-volume facilities for pancreatic surgery. The study was approved by the ethical committees of participating sites, and performed in accordance with the Declaration of Helsinki and Good Clinical Practice guidelines. All patients were required to give written informed consent.
Inclusion criteria were: age at least 18 years and histologically confirmed sporadic, resectable or potentially resectable, well differentiated NF-PanNET with positive uptake on 68Ga-labelled DOTA (68Ga-DOTA) PET. Findings with 68Ga-DOTA uptake higher than physiological biodistribution were considered as positive. Additionally, eligible patients were required to have at least one high-risk feature, including: radiological tumour size over 4 cm; Ki-67 index exceeding 10%; adjacent organ involvement; vascular invasion (radiological evidence of vessel wall infiltration, excluding superior mesenteric vein/portal vein invasion greater than 180° and/or coeliac trunk/superior mesenteric artery invasion); mesenteric, portal, or splenic vein thrombosis; single resectable liver metastasis; enlarged, hypervascularized lymph nodes showing uptake on 68Ga-DOTA PET. Exclusion criteria were: genetic syndromes; functioning pancreatic neuroendocrine tumour; extra-abdominal metastases or peritoneal metastases; any previous treatment directed at NF-PanNET; Karnofsky Performance Status below 90; ASA fitness grade higher than III; inadequate bone marrow, liver and kidney function. This study was reported following STROBE guidelines.
Procedures
Patients received 177Lu-DOTATATE at a cumulative dose of 29 600 MBq, divided into four cycles of 7400 MBq each, scheduled at 6–8-week intervals unless unacceptable toxic effects occurred. Following each 177Lu-DOTATATE administration, patients stayed in hospital for at least 24 h. To verify the correct biodistribution of the radiopharmaceutical and its uptake into the target lesions, whole-body scans were performed 16–24 h after 177Lu-DOTATATE injection. During the first and fourth cycle, single-photon emission CT (SPECT) or SPECT–CT was used for more accurate assessments. These assessments were conducted to determine the percentage of radiopharmaceutical uptake and half-life as well as to calculate absorbed dose, effective biological dose, and equivalent uniform dose in both target lesions and critical organs. Three months after the last cycle of 177Lu-DOTATATE, all patients underwent restaging with contrast-enhanced CT (CE-CT) and 68Ga-DOTA PET, in accordance with the protocol. Surgical intervention was recommended to be carried out within 2 weeks of restaging. The extent of pancreatic resection was determined by tumour site, and included resection of involved adjacent organs and/or vessels. When a single liver metastasis was present, concurrent liver resection was performed.
Endpoints
Safety was defined as the primary endpoint, and assessed by the rates of postoperative mortality and morbidity within 90 days or during the hospital stay. Postoperative complications were categorized using the Clavien–Dindo classification. Postoperative pancreatic fistula (POPF) was graded according to the International Study Group on Pancreatic Surgery criteria. The occurrence of bile/chyle leak, delayed gastric emptying, and abdominal collection was also recorded. Duration of hospital stay was defined as the interval from surgery to the date of discharge.
Secondary endpoints were the rate of objective radiological response to 177Lu-DOTATATE, and quality of life (QoL) after neoadjuvant therapy and surgery. The objective radiological response was evaluated by CE-CT 12 weeks after the final 177Lu-DOTATATE cycle. This was measured in accordance with the Response Evaluation Criteria in Solid Tumours, version 1.1. Responses to 177Lu-DOTATATE were classified as complete response (CR), partial response (PR), stable disease (SD), and progressive disease (PD). Local investigators assessed the objective response, defined as the percentage of patients exhibiting PR or CR as best overall response. Changes in maximum standardized uptake value (SUVmax) on pretreatment and post-treatment 68Ga-DOTA PET were reported. Pathological assessments included analysis of fibrosis, microvascular/perineural invasion, necrosis, nodal status, and status of resection margins (R0, margin over 1 mm; R1, margin 0–1 mm; R2, macroscopic residual tumour). Tumour staging was undertaken according to the European Neuroendocrine Tumor Society and AJCC TNM staging systems,. Tumour grade was assigned according to the 2017 WHO classification as G1 (Ki-67 index below 3%), G2 (Ki-67 index 3–20%) or G3 (Ki-67 index over 20%). Patient QoL was measured using the European Organisation for Research and Treatment of Cancer QoL questionnaire QLQ-C30.
