Introduction
In developed countries, bioprostheses have been considered the valve substitute of choice in elderly patients, especially in those who are 65 years or older [,,,]. A growing number of people optimize their choice of bioprosthesis over mechanical prostheses as a result of freedom from anticoagulation therapy and associated complications such as hemorrhages and thromboembolic events [,]. The second-generation Carpentier-Edwards Perimount (CE-P) bovine pericardial bioprosthesis (Edwards Lifesciences, Irvine, CA, USA) has shown excellent safety and efficacy in long-term clinical follow-up investigations [,,,,,]. In addition, age has been reported as an influential factor for bioprosthesis durability, survival, and the risks that are associated with reoperation [,,]. In China, mechanical prostheses are still predominant for cardiac valve replacements in clinical practice; in fact, the patients' age at the time of implantation is relatively younger than in industrialized societies [,,]. Unlike in Western countries, where the etiologic spectrum is dominated by degenerative heart disease, the majority of Chinese patients receive valve replacement as a result of rheumatic heart disease [,]. Very few studies have presented mid- to long-term outcomes of patients who have undergone bioprosthetic valve replacement in China, especially when the influence of age at surgery and etiology was assessed.
We herein report the safety and efficacy results of a 9- to 15-year follow-up investigation among patients who had received CE-P bioprostheses for valve replacement in aortic, mitral, tricuspid, or a combination of these positions with a primary focus on the occurrence of structural valve deterioration (SVD) and reoperation for SVD, as well as on survival and reoperation, among different age and etiology groups.
Subjects and Methods
This study was approved by the Ethics Committee of Guangdong General Hospital. From 2001 to 2007, a total of 4,726 patients received prosthetic valve implantation at Guangdong General Hospital, including 3,910 with mechanical valves, 812 with bioprostheses, and 4 with the implantation of both mechanical and bioprosthetic valves. A total of 253 patients who were implanted with CE-P bioprostheses (types 6900P and 2900; Fig. 1) during the same period with recent medical data and complete medical records were identified. Among them, 13 were excluded from final analysis, since complete data could not be obtained for these patients as a result of old age, immobility, or refusal of direct contact of the patient by family members. Therefore, the overall follow-up rate for this study was 94.8% (240/253), with a final inclusion of 225 patients for analysis.
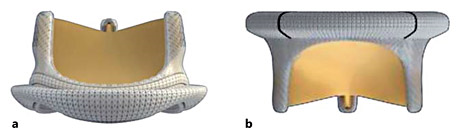
Fig. 1
Illustration of the Carpentier-Edwards Perimount bioprosthesis for aortic valve replacement (a) and mitral valve replacement (b).
Standard surgical procedures of cardiac valve replacement were adopted for all patients. In brief, a median sternotomy was conducted after the use of general anesthesia. The surgery was performed under moderate hypothermia (28-30°C) with a cardiopulmonary bypass. Blood or crystalloid cardioplegia was applied in the aortic root or coronary ostia for myocardial protection, as described in previous studies []. Valves were sutured with everting mattress or horizontal mattress sutures. All concomitant procedures were performed prior to valve implantation. Postoperatively, oral anticoagulants were prescribed to all patients for the first 3 months, and then terminated in those who were not indicated for atrial fibrillation; antiplatelet therapy was continually prescribed for patients with concomitant coronary artery bypass grafting.
For the purpose of this study, the patients were scheduled to return to Guangdong General Hospital for both clinical and echocardiographic examinations; for those who had difficulty returning to Guangdong General Hospital, the follow-up was completed at local hospitals. The mean follow-up duration, when not accounting for deaths, was 127.4 ± 20.4 months; when deaths were accounted for, it was 101.4 ± 45.9 months.
