Introduction
Patients with clinically node-positive (cN+) breast cancer often receive neoadjuvant systemic therapy (NST). The best-case scenario is achieving a pCR, defined by the absence of remaining in-breast tumour (that is breast pCR) and/or axillary lymph node metastases (ALNMs; that is axillary pCR). On average, 37% of cN+ breast cancer patients treated with NST achieve an axillary pCR, with the rates varying between breast cancer molecular subtypes. A systematic review and meta-analysis by Samiei et al. showed that the probability of achieving an axillary pCR is 18% for hormone receptor (HR)+/human epidermal growth factor receptor 2 (HER2)−, 45% for HR+/HER2+, 60% for HR−/HER2+, and 48% for triple-negative breast cancer.
Axillary lymph node dissection (ALND) has been the standard treatment for cN+ breast cancer with remaining axillary disease after NST, but is not expected to benefit patients with an axillary pCR. High diagnostic accuracy is crucial for identifying axillary pCR after NST, to avoid omission of axillary surgery in the case of chemotherapy-resistant ALNMs. However, current imaging modalities are not very accurate with regard to identifying axillary pCR for cN+ patients after NST and thus carry an unacceptable risk of failing to detect residual disease. Less invasive axillary restaging procedures than ALND are needed to identify axillary pCR, and to adjust axillary treatment strategies accordingly,, with identification of the optimal axillary treatment strategy being debated.
Baseline disease extent, based on the number of ALNMs before NST, combined with axillary treatment response detected by less invasive axillary restaging procedures is used to guide axillary treatment strategies,. Axillary pCR has been shown to be strongly dependent on breast cancer molecular subtype. Therefore, incorporating molecular subtype into axillary treatment strategies may optimize patient-tailored management and enable further de-escalation of axillary treatment.
Therefore, the aim of this study was to assess whether axillary disease extent according to baseline [18F]fluorodeoxyglucose (FDG) PET/CT and breast cancer molecular subtype are predictors of axillary pCR for cN+ patients treated with NST in a retrospective analysis of the prospective Radioactive Iodine Seed placement in the Axilla with Sentinel lymph node biopsy (RISAS) trial.
Methods
Study design and patients
cN+ breast cancer patients who participated in the prospective RISAS trial (NCT02800317; METC number 2016-412) were included. In the RISAS trial, the diagnostic accuracy of the RISAS procedure was investigated in 13 institutions in the Netherlands between March 2017 and December 2019. RISAS participants underwent the RISAS procedure followed by completion ALND (cALND). The presence of ALNMs at the time of breast cancer diagnosis was confirmed by either core needle biopsy or fine needle aspiration. Patients who were eligible for participation in the RISAS trial were excluded in the case of infraclavicular or supraclavicular lymph node metastases (cN3a or cN3c), distant (oligo)metastases, a sentinel lymph node biopsy performed before NST, or previous radiotherapy to the ipsilateral axilla. Informed consent was obtained from all participants.
This study includes RISAS participants who underwent baseline [18F]FDG PET/CT imaging before the start of NST. Data on age, tumour size, tumour localization, clinical stage, histopathological subtype, molecular subtype, and treatment response were collected. Due to the retrospective analysis, the necessity to obtain additional informed consent was waived by all local medical ethics committees of the participating institutions.
[18F]fluorodeoxyglucose PET/CT imaging
All patients underwent whole-body [18F]FDG PET/CT imaging before NST initiation, using a standard acquisition protocol, in accordance with the practices of the institutions involved in this study. [18F]FDG PET/CT imaging was performed according to European Association of Nuclear Medicine (EANM) guidelines. All patients had to fast for at least 4–6 h before injection of intravenous [18F]FDG. The blood glucose levels of all patients were less than 11 mmol/l. After glucose testing, an intravenous [18F]FDG injection was administered to all patients. After a resting interval of 45–60 min whole-body [18F]FDG PET/CT imaging was performed.