Statistical analysis
All patients who received at least one cycle of 177Lu-DOTATATE and had their disease evaluated were considered for the primary endpoint. In a previous study, 57% of patients had an uneventful postoperative course after neoadjuvant PRRT followed by surgical resection, compared with 39% of patients who underwent upfront surgery. Based on these findings, using the single-stage phase II sample size method, it was calculated that a sample size of 30 patients was needed to evaluate whether the proportion of patients without postoperative complications (defined as responses) would be at least 57% or at most 39%. If 15 or more patients did not experience postoperative complications, the hypothesis that the proportion of responses is 39% or less would be rejected with a target error rate of 0.1 and a power of 80%.
Categorical variables are reported as absolute numbers and percentages. Continuous data are reported as mean(s.d.) or median (i.q.r.), as appropriate. QoL and symptom scores were calculated as described previously. Statistical analyses were carried out using SPSS® version 26.0 for Mac® (IBM, Armonk, NY, USA).
Results
Study population
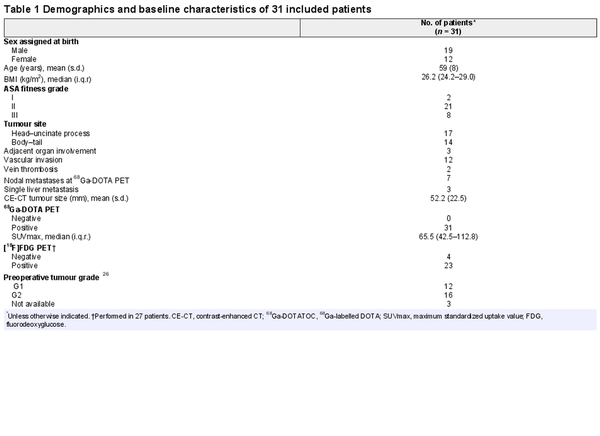
Overall, 34 patients were screened, of whom 31 met the eligibility criteria and were enrolled; they received at least 1 cycle of 177Lu-DOTATATE. Three patients were excluded during screening. Additionally, two enrolled patients decided against surgical intervention after 177Lu-DOTATATE treatment. The remaining 29 patients underwent surgical exploration according to the protocol (Fig. 1). All these patients exhibited at least one high-risk feature (Table S1). Vascular invasion was present in 12 patients. Nodal metastases were observed at 68Ga-DOTA PET in seven patients (Tables 1 and S1). The mean(s.d.) radiological tumour diameter was 52(22.5) mm. Consistently, all 31 patients demonstrated positive uptake at 68Ga-DOTA PET, yielding a median SUVmax of 65.5 (i.q.r. 42.5–112.81). Overall, 27 patients underwent [18F]fluorodeoxyglucose PET, with positive uptake in 23.
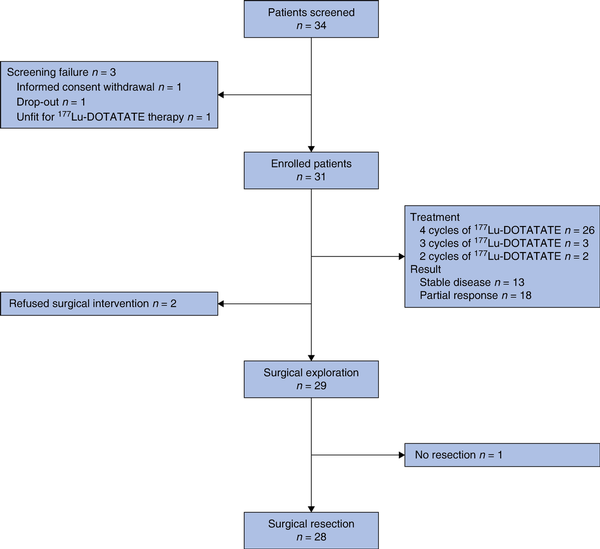
Fig. 1
Study flow chart
177Lu-DOTATATE, 177Lu-labelled DOTA0-octreotate.