Early mortality is defined as mortality within the first 30 days following surgery or before discharge, whereas late mortality refers to death beyond the first 30 days following surgery or after discharge. SVD, defined as a dysfunction of valve opening and closing movements, is diagnosed by echocardiographic reexamination during a patient's follow-up period. The definitions of mortality, as well as valve-related complications including SVD, thromboembolism, bleeding, operated valve endocarditis, and nonstructural dysfunctions such as paravalvular leakage, were solely based on the guidelines for reporting mortality and morbidity after cardiac valve interventions [].
Categorical data are summarized as frequencies and percentages, whereas continuous data are presented as mean (±SD), or median and range. Differences in percentages were tested with the χ2 test or Fisher's exact test. The Kaplan-Meier survival analysis, accompanied by a log-rank test, was adopted to estimate SVD and survival, for which the potential risk factors were identified with multivariate Cox proportional hazards regression through the stepwise method. The significance level α was set at 0.05 and the statistical analyses were performed using SAS software (version 9.1.3; SAS Institute, Cary, NC, USA).
Results
The baseline and operative characteristics are shown in Table 1. The patients included in this study had a young age at implantation (61.2 ± 11.5 years) and a unique etiologic spectrum: the most common etiology for cardiac valve replacement was rheumatic disease (55.1%), followed by degenerative disease (22.7%). Due to the high proportion of patients with rheumatic heart disease, approximately one-fifth of the patients (21.3%) underwent double valve replacement (DVR) in this study.
Postoperatively, over half (58.2%) of the patients were New York Heart Association (NYHA) class I or II. A total of 86 deaths (38.2%; N = 225) occurred, of which 14 (6.2%) were early and 72 (32.0%) were late. Thirty-eight of these deaths (44.2%) were cardiac, 23 (26.7%) were noncardiac, 1 (1.2%) was non-valve-related, and the remaining 24 (27.9%) were of unknown etiology.
The median survival time was 12.6 years (range: 0.01-13.86) for patients with mitral valve replacement (MVR; N = 96), whereas it was not calculable for the groups with aortic valve replacement (AVR) and DVR in both aortic and mitral positions: the rate of survival was never ≤50% for either group. The survival rate was 86.46% (95% CI: 77.82-91.90), 81.58% (95% CI: 70.89-88.65), and 74.42% (95% CI: 63.80-82.34) at 5 years, and 64.39% (95% CI: 52.96-73.72), 66.19% (95% CI: 53.63-76.08), and 55.85% (95% CI: 43.07-66.84) at 10 years, for the groups with MVR, AVR, and DVR, respectively. At 15 years, it was 48.37% (95% CI: 30.18-64.38), 57.33% (95% CI: 42.68-69.52), and 46.54% (95% CI: 30.71-60.92) for the three groups, respectively (Fig. 2).
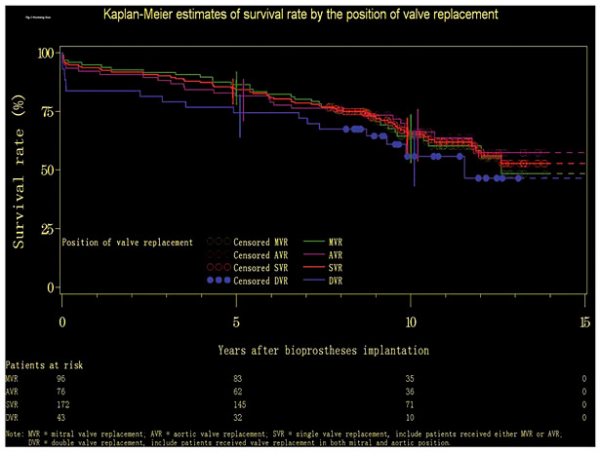
Fig. 2
Kaplan-Meier estimates of survival rates by the position of valve replacement.