All [18F]FDG PET/CT images were centrally reviewed on a dedicated commercially available workstation (syngo.via 6.4; Siemens Healthcare, Erlangen, Germany) and a minority of the [18F]FDG PET/CT images were reconstructed according to European Association of Nuclear Medicine Research Ltd (EARL) guidelines. Each visible ALNM on an [18F]FDG PET/CT image was scored using a four-point confidence scale (0, similar to surrounding lymph nodes; 1, slightly more intense than other lymph nodes; 2, moderately intense; and 3, very intense). First, the number of hypermetabolic ALNMs (score 1–3) was noted from the original report and patients were classified as having either limited (1–3 hypermetabolic ALNMs) or advanced (greater than or equal to 4 hypermetabolic ALNMs) baseline axillary disease. Thereafter, all [18F]FDG PET/CT images were reviewed by a final-year resident in radiology and nuclear medicine (T.J.A.v.N.), with 4 years of clinical experience in [18F]FDG PET/CT and breast imaging (reviewer 1). The number of hypermetabolic ALNMs from the original report was compared with the number of hypermetabolic ALNMs determined by reviewer 1. When the number of hypermetabolic ALNMs was missing from the original report or there was a discrepancy between the original report and reviewer 1 regarding the number of hypermetabolic ALNMs, another nuclear medicine physician (C.M.), with more than 10 years of experience in nuclear imaging, reviewed the images to obtain consensus on the number of hypermetabolic ALNMs.
Neoadjuvant systemic therapy
The NST regimen was based on the Dutch national breast cancer guidelines that were in effect at the time of the RISAS trial. Generally, anthracycline- and/or taxane-based regimens were administered. HER2-positive breast cancer patients also received targeted therapy (trastuzumab alone or trastuzumab and pertuzumab).
Histopathology
All excised axillary lymph nodes were stained with haematoxylin and eosin. On-site use of immunohistochemistry was not obligatory. The absence of any residual disease (that is isolated tumour cells, micrometastases, and macrometastases) was defined as axillary pCR. As part of the prospective RISAS trial, all RISAS lymph nodes negative with regard to haematoxylin and eosin staining underwent immunohistochemistry and additional sectioning for central pathology review as previously described.
Statistical analysis
Proportions of patients with axillary pCR were compared between subgroups of patients with limited and advanced axillary disease according to baseline [18F]FDG PET/CT and subgroups of patients with different breast cancer molecular subtypes. The chi-squared test and Fisher’s exact test were used for significance testing. To evaluate the predictive effect of axillary disease extent according to baseline [18F]FDG PET/CT and molecular subtype on the probability of achieving axillary pCR after NST, univariable logistic regression analyses were performed. Discriminative ability is expressed using ORs with 95% confidence intervals. The predicted probabilities of the univariable models were used for the construction of receiver operating characteristic (ROC) curves and the calculation of corresponding area under the curve (AUC) values. A test for equality of paired ROC curves was used (‘roccomp command’ in STATA). Statistical analyses were performed using SPSS® (IBM, Armonk, NY, USA; 26.0) and STATA (release 14). All statistical tests were two-sided and P < 0.050 was considered statistically significant.
Results
Baseline and treatment characteristics
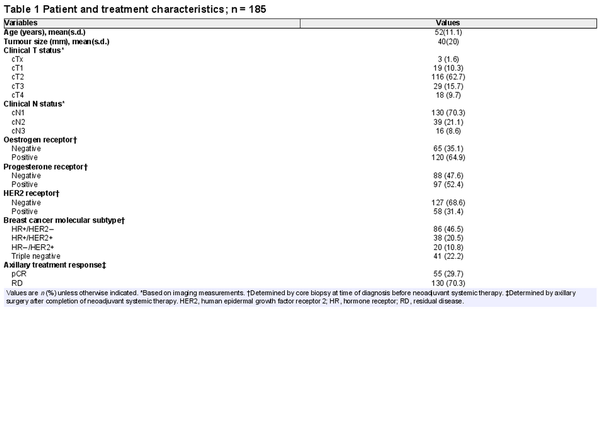
A total of 208 cN+ breast cancer patients were prospectively enrolled in the RISAS trial and underwent the RISAS procedure followed by cALND. For 88.9% (185 of 208) of patients, baseline [18F]FDG PET/CT imaging was performed. The mean patient age was 52 years and the mean tumour size was 40 mm. Limited baseline axillary disease was seen in 62.7% (116 of 185) and advanced axillary disease in 37.3% (69 of 185) of patients. Primary tumours were HR+/HER2− in 46.5% (86 of 185), HR+/HER2+ in 20.5% (38 of 185), HR−/HER2+ in 10.8% (20 of 185), and triple negative in 22.2% (41 of 185) of patients (Fig. 1). Overall, 29.7% (55 of 185) of patients achieved an axillary pCR. Clinicopathological characteristics are listed in Table 1.