177Lu-DOTATATE administration and objective radiological response
Twenty-six patients completed the full protocol of four 177Lu-DOTATATE cycles without significant toxicity. In the remaining five patients, discontinuation of treatment was primarily due to severe adverse events (3 patients) including proteinuria (after 2 cycles), thrombocytopenia (after 3 cycles), and severe nausea associated with facial burning sensation (after 3 cycles). One patient was limited to three cycles to avert potential renal toxicity, based on dosimetry evaluations. One patient chose to stop after two cycles, based on personal preference. Despite treatment discontinuation, all these patients underwent surgery with primary tumour resection. Eighteen of 31 patients had a PR. The other 13 patients did not show any relevant changes in tumour diameter (Fig. 2). All three patients with preoperative evidence of a single liver metastasis had a PR. No patient had a PD or CR (Table S2).
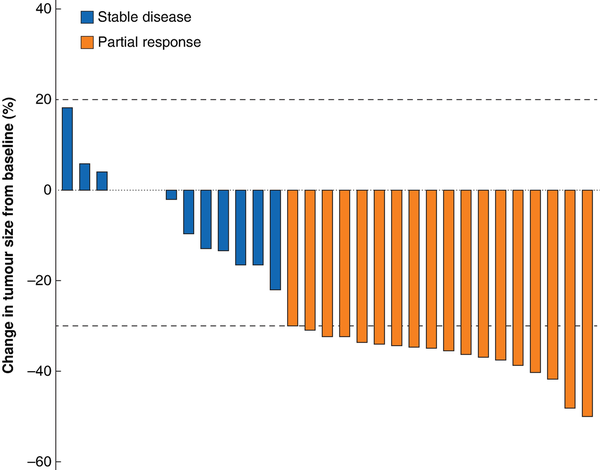
Fig. 2
Waterfall plot showing radiological tumour response
Tumour response was evaluated using Response Evaluation Criteria in Solid Tumours (version 1.1).
Intraoperative details and postoperative outcomes
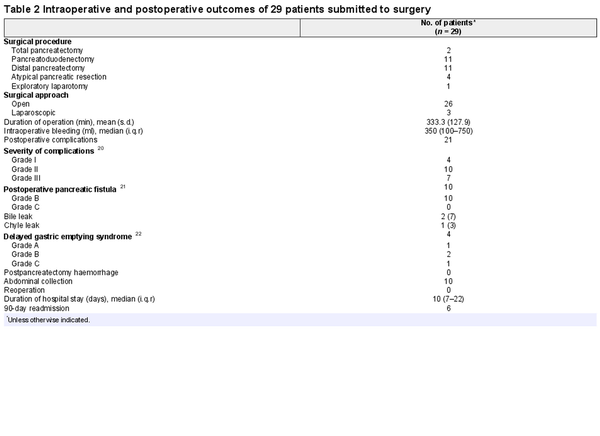
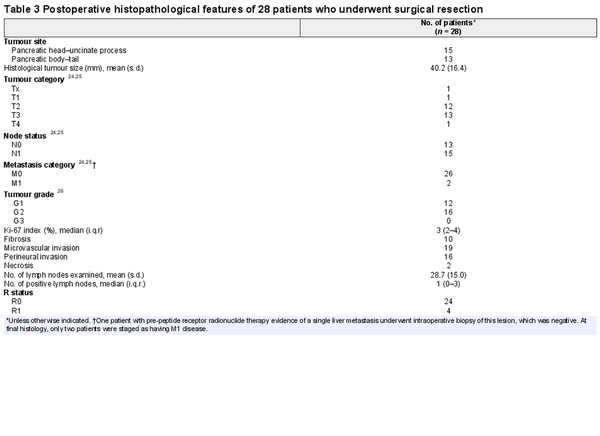
Surgical resection was performed 4 months after the last 177Lu-DOTATATE cycle (median 119 (i.q.r. 113–142.5) days) in 28 of 29 patients. One tumour was not resectable during surgical exploration owing to extensive vascular infiltration (Table 2). This patient had several preoperative high-risk features, including tumour size larger than 4 cm, Ki-67 index over 10%, adjacent organ invasion, and vascular involvement, and completed four cycles of PRRT. Vein resection was performed in four patients, whereas portal thrombectomy was required in one patient. Postoperative complications occurred in 21 of 29 patients. Severe complications (only grade III) occurred in seven patients. There were no reoperations and no patient died from postoperative complications. POPF (10 patients) was the most common complication. Postpancreatectomy haemorrhage did not occur. The median duration of hospital stay was 10 (i.q.r. 7–22) days. Table 3 depicts the histopathological characteristics of 28 resected tumours. More than half of the patients had nodal involvement (N1). Of seven patients with positive lymph nodes identified at 68Ga-DOTA PET, final histology confirmed nodal metastases in four patients. Fibrosis was observed in 10 of 28 surgical specimens. Surgical margin status was classified as R0 and R1 in 24 and 4 patients respectively.