Multivariate Cox proportional hazards regression analysis showed that for patients who underwent MVR, the hazard ratio (HR) of mortality for those ≥70 years was significantly higher than that for the youngest age group (HR = 9.350; 95% CI: 2.628-33.273; p = 0.0006). The HR of mortality was not statistically different between the 60- to 69-year and the <60-year age groups or between the ≥70-year and the 60- to 69-year age groups. Concomitant procedures were found to have an influence on the survival of patients who underwent AVR: the reported HR of mortality for those who had not undergone concomitant procedures was 3.438 times higher than that for those who had undergone the procedure (95% CI: 1.248-9.472; p = 0.0169). None were identified as statistically significant factors that affected the survival of those who underwent DVR in both aortic and mitral positions.
A total of 35 patients (15.6%; N = 225) experienced bleeding events: in 26 cases (74.3%) these events occurred during the intraoperative period, and in the remaining 9 cases (25.7%) they occurred during the follow-up period. Other valve-related complications included thromboembolic events, perivalvular leakage, and endocarditis, which were reported in 2 (0.9%), 4 (1.8%), and 6 (2.7%) of the patients, respectively. These valve-related complications all occurred during the follow-up period.
Among the 225 patients, 43 (19.1%) developed SVD during the follow-up period: 17 (39.5%) had MVR, 12 (27.9%) had AVR, 1 (2.3%) had tricuspid valve replacement, and the remaining 13 (30.2%) had DVR in both aortic and mitral positions. The median time to freedom from SVD was 12.5 years (range: 0.01-13.13), 13.2 years (range: 0.02-13.82), and 11.2 years (range: 0.00-13.09), respectively, for patients with MVR, AVR, and DVR. The 5-year actuarial freedom from SVD was 100%, and the 10-year actuarial freedom from SVD was 83.94 ± 5.16%, 90.76 ± 4.41%, and 68.16 ± 10.74% for patients with MVR, AVR, and DVR, respectively. At 15 years, the actuarial freedom from SVD was 30.62 ± 15.09% for patients with MVR.
The median time to freedom from SVD for patients with MVR was 10.6 years (range: 0.57-13.13), 12.7 years (range: 1.13-13.03), and 12.5 years (range: 0.01-12.50) for the age groups of <60, 60-69, and ≥70 years, respectively. Actuarial freedom from SVD at 5 years was 100% for all three age groups, and at 10 years it remained 100% for the oldest age group and was reduced to 67.67 ± 14.10 and 87.93 ± 5.07% for the age groups of <60 and 60-69 years, respectively. At 15 years, it was 22.56 ± 17.86% for the age group of <60 years and 33.49 ± 24.40% for the age group of 60-69 years (Fig. 3). No statistical difference was observed for the rates of SVD occurrence between the three age groups (p = 0.293).
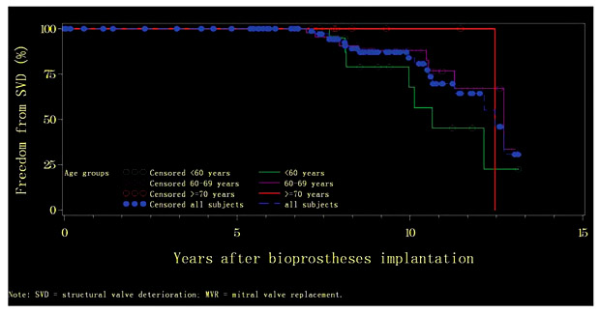
Fig. 3
Kaplan-Meier curves of actuarial freedom from structural valve deterioration (SVD) by age group among subjects with mitral valve replacement (MVR).
For patients with AVR, the median time to freedom from SVD was 9.5 years (range: 0.06-10.18) and 13.2 years (range: 0.02-13.82) for the age groups of <60 and 60-69 years, respectively, but it was not calculable for the group ≥70 years. Actuarial freedom from SVD at 5 years was 100% for all three age groups, and at 10 years it remained 100% for the oldest age group and was reduced to 40.00 ± 29.66 and 94.29 ± 3.92% for the age groups of <60 and 60-69 years, respectively. At 15 years, it was 40.00 ± 29.66% for the age group of <60 years and 66.67 ± 27.22% for the age group of ≥70 years (Fig. 4). The results of log-rank testing indicate a significant difference in terms of SVD occurrence rates between the three age groups among patients with AVR (p = 0.004).