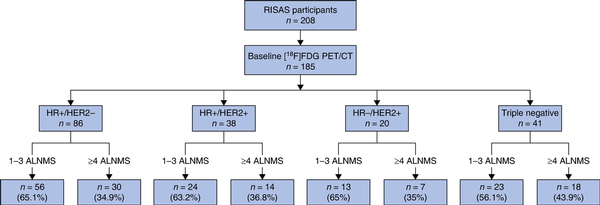
Fig. 1
Distribution of patients with different breast cancer molecular subtypes with either limited or advanced axillary disease according to baseline [18F]fluorodeoxyglucose PET/CT
RISAS, Radioactive Iodine Seed placement in the Axilla with Sentinel lymph node biopsy; FDG, fluorodeoxyglucose; HR, hormone receptor; HER2, human epidermal growth factor receptor 2; ALNMs, axillary lymph node metastases.
Axillary pCR stratified by baseline axillary disease extent and breast cancer molecular subtype
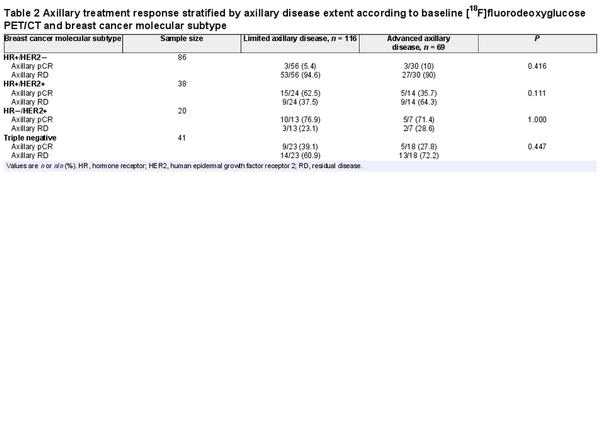
There was no significant difference in axillary pCR rates between patients with limited and advanced baseline axillary disease (31.9% (37 of 116) versus 26.1% (18 of 69) respectively (P = 0.403)) according to [18F]FDG PET/CT. However, axillary pCR rates varied significantly amongst molecular subtypes. The highest axillary pCR rate was seen for HR−/HER2+ patients (75% (15 of 20)), followed by HR+/HER2+ patients (52.6% (20 of 38)), triple-negative patients (34.1% (14 of 41)), and HR+/HER2− patients (7% (6 of 86)) (P < 0.001).
Within each of the molecular subtypes, the axillary pCR rates for limited and advanced baseline axillary disease did not differ significantly (5.4% (3 of 56) versus 10% (3 of 30) (P = 0.416) for HR+/HER2− patients, 62.5% (15 of 24) versus 35.7% (5 of 14) (P = 0.111) for HR+/HER2+ patients, 76.9% (10 of 13) versus 71.4% (5 of 7) (P = 1.000) for HR−/HER2+ patients, and 39.1% (9 of 23) versus 27.8% (5 of 18) (P = 0.447) for triple-negative patients) (Table 2). For HR+/HER2− patients, only 3.5% (3 of 86) had limited baseline axillary disease and axillary pCR, compared with 39.5% (15 of 38) of HR+/HER2+ patients, 50% (10 of 20) of HR−/HER2+ patients, and 22% (9 of 41) of triple-negative patients (Fig. 1 and Table 2).
Univariable logistic regression
Two separate univariable logistic regression models were used to evaluate the predictive effect on the probability of achieving axillary pCR after NST; one with axillary disease extent according to baseline [18F]FDG PET/CT as an independent variable and one with breast cancer molecular subtype as an independent variable. The OR for axillary pCR associated with limited versus advanced baseline axillary disease extent was 0.75 (95% c.i. 0.38 to 1.46) (P = 0.404). Molecular subtype was designated as the categorical variable, with patients with HR+/HER2− tumours designated as the reference category. Significantly increased ORs of 14.82 (95% c.i. 5.21 to 42.16) for patients with HR+/HER2+ tumours, 40 (95% c.i. 10.87 to 148.05) for patients with HR−/HER2+ tumours, and 6.91 (95% c.i. 2.42 to 19.78) for patients with triple-negative tumours were found (P < 0.001). Figure 2 shows the ROC curves based on the predicted probabilities of each model. The AUC for axillary disease extent according to baseline [18F]FDG PET/CT was 0.533 (95% c.i. 0.442 to 0.623) (P = 0.485) and the AUC for molecular subtype was 0.809 (95% c.i. 0.740 to 0.877) (P < 0.001), and the difference between these AUCs was statistically significant (P < 0.001) (Fig. 2).