Quality of life
Initially, patients reported good QoL with a mean(s.d.) global health status score of 85.4(17.5); functional scores were high and symptom scores low. After 177Lu-DOTATATE treatment, the global health status score remained stable (83.0(16.6); P = 1.000), with similar functional scores and symptom scores marginally increasing. QoL decreased after surgery; the mean global health status score was 70.2(14.2) (P = 0.001), with a consistent decline in functional scores and increase in symptom scores (Table S3).
Discussion
This prospective study has shown that neoadjuvant 177Lu-DOTATATE is safe and effective for patients with resectable or potentially resectable NF-PanNETs at high risk of disease recurrence. NF-PanNETs display variable biological behaviour, ranging from indolent, small, and asymptomatic tumours, which can be managed through active surveillance, to aggressive lesions requiring radical surgical treatment. The recurrence rate after surgery is around 20–30%. No established adjuvant therapy protocols are available to reduce this risk,. As for other malignancies, neoadjuvant therapy could be beneficial in decreasing the risk and facilitating surgical resection, especially in patients with borderline resectable tumours. In this study, the safety of a neoadjuvant approach using 177Lu-DOTATATE was demonstrated, although this treatment did not help reduce the rate of postoperative complications. In fact, the rate was higher than that reported in a previous retrospective experience with a surprisingly reduced rate of postoperative complications in patients undergoing neoadjuvant PRRT. The complication rate, however, aligned with the complexity of the procedures performed. The number of severe complications was limited, with POPF being the most common complication. Patients with NF-PanNETs generally have a higher risk of POPF than those undergoing pancreatic resection for adenocarcinoma, owing to specific features of the remnant pancreas. Discordant results compared with the aforementioned study may have several explanations. The retrospective study included patients submitted to various lines of therapy and PRRT with different radioligands, including 90Y-DOTATOC. It is possible that these different therapies might have contributed to fibrosis in the pancreatic remnant. Patients with more advanced NF-PanNETs were included, which may have led to wider pancreatic ducts and chronic obstructive pancreatitis, potentially reducing the POPF risk.