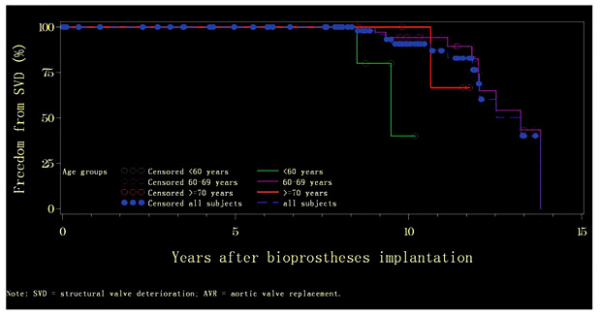
Fig. 4
Kaplan-Meier curves of actuarial freedom from structural valve deterioration (SVD) by age group among subjects with aortic valve replacement (AVR).
All the patients with DVR who experienced SVD were <70 years old. The median time to freedom from SVD was 8.7 years (range: 4.39-9.61) and 11.3 years (range: 0.00-13.09) for the age groups of <60 and 60-69 years, respectively. Actuarial freedom from SVD at 5 years was 100% for both groups, and at 10 years it was 37.50 ± 20.35 and 79.48 ± 11.29% for the age groups of <60 and 60-69 years, respectively. At 15 years, it remained 37.50 ± 20.35% for the age group of <60 years (Fig. 5). The rates of SVD occurrence did not differ between the two age groups.
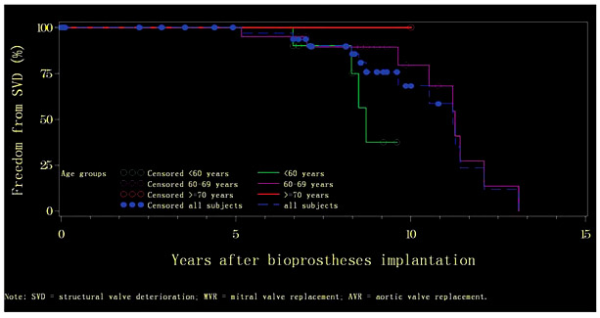
Fig. 5
Kaplan-Meier curves of actuarial freedom from structural valve deterioration (SVD) by age group among subjects with both mitral valve replacement (MVR) and aortic valve replacement (AVR).
In patients who underwent valve replacement due to rheumatic, degenerative, endocarditic, ischemic, and other causes of valve disease, the proportions having developed SVD during the follow-up period were 22.6% (N = 124), 15.7% (N = 51), 16.7% (N = 6), 16.7% (N = 18), and 11.5% (N = 26), respectively. The median time to freedom from SVD was 12.1 years (range: 0.00-13.82), 12.5 years (range: 0.01-13.33), and 11.3 years (range: 0.05-12.45), respectively, for patients with a disease of rheumatic, degenerative, or ischemic etiology, whereas it was not calculable for those with a disease of endocarditic or another etiology, since the rate of freedom from SVD was not reduced to (or less than) 50% in both groups. Actuarial freedom from SVD at 5 years was 100% for all groups; at 10 years, it was over 80% for all but those with an ischemic cause (75 ± 21.65%). The results for total SVD occurrence, median time to SVD, and incidence of SVD at 5, 10, and 15 years according to etiology are presented separately for the patients who underwent MVR, AVR, and DVR in Table 2.
A higher age at the time of implantation was a significant risk factor for SVD, as identified in patients with AVR (p = 0.01). The HR of SVD occurrence for patients with AVR in the age group of <60 years was 14.921 times higher than that in the age group of 60-69 years (95% CI: 1.908-116.665; p = 0.01); however, no significant difference was found between the age groups of 60-69 years and ≥70 years. For patients with MVR, the preoperative NYHA functional class was found to have an influence on SVD occurrence (p = 0.0195): the HR of those patients who had belonged to NYHA functional class II preoperatively was 4,395 times (95% CI: 1.269-15.228) higher than that of the NYHA class III group.