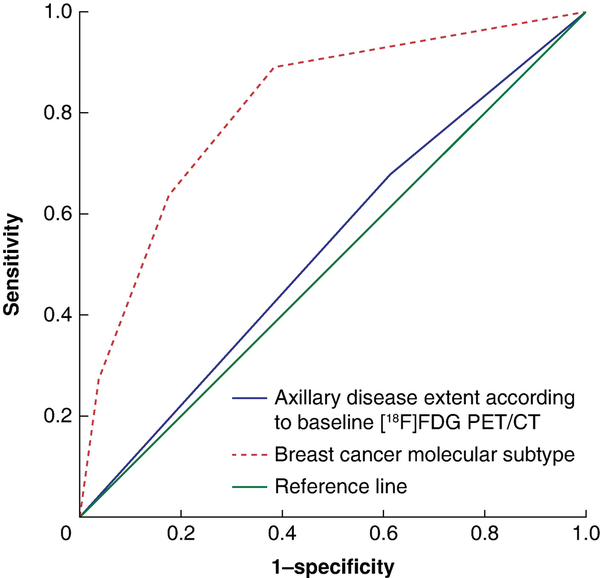
Fig. 2
Receiver operating characteristic (ROC) curves evaluating the predictive effect of axillary disease extent according to baseline [18F]fluorodeoxyglucose PET/CT and breast cancer molecular subtype on the probability of achieving axillary pCR
FDG, fluorodeoxyglucose.
Discussion
This study investigated the predictive effect of axillary disease extent according to baseline [18F]FDG PET/CT and breast cancer molecular subtype on the probability of achieving axillary pCR after NST. The breast cancer molecular subtype is significantly correlated with axillary pCR, whereas axillary disease extent according to baseline [18F]FDG PET/CT is not.
Previous studies have already demonstrated the importance of molecular subtype in the evaluation of axillary response after NST in breast cancer,. The axillary pCR rates for HR+/HER2−, HR+/HER2+, HR−/HER2+, and triple-negative breast cancer reported in the present study are well in line with previous findings,,.
Axillary disease extent according to baseline [18F]FDG PET/CT does not appear to be a useful predictor of axillary pCR after NST. The axillary pCR rates for limited and advanced baseline axillary disease did not differ significantly overall (31.9% versus 26.1% respectively) and this finding was consistent among all molecular subtypes. This is line with a study by Ng et al., who found no significant difference in axillary pCR rates between patients with low (1–2 abnormal ALNMs) or high (greater than or equal to 3 abnormal ALNMs) axillary disease according to baseline ultrasonography (44% versus 42% respectively). A study by van Loevezijn et al. reported axillary pCR rates of 32% for patients with low (less than 4 hypermetabolic ALNMs) and 44% for patients with high (greater than or equal to 4 hypermetabolic ALNMs) axillary disease extent according to baseline [18F]FDG PET/CT. A study by Garcia-Tejedor et al. reported an axillary pCR rate of 40% in the case of advanced axillary disease according to baseline ultrasonography (that is at least three suspicious ALNMs) and even higher axillary pCR rates for HER2-positive (68%) and triple-negative (45%) patients.
Although axillary disease extent is not a significant predictor of axillary pCR, it is used in clinical practice to guide adjuvant axillary treatment. This is illustrated by an axillary treatment protocol that combines the number of hypermetabolic ALNMs according to baseline [18F]FDG PET/CT with the Marking Axillary lymph nodes with Radioactive Iodine seeds (MARI) procedure. This protocol recommends no further treatment for patients with limited axillary disease (1–3 hypermetabolic ALNMs) and axillary pCR according to the MARI procedure. For patients with advanced axillary disease (greater than or equal to 4 hypermetabolic ALNMs) according to baseline [18F]FDG PET/CT and axillary pCR according to the MARI procedure, axillary radiotherapy is recommended. For patients with limited baseline axillary disease and axillary residual disease according to the MARI procedure, axillary radiotherapy is also recommended, whereas, for patients with advanced baseline axillary disease and residual disease according to the MARI procedure, both ALND and axillary radiotherapy are recommended,. It is questionable whether an axillary treatment protocol that combines the number of hypermetabolic ALNMs according to baseline [18F]FDG PET/CT and pathological axillary response to NST is entirely justified, as only molecular subtype was a significant predictor of axillary response to NST in the present study. Almost all (96.5%) of the HR+/HER2− patients in this study would have an indication for ALND ± axillary radiotherapy, compared with 50–78% of patients with other molecular subtypes. Therefore, the introduction of information on molecular subtype may be highly relevant in axillary treatment protocols.