Of note is the low toxicity of 177Lu-DOTATATE. Most patients completed all four cycles, and only three discontinued treatment because of adverse events. The occurrence of adverse events did not prevent subsequent surgical resection. The favourable tolerability of 177Lu-DOTATATE is consistent with current evidence, particularly with results from the NETTER-1 study, and is evident from the QoL scores, which did not deteriorate following treatment. It is important to emphasize the lack of disease progression during neoadjuvant treatment. Post-PRRT restaging was crucial in determining the type of surgical resection needed, especially in assessing the need for vascular resection. Consistently, few patients ultimately required venous resection, compared with 12 patients initially found to have vascular involvement. Only one patient, with SD after PRRT, had a tumour deemed non-resectable at operation. In this patient, both pre-PRRT and preoperative imaging probably underestimated the extent of vascular involvement. Even more important than lack of progression is the chance of response. A PR was observed in almost 60% of patients reflecting the potential of preoperative PRRT in reducing disease burden. The PR rate was notably higher than that reported in the NETTER study, which reported an overall response rate of 18%. The latter study included only patients with midgut tumours, which complicates comparison. Previous series, assessing the efficacy of PRRT in patients with advanced pancreatic neuroendocrine tumours, however, reported a radiological response rate of between 25 and 35%, which is clearly lower. The present study included a population of patients with localized disease. It is therefore plausible that the therapy might have been more effective owing to a lower disease burden, a factor proven to be associated with better response to PRRT,.
In the present study, ORR represented the only efficacy endpoint, as no long-term oncological outcomes were analysed. The hypothesis is that neoadjuvant treatment facilitates surgical resection and reduces the risk of early postoperative recurrence. However, early recurrence cannot be ruled out, and preoperative PRRT treatment will influence treatment decisions at the time of recurrence. Patients who had a favourable ORR (PR) previously are likely to be candidates for additional cycles of 177Lu-DOTATATE (if the interval is sufficient), whereas those who did not respond well to previous treatment require alternative management strategies.
Post-PRRT histological findings were also assessed. Nodal metastases were observed in approximately half of patients. This finding is consistent with existing literature,, which documented a rate of nodal metastases of NF-PanNETs of between 50 and 60% in patients undergoing surgery. The present study focused on a population selected for aggressive tumours, where an even higher frequency of N1 tumours was anticipated. A previous series showed nodal metastases in 74% of patients undergoing upfront surgery for NF-PanNETs at high risk of recurrence. The authors hypothesize that preoperative 177Lu-DOTATATE may sterilize nodal metastases in some patients. The relatively high rate of fibrosis is another noteworthy finding. A previous retrospective study demonstrated that PRRT was associated with an increase in the percentage of stroma. This finding likely represents the pathological response to therapy and could have future implications related to the immune landscape of these neoplasms. This rise in fibrotic content did not correlate with increased intraoperative difficulty.
Limitations of this study include lack of a control group owing to the single-arm prospective design. Inclusion criteria were heterogeneous, particularly regarding high-risk features. Most of these predictive factors can be obtained only after lesion removal and histological examination. The recent recognition of molecular factors, such as DAXX/ATRX status, which can be assessed on preoperative samples, will enhance the ability to stratify these patients before operation. Patients underwent different surgical interventions, both pancreatoduodenectomy and distal pancreatectomy. These two surgical procedures carry a distinct burden of complications and a different risk of POPF. Finally, as the ORR was the only efficacy endpoint considered and the sample size was small, conclusions about survival cannot be drawn. An international RCT with disease-free survival as primary endpoint should be designed to determine the utility, long-term safety, and overall survival benefit of neoadjuvant 177Lu-DOTATATE in patients with NF-PanNETs.