Thirteen patients (5.78%; N = 225) underwent reoperation, including 8 with single valve replacement (6 for MVR, 1 for AVR, and 1 for tricuspid valve replacement), 4 with DVR in aortic and mitral positions, and 1 with three-valve replacement in aortic, mitral, and tricuspid positions. Of the 13 patients, 8 (61.5%) were reoperated on as a result of SVD, whereas of the 5 patients (38.5%) who did not develop SVD but who underwent a reoperation, 3 (60%; N = 5) underwent the procedure as a result of endocarditis, 1 (20%; N = 5) had it because of a stitching error, and 1 (20%; N = 5) had it due to thromboembolism.
Discussion
This long-term follow-up study evaluated the durability of the CE-P valve in terms of clinical effectiveness among Chinese patients who had received valve replacements in aortic, mitral, tricuspid, or a combination of these positions. The patients included in this study had a younger age (61.2 ± 11.5 years) at implantation than patients in previous reports [,,]. A different etiologic spectrum was also observed: while degenerative heart disease was the main cause of valve replacement in Western countries [,], more than half of the patients (55.1%) had rheumatic heart disease in this study. Due to the small sample size, our results could not reveal an explicit association between SVD and etiology for valve replacement; for this, in the future more studies on large samples are required to gain a better understanding of the possibility of such associations.
Our study found a survival rate of 86.46, 81.58, and 74.42% at 5 years, 64.39, 66.19, and 55.85% at 10 years, and 48.37, 57.33, and 46.54% at 15 years for the groups with MVR, AVR, and DVR, respectively, as compared to the overall 5-, 10-, and 15-year survival rates (71.7, 66.9, and 55.5%) of 82 patients having received valve replacement with a standard Carpentier-Edwards porcine valve in Taiwan []. In another series including 227 patients who had received epichlorohydrin-modified glutaraldehyde-treated porcine valves (Bio-Rens Porcine Valve; BalMedic, Beijing, China) for aortic, mitral, tricuspid, and double (mitral and aortic) valve replacements in China, Wei et al. [] found that the 10-year survival rate was 83.1% for AVR, 78.8% for MVR, and 54.3% for DVR. At 15 years, it corresponded to 58.1% for AVR, 49.0% for MVR, and 40.7% for DVR. The relatively higher rate of survival at 10 years for AVR and MVR in the study of Wei et al. [], as compared to ours, could be attributable to the younger age at implantation in their study (mean: 42 ± 7.9 years). Our results for survival were consistent with those of the study by Poirer et al. [], which pertained to the CE-P valve. Their actuarial survival at 5, 10, and 15 years was higher for single valve replacement in either the aortic or the mitral position than for DVR in both positions, and survival following MVR at 5 years was higher than that following AVR, even though it decreased at a faster rate [].
In this study, the 5-year actuarial freedom from SVD was 100%, and the 10-year actuarial freedom from SVD was 83.94, 90.76, and 68.16% for patients with MVR, AVR, and DVR, respectively. At 15 years, it was 30.62% for patients with MVR. Zhu et al. [] reported an overall valve durability rate of 75% at 10 years for 520 Chinese patients implanted with the bovine pericardial xenograft modeled after a similar type of prosthesis manufactured by Shiley Inc. (Irvine, CA, USA). However, a study involving the implantation of Hancock II porcine bioprostheses (Medtronic Inc., Minneapolis, MN, USA) into 647 Chinese patients in aortic, mitral, and both positions, respectively, revealed that the 5- and 10-year freedom from SVD was as high as 98.9 and 94.3% for AVR, 97.0 and 95.5% for MVR, and 98.0 and 93.7% for DVR []. Similar findings were also made in the study of Wei et al. [], where 227 patients were implanted with epichlorohydrin-modified glutaraldehyde-treated porcine valves, with the 5- and 10-year freedom from SVD being 96.4 and 91.1% for AVR, 96.3 and 88.3% for MVR, and 100 and 66.7% for DVR. However, as the patient characteristics and operative procedures differed between the aforementioned studies, and since there is a lack of direct comparison between CE-P and other bioprostheses in a homogeneous population, conclusions could not be drawn on the superiority of any particular bioprosthesis.