The present study focused on predictors of axillary pCR for cN+ patients treated with NST. However, previous studies have indicated that axillary pCR after NST does not have the same impact for all molecular subtypes,. Especially for those with HR+/HER2− tumours, the prognosis, in terms of recurrence-free and overall survival, for patients with axillary pCR seems similar to that for patients with residual disease in up to three axillary lymph nodes in surgical specimens after NST,. This might provide an additional argument to pay attention to molecular subtype in axillary treatment protocols.
Apart from axillary treatment protocols, the importance of information on breast cancer molecular subtype might also be considered in ongoing studies. At this moment, various clinical trials are investigating the best way to manage axillary residual disease after NST and surgery. The Alliance A011202 (NCT01901094) and TAXIS (NCT03513614) trials are investigating whether axillary radiotherapy and tailored axillary surgery combined with regional nodal irradiation can safely replace cALND for patients with axillary residual disease after NST,. Additionally, the MINIMAX (NCT04486495) and AXSANA (NCT04373655) registry trials are assessing the impact of different axillary restaging procedures, the oncological safety, and the impact on quality of life for cN+ patients after NST,. As cALND is more frequently being omitted for cN+ patients with axillary residual disease after NST, the results of these trials are highly anticipated. It would be highly beneficial for ongoing and future studies to include information on molecular subtype in their designs.
This study has some limitations. Axillary disease extent was only evaluated according to baseline [18F]FDG PET/CT and results from this study may not be generalizable to settings wherein pathological confirmation or other imaging modalities, such as axillary ultrasonography or MRI, are used for evaluation of the number of ALNMs at baseline. However, baseline [18F]FDG PET/CT is known to accurately stage regional and distant disease dissemination and has a positive predictive value of 98% with regard to detecting ALNMs. Moreover, 13 institutions participated in the prospective multicentre RISAS trial. Only a minority of the [18F]FDG PET/CT images were reconstructed according to EARL guidelines, allowing data pooling with regard to the metabolic information in each [18F]FDG PET/CT image. Consequently, it was not possible to combine measurements on metabolic parameters such as standardized uptake values between the participating centres.
This study demonstrates that molecular subtype is a significant predictor of axillary pCR after NST, whereas axillary disease extent according to baseline [18F]FDG PET/CT is not. Information on molecular subtype may be relevant to guide further axillary treatment for cN+ patients treated with NST.
Collaborators
L. de Beer (Martini Hospital, Groningen, the Netherlands); E.G. Boerma (Maastricht University Medical Center+, Maastricht, the Netherlands); M. Boskamp (Wilhelmina Hospital, Assen, the Netherlands); E.M.J. Brouwers-Kuyper (Albert Schweitzer Hospital, Dordrecht, the Netherlands); C.M.E. Contant (Maasstad Hospital, Rotterdam, the Netherlands); A.W.F. du Mée (Amphia Hospital, Breda, the Netherlands); H.J. Heijmans (Hospital Group Twente, Breast Clinic Oost-Nederland, Hengelo, the Netherlands); S. Ho-Han (Albert Schweitzer Hospital, Dordrecht, the Netherlands); F. Hulsebosch (Franciscus Gasthuis & Vlietland, Schiedam, the Netherlands); A. Jager (Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands); J.A.J. Janssen (Ikazia Hospital, Rotterdam, the Netherlands); B.L.R. Kam (Ikazia Hospital, Rotterdam, the Netherlands); W. Kelder (Martini Hospital, Groningen, the Netherlands); T.M.A.L. Klem (Franciscus Gasthuis & Vlietland, Schiedam, the Netherlands); K.P. Koopmans (Martini Hospital, Groningen, the Netherlands); M.B.I. Lobbes (Zuyderland Medical Center, Sittard-Geleen, the Netherlands); M.B.E. Menke-Pluijmers (Albert Schweitzer Hospital, Dordrecht, the Netherlands); C. de Monye (Erasmus Medical Center, Rotterdam, the Netherlands); P. Sars (Bravis Hospital, Roosendaal, the Netherlands); L.H.M. Smit (Treant Zorggroep Hospital, Hoogeveen, the Netherlands); E. van Haaren (Zuyderland Medical Center, Sittard-Geleen, the Netherlands); D. van Klaveren (Erasmus Medical Center, Rotterdam, the Netherlands); J. Veltman (Hospital Group Twente, Breast Clinic Oost-Nederland, Hengelo, the Netherlands); C. Verhoef (Erasmus Medical Center Cancer Institute, Rotterdam, the Netherlands); W.J. Vles (Ikazia Hospital, Rotterdam, the Netherlands).