References
- 1. Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al Phase 3 trial of 177Lu-Dotatate for midgut neuroendocrine tumors. N Engl J Med 2017;376:125–135
- 2. Bodei L, Kidd M, Paganelli G, Grana CM, Drozdov I, Cremonesi M, et al Long-term tolerability of PRRT in 807 patients with neuroendocrine tumours: the value and limitations of clinical factors. Eur J Nucl Med Mol Imaging 2015;42:5–19
- 3. Paganelli G, Sansovini M, Nicolini S, Grassi I, Ibrahim T, Amadori E, et al 177Lu-PRRT in advanced gastrointestinal neuroendocrine tumors: 10-year follow-up of the IRST phase II prospective study. Eur J Nucl Med Mol Imaging 2021;48:152–160
- 4. Pusceddu S, Prinzi N, Tafuto S, Ibrahim T, Filice A, Brizzi MP, et al Association of upfront peptide receptor radionuclide therapy with progression-free survival among patients with enteropancreatic neuroendocrine tumors. JAMA Netw Open 2022;5:e220290
- 5. Brabander T, Van Der Zwan WA, Teunissen JJM, Kam BLR, Feelders RA, De Herder WW, et al Long-term efficacy, survival, and safety of [177Lu-DOTA0, Tyr3]octreotate in patients with gastroenteropancreatic and bronchial neuroendocrine tumors. Clin Cancer Res 2017;23:4617–4624
- 6. Sansovini M, Severi S, Ianniello A, Nicolini S, Fantini L, Mezzenga E, et al Long-term follow-up and role of FDG PET in advanced pancreatic neuroendocrine patients treated with 177Lu-DOTATATE. Eur J Nucl Med Mol Imaging 2017;44:490–499
- 7. Kipnis ST, Hung M, Kumar S, Heckert JM, Lee H, Bennett B, et al Laboratory, clinical, and survival outcomes associated with peptide receptor radionuclide therapy in patients with gastroenteropancreatic neuroendocrine tumors. JAMA Netw Open 2021;4:e212274
- 8. Landoni L, Marchegiani G, Pollini T, Cingarlini S, D’Onofrio M, Capelli P, et al The evolution of surgical strategies for pancreatic neuroendocrine tumors (Pan-NENs). Ann Surg 2019;269:725–732
- 9. Genç CG, Jilesen AP, Partelli S, Falconi M, Muffatti F, Van Kemenade FJ, et al A new scoring system to predict recurrent disease in grade 1 and 2 nonfunctional pancreatic neuroendocrine tumors. Ann Surg 2018;267:1148–1154
- 10. Genç CG, Falconi M, Partelli S, Muffatti F, van Eeden S, Doglioni C, et al Recurrence of pancreatic neuroendocrine tumors and survival predicted by Ki67. Ann Surg Oncol 2018;25:2467–2474
- 11. Andreasi V, Ricci C, Partelli S, Guarneri G, Ingaldi C, Muffatti F, et al Predictors of disease recurrence after curative surgery for nonfunctioning pancreatic neuroendocrine neoplasms (NF-PanNENs): a systematic review and meta-analysis. J Endocrinol Invest 2022;45:705–718
- 12. Zaidi MY, Lopez-Aguiar AG, Switchenko JM, Lipscomb J, Andreasi V, Partelli S, et al A novel validated recurrence risk score to guide a pragmatic surveillance strategy after resection of pancreatic neuroendocrine tumors: an international study of 1006 patients. Ann Surg 2019;270:422–433
- 13. Partelli S, Andreasi V, Peralta Ferreira M, Palumbo D, Muffatti F, Battistella A, et al Prognostic significance and predictors of nodal recurrence after surgery for non-functioning pancreatic neuroendocrine tumors. Ann Surg Oncol 2023;30:3466–3477
- 14. Opalińska M, Sowa-Staszczak A, Grochowska A, Olearska H, Hubalewska-Dydejczyk A. Value of peptide receptor radionuclide therapy as neoadjuvant treatment in the management of primary inoperable neuroendocrine tumors. Front Oncol 2021;11:687925
- 15. Chen H, Zhu C, He X, Dong W., Sun L. Neoadjuvant on the gastroenteropancreatic neuroendocrine tumors: a systematic review and meta-analysis. DOI:
- 16. van Vliet EI, van Eijck CH, de Krijger RR, Nieveen van Dijkum EJ, Teunissen JJ, Kam BL, et al Neoadjuvant treatment of nonfunctioning pancreatic neuroendocrine tumors with [177Lu-DOTA0, Tyr3]octreotate. J Nucl Med 2015;56:1647–1653