The results for 5-, 10-, and 15-year freedom from SVD according to age group are consistent with the findings of other studies on CE-P valves [,,,]. The study by Marchand et al. [] revealed that for patients who had undergone either isolated MVR or combined MVR and AVR, the freedom from SVD at 5, 10, and 14 years was 99.3 ± 0.7, 83.8 ± 3.3, and 59.2 ± 6.6%, respectively, for those ≤60 years of age, 100, 94.6 ± 2.2, and 76.0 ± 6.3% for those 61-70 years of age, and 100% at both 5 and 10 years for those >70 years of age. In a study of 313 Japanese patients who had CE-P bioprostheses for AVR, the rate of freedom from SVD at 20 years was 54.2 ± 18% for those who were 60-69 years old and 29.5 ± 15% for those who were <60 years old []. These findings, similar to ours, suggested (1) that freedom from SVD differed between age groups, as seen in the patients ≥70 years, who infrequently developed SVD at 10 years or more following valve replacement, and (2) that SVD was more likely to occur in patients of the age group <60 years. Showing consistency with other studies [,,], our study found being in a higher age group at the time of implantation to be a significant risk factor for SVD among patients with AVR. A higher risk of SVD was observed for patients <60 years of age in relation to those 60-69 years of age, but no statistically significant differences were found between those 60-69 years old and those ≥70 years old [,,], indicating the durability of the CE-P bioprosthesis for both the age groups of 60-69 and ≥70 years. Further stratified by the position of valve replacement, our results, along with those of Poirier et al. [], suggested that the long-term durability of the CE-P bioprosthesis was superior in patients with AVR in comparison to those with MVR. Bourguignon et al. [,] also found an overall actuarial freedom from SVD at 20 years of 48.5 ± 4.6% for patients who had received the CE-P bioprosthesis for AVR [] compared to a rate of 16.9 ± 3.9% at 20 years in another study on patients who had received the CE-P bioprosthesis for MVR [].
Of the 13 patients that underwent reoperation, a higher proportion received MVR (6/13) as compared to AVR (1/13). The existing studies consistently demonstrated a higher rate of freedom from reoperation in aortic positions: Chan et al. [] reported a 15-year freedom from reoperation of 78% following AVR and of 62% following MVR in patients >60 years of age, while the freedom from reoperation at 10 years was 91 and 76%, respectively, for the AVR and MVR groups in the study of Poirier et al. []. Indeed, it is recommended that bioprosthesis use is optimal in the age group of ≥60 years for AVR and >70 years for MVR. In the age group of 60-70 years, the benefits of mechanical prostheses outweigh the risk of reoperation due to SVD among patients who have undergone MVR, and the benefits of bioprostheses outweigh the risk of anticoagulant hemorrhage in patients who have undergone AVR [,].
From 2001 to 2007, 4,726 patients underwent prosthetic valve implantation in our hospital; of those, 3,910 received mechanical valves and 812 received bioprostheses. In another Chinese study, during a 9-year period from 2004 to 2013, Wang et al. [] reported 4,791 mechanical prosthesis and 647 bioprosthesis implantations. In China, mechanical prosthesis is still the predominant choice of valvular type, mainly for two reasons. Firstly, rheumatic heart disease is the primary pathological type of heart valve disease, which generally occurs at a younger age than does the degenerative type. Secondly, even if recommended bioprosthetic valve replacement, most patients prefer to choose mechanical prostheses considering the risk and cost of reoperation []. However, our study demonstrated a satisfactory rate of survival, freedom from SVD, and rate of reoperation in this relatively young patient cohort.