References
- 1. Cao S, Liu X, Cui J, Liu X, Zhong J, Yang Z, et al Feasibility and reliability of sentinel lymph node biopsy after neoadjuvant chemotherapy in breast cancer patients with positive axillary nodes at initial diagnosis: an up-to-date meta-analysis of 3,578 patients. Breast 2021;59:256–269
- 2. van Nijnatten TJ, Simons JM, Smidt ML, van der Pol CC, van Diest PJ, Jager A, et al A novel less-invasive approach for axillary staging after neoadjuvant chemotherapy in patients with axillary node-positive breast cancer by combining radioactive iodine seed localization in the axilla with the sentinel node procedure (RISAS): a Dutch prospective multicenter validation study. Clin Breast Cancer 2017;17:399–402
- 3. Esgueva A, Siso C, Espinosa-Bravo M, Sobrido C, Miranda I, Salazar JP, et al Leveraging the increased rates of pathologic complete response after neoadjuvant treatment in breast cancer to de-escalate surgical treatments. J Surg Oncol 2021;123:71–79
- 4. Hong J, Tong Y, He J, Chen X, Shen K. Association between tumour molecular subtype, clinical stage and axillary pathological response in breast cancer patients undergoing complete pathological remission after neoadjuvant chemotherapy: potential implications for de-escalation of axillary surgery. Ther Adv Med Oncol 2021;13:1758835921996673
- 5. Samiei S, van Nijnatten TJ, de Munck L, Keymeulen KB, Simons JM, Kooreman LF, et al Correlation between pathologic complete response in the breast and absence of axillary lymph node metastases after neoadjuvant systemic therapy. Ann Surg 2020;271:574–580
- 6. Samiei S, Simons JM, Engelen SME, Beets-Tan RGH, Classe JM, Smidt ML, et al Axillary pathologic complete response after neoadjuvant systemic therapy by breast cancer subtype in patients with initially clinically node-positive disease a systematic review and meta-analysis. JAMA Surg 2021;156:e210891
- 7. Simons JM, van Nijnatten TJA, van der Pol CC, van Diest PJ, Jager A, van Klaveren D, et al Diagnostic accuracy of radioactive iodine seed placement in the axilla with sentinel lymph node biopsy after neoadjuvant chemotherapy in node-positive breast cancer. JAMA Surg 2022;157:991–999
- 8. Samiei S, de Mooij CM, Lobbes MB, Keymeulen KB, van Nijnatten TJ, Smidt ML. Diagnostic performance of noninvasive imaging for assessment of axillary response after neoadjuvant systemic therapy in clinically node-positive breast cancer: a systematic review and meta-analysis. Ann Surg 2021;273:694–700
- 9. Aktaş A, Gürleyik MG, Aksu SA, Aker F, Güngör S. Diagnostic value of axillary ultrasound, MRI, and 18F-FDG-PET/CT in determining axillary lymph node status in breast cancer patients. Eur J Breast Health 2022;18:37–47
- 10. de Wild SR, Simons JM, Vrancken Peeters MTFD, Smidt ML, Koppert LBMINIMAX Group. MINImal vs. MAXimal invasive axillary staging and treatment after neoadjuvant systemic therapy in node positive breast cancer: protocol of a Dutch multicenter registry study (MINIMAX). Clin Breast Cancer 2022;22:e59–e64
- 11. Koolen B, Donker M, Straver M, van der Noordaa M, Rutgers E, Valdes Olmos R, et al Combined PET–CT and axillary lymph node marking with radioactive iodine seeds (MARI procedure) for tailored axillary treatment in node-positive breast cancer after neoadjuvant therapy. Br J Surg 2017;104:1188–1196