- 17. Xie H, Liu J, Yadav S, Keutgen XM, Hobday TJ, Strosberg JR, et al The role of perioperative systemic therapy in localized pancreatic neuroendocrine neoplasms. Neuroendocrinology 2020;110:234–245
- 18. Partelli S, Bertani E, Bartolomei M, Perali C, Muffatti F, Grana CM, et al Peptide receptor radionuclide therapy as neoadjuvant therapy for resectable or potentially resectable pancreatic neuroendocrine neoplasms. Surgery 2018;163:761–767
- 19. von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP. The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Int J Surg 2014;12:1495–1499
- 20. Dindo D, Demartines N, Clavien PA. Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 2004;240:205–213
- 21. Bassi C, Marchegiani G, Dervenis C, Sarr M, Abu Hilal M, Adham M, et al The 2016 update of the international study group (ISGPS) definition and grading of postoperative pancreatic fistula: 11 years after. Surgery 2017;161:584–591
- 22. Wente MN, Bassi C, Dervenis C, Fingerhut A, Gouma DJ, Izbicki JR, et al Delayed gastric emptying (DGE) after pancreatic surgery: a suggested definition by the International Study Group of Pancreatic Surgery (ISGPS). Surgery 2007;142:761–768
- 23. Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer 2009;45:228–247
- 24. Rindi G, Klöppel G, Alhman H, Caplin M, Couvelard A, de Herder WW, et al TNM staging of foregut (neuro)endocrine tumors: a consensus proposal including a grading system. Virchows Arch 2006;449:395–401
- 25. Brierly JD, Gospodarowicz MK, Witteking C (eds). TNM Classification of Malignant Tumours (8th edn). Oxford, Hoboken: John Wiley & Sons, 2017
- 26. Lloyd RV, Osamura RY, Klöppel G, RJ. WHO Classification of Tumours of Endocrine Organs (4th edn). IARC Press, 2017
- 27. Aaronson NK, Ahmedzai S, Bergman B, Bullinger M, Cull A, Duez NJ, et al The European Organization for Research and Treatment of Cancer QLQ-C30: a quality-of-life instrument for use in international clinical trials in oncology. J Natl Cancer Inst 1993;85:365–376
- 28. Partelli S, Massironi S, Zerbi A, Niccoli P, Kwon W, Landoni L, et al Management of asymptomatic sporadic non-functioning pancreatic neuroendocrine neoplasms no larger than 2 cm: interim analysis of prospective ASPEN trial. Br J Surg 2022;109:1186–1190
- 29. Titan AL, Norton JA, Fisher AT, Foster DS, Harris EJ, Worhunsky DJ, et al Evaluation of outcomes following surgery for locally advanced pancreatic neuroendocrine tumors. JAMA Netw Open 2020;3:e2024318
- 30. Jensen RT, Bodei L, Capdevila J, Couvelard A, Falconi M, Glasberg S, et al Unmet needs in functional and nonfunctional pancreatic neuroendocrine neoplasms. Neuroendocrinology 2019;108:26–36
- 31. Albers MB, Almquist M, Bergenfelz A, Nordenström E. Complications of surgery for gastro-entero-pancreatic neuroendocrine neoplasias. Langenbecks Arch Surg 2020;405:137–143
- 32. Partelli S, Tamburrino D, Cherif R, Muffatti F, Moggia E, Gaujoux S, et al Risk and predictors of postoperative morbidity and mortality after pancreaticoduodenectomy for pancreatic neuroendocrine neoplasms: a comparative study with pancreatic ductal adenocarcinoma. Pancreas 2019;48:504–509
- 33. Atema JJ, Jilesen APJ, Busch ORC, Van Gulik TM, Gouma DJ, Nieveen Van Dijkum EJM. Pancreatic fistulae after pancreatic resections for neuroendocrine tumours compared with resections for other lesions. HPB (Oxford) 2015;17:38–45
- 34. Hedges EA, Khan TM, Babic B, Nilubol N. Predictors of post-operative pancreatic fistula formation in pancreatic neuroendocrine tumors: a national surgical quality improvement program analysis. Am J Surg 2022;224:1256–1261
- 35. Schiavo Lena M, Partelli S, Castelli P, Andreasi V, Smart CE, Pisa E, et al Histopathological and immunophenotypic changes of pancreatic neuroendocrine tumors after neoadjuvant peptide receptor radionuclide therapy (PRRT). Endocr Pathol 2020;31:119–131