This is the first study that systematically evaluated the safety and clinical effectiveness of CE-P bovine pericardial valves in Chinese patients with valve replacements. Due to the limited sample size (N = 225), the results of this study might not be generalizable to the entire Chinese population. However, our study can serve as a basis for further long-term and large-scale studies that evaluate the clinical effectiveness and safety of bovine pericardial bioprostheses in Chinese patients.
We found favorable results for the long-term safety and efficacy of the CE-P valve in Chinese patients, especially among those ≥60 years of age. The CE-P bioprosthesis is most suitable for use in elderly patients ≥70 years of age who have undergone either MVR or AVR, with low rates of SVD and reoperation and a prolonged valve durability in relation to the actuarial time of survival. Among patients 60-69 years old, the CE-P valve may be more appropriate for use in those who have undergone AVR, as can be seen from the low incidence of SVD and reoperation observed; however, more studies are needed to further confirm the performance of CE-P bioprostheses in patients who have undergone MVR in the same age group.
Acknowledgements
The authors want to thank Ms. Huimin Wen for data collection and collation, Dr. Hezhi Li for review of echocardiographic assessments and translation, and Ms. Ying Li, Ms. Yiwen Huang, and Dr. Jie Mao for writing and editing.
Conflict of Interest
None declared.
References
- 1. Biglioli P, Spampinato N, Cannata A, Musumeci A, Parolari A, Gagliardi C, Alamanni F: Long-term outcomes of the Carpentier-Edwards pericardial valve prosthesis in the aortic position: effect of patient age. J Heart Valve Dis 2004;13(suppl 1):S49-S51.
- 2. McClure RS, Narayanasamy N, Wiegerinck E, Lipsitz S, Maloney A, Byrne JG, Aranki SF, Couper GS, Cohn LH: Late outcomes for aortic valve replacement with the Carpentier-Edwards pericardial bioprosthesis: up to 17-year follow-up in 1,000 patients. Ann Thorac Surg 2010;89:1410-1416.
- 3. Banbury MK, Cosgrove DM, White JA, Blackstone EH, Frater RW, Okies JE: Age and valve size effect on the long-term durability of the Carpentier-Edwards aortic pericardial bioprosthesis. Ann Thorac Surg 2001;72:753-757.
- 4. Marchand MA, Aupart MR, Norton R, Goldsmith IR, Pelletier LC, Pellerin M, Dubiel T, Daenen WJ, Herijgers P, Casselman FP, Holden MP, David TE: Fifteen-year experience with the mitral Carpentier-Edwards PERIMOUNT pericardial bioprosthesis. Ann Thorac Surg 2001;71(suppl):S236-S239.
- 5. Rahimtoola SH: Choice of prosthetic heart valve for adult patients. J Am Coll Cardiol 2003;41:893-904.
- 6. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Barón-Esquivias G, Baumgartner H, Borger MA, Carrel TP, De Bonis M, Evangelista A, Falk V, Lung B, Lancellotti P, Pierard L, Price S, Schäfers HJ, Schuler G, Stepinska J, Swedberg K, Takkenberg J, Von Oppell UO, Windecker S, Zamorano JL, Zembala M: Guidelines on the management of valvular heart disease (version 2012): the Joint Task Force on the Management of Valvular Heart Disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur J Cardiothorac Surg 2012;42:S1-S44.
- 7. Bourguignon T, Bouquiaux-Stablo AL, Candolfi P, Mirza A, Loardi C, May MA, El-Khoury R, Marchand M, Aupart M: Very long-term outcomes of the Carpentier-Edwards Perimount valve in aortic position. Ann Thorac Surg 2015;99:831-837.