- 12. van der Noordaa MEM, van Duijnhoven FH, Straver ME, Groen EJ, Stokkel M, Loo CE, et al Major reduction in axillary lymph node dissections after neoadjuvant systemic therapy for node-positive breast cancer by combining PET/CT and the MARI procedure. Ann Surg Oncol 2018;25:1512–1520
- 13. Aukema TS, Straver ME, Peeters M-JTV, Russell NS, Gilhuijs KG, Vogel WV, et al Detection of extra-axillary lymph node involvement with FDG PET/CT in patients with stage II–III breast cancer. Eur J Cancer 2010;46:3205–3210
- 14. NABON Breast Cancer Audit. National Breast Cancer Guideline. 2017. https://richtlijnendatabase.nl/richtlijn/borstkanker/behandeling_invasief_carcinoom/neoadjuvante_behandeling/systemische_therapie.html
- 15. He ZY, Wu SG, Yang Q, Sun JY, Li FY, Lin Q, et al Breast cancer subtype is associated with axillary lymph node metastasis: a retrospective cohort study. Medicine (Baltimore) 2015;94:e2213
- 16. Zetterlund L, Celebioglu F, Hatschek T, Frisell J, de Boniface J. Long-term prognosis in breast cancer is associated with residual disease after neoadjuvant systemic therapy but not with initial nodal status. Br J Surg 2021;108:583–589
- 17. Ng S, Sabel MS, Hughes TM, Chang AE, Dossett LA, Jeruss JS. Impact of breast cancer pretreatment nodal burden and disease subtype on axillary surgical management. J Surg Res 2021;261:67–73
- 18. van Loevezijn AA, van der Noordaa ME, Stokkel MP, van Werkhoven ED, Groen EJ, Loo CE, et al Three-year follow-up of de-escalated axillary treatment after neoadjuvant systemic therapy in clinically node-positive breast cancer: the MARI-protocol. Breast Cancer Res Treat 2022;193:37–48
- 19. Garcia-Tejedor A, Fernandez-Gonzalez S, Ortega R, Gil-Gil M, Perez-Montero H, Fernandez-Montolí E, et al Can we avoid axillary lymph node dissection in N2 breast cancer patients with chemo-sensitive tumours such as HER2 and TNBC? Breast Cancer Res Treat 2021;185:657–666
- 20. Fayanju OM, Ren Y, Thomas SM, Greenup RA, Plichta JK, Rosenberger LH, et al The clinical significance of breast-only and node-only pathologic complete response (pCR) after neoadjuvant chemotherapy (NACT): a review of 20,000 breast cancer patients in the National Cancer Data Base (NCDB). Ann Surg 2018;268:591–601
- 21. Laot L, Laas E, Girard N, Dumas E, Daoud E, Grandal B, et al The prognostic value of lymph node involvement after neoadjuvant chemotherapy is different among breast cancer subtypes. Cancers (Basel) 2021;13:171
- 22. Comparison of Axillary Lymph Node Dissection with Axillary Radiation for Patients with Node-Positive Breast Cancer Treated with Chemotherapy. ClinicalTrials.gov identifier: NCT01901094. 2023. https://clinicaltrials.gov/study/NCT01901094#publications
- 23. Henke G, Knauer M, Ribi K, Hayoz S, Gérard MA, Ruhstaller T, et al Tailored axillary surgery with or without axillary lymph node dissection followed by radiotherapy in patients with clinically node-positive breast cancer (TAXIS): study protocol for a multicenter, randomized phase-III trial. Trials 2018;19:1–28
- 24. Gasparri ML, de Boniface J, Poortmans P, Gentilini OD, Kaidar-Person O, Banys-Paluchowski M, et al Axillary surgery after neoadjuvant therapy in initially node-positive breast cancer: international EUBREAST survey. Br J Surg 2022;109:857–863
- 25. Koolen BB, Valdés Olmos RA, Elkhuizen PH, Vogel WV, Vrancken Peeters M-JT, Rodenhuis S, et al Locoregional lymph node involvement on 18F-FDG PET/CT in breast cancer patients scheduled for neoadjuvant chemotherapy. Breast Cancer Res Treat 2012;135:231–240