- 8. Bourguignon T, El Khoury R, Candolfi P, Loardi C, Mirza A, Boulanger-Lothion J, Bouquiaux-Stablo-Duncan AL, Espitalier F, Marchand M, Aupart M: Very long-term outcomes of the Carpentier-Edwards Perimount aortic valve in patients aged 60 or younger. Ann Thorac Surg 2015;100:853-859.
- 9. Chan V, Malas T, Lapierre H, Boodhwani M, Lam BK, Rubens FD, Hendry PJ, Masters RG, Goldstein W, Mesana TG, Ruel M: Reoperation of left heart valve bioprostheses according to age at implantation. Circulation 2011;124(suppl):S75-S80.
- 10. Han QQ, Xu ZY, Zhang BR, Zou LJ, Hao JH, Huang SD: Primary triple valve surgery for advanced rheumatic heart disease in Mainland China: a single-center experience with 871 clinical cases. Eur J Cardiothorac Surg 2007;31:845-850.
- 11. Wang Y, Chen S, Hu XJ, Shi JW, Dong NG: Mid- to long-term clinical outcomes of Hancock II bioprosthesis in Chinese population. Chin Med J (Engl) 2015;128:3317-3323.
- 12. Wei X, Yi W, Chen W, Ma X, Lau WB, Wang H, Yi D: Clinical outcomes with the epichlorohydrin-modified porcine aortic heart valve: a 15-year follow-up. Ann Thorac Surg 2010;89:1417-1424.
- 13. van der Straaten EP, Rademakers LM, van Straten AH, Houterman S, Tan ME, Soliman Hamad MA: Mid-term haemodynamic and clinical results after aortic valve replacement using the Freedom Solo stentless bioprosthesis versus the Carpentier Edwards Perimount stented bioprosthesis. Eur J Cardiothorac Surg 2016;49:1174-1180.
- 14. Akins CW, Miller DC, Turina MI, Kouchoukos NT, Blackstone EH, Grunkemeier GL, Takkenberg JJ, David TE, Butchart EG, Adams DH, Shahian DM, Hagl S, Mayer JE, Lytle BW: Guidelines for reporting mortality and morbidity after cardiac valve interventions. Ann Thorac Surg 2008;58:1490-1495.
- 15. Forcillo J, Pellerin M, Perrault LP, Cartier R, Bouchard D, Demers P, Carrier M: Carpentier-Edwards pericardial valve in the aortic position: 25-years experience. Ann Thorac Surg 2013;96:486-493.
- 16. Bourguignon T, Bouquiaux-Stablo AL, Loardi C, Mirza A, Candolfi P, Marchand M, Aupart MR: Very late outcomes for mitral valve replacement with the Carpentier-Edwards pericardial bioprosthesis: 25-year follow-up of 450 implantations. J Thorac Cardiovasc Surg 2014;148:2004-2011.e1.
- 17. Chen YF, Lee CS, Lin CC, Su SF, Chen ML, Hsieh CC, Chen HM, Chiu CC, Lu YH, Liang HY, Yen HW, Hwang YS, Lin YT: Twenty-year follow-up of the Carpentier-Edwards standard porcine bioprosthesis in the oriental population. J Cardiovasc Surg (Torino) 2003;44:691-699.
- 18. Poirier NC, Pelletier LC, Pellerin M, Carrier M: 15-year experience with the Carpentier-Edwards pericardial bioprosthesis. Ann Thorac Surg 1998;66(suppl):S57-S61.
- 19. Zhu XD, Guo JQ, Chen YC, Tang CJ, Xue GX: Ten-year experience with pericardial xenograft valves. J Thorac Cardiovasc Surg 1988;95:572-576.
- 20. Nishida T, Sonoda H, Oishi Y, Tatewaki H, Tanoue Y, Shiokawa Y, Tominaga R: Long-term results of aortic valve replacement with mechanical prosthesis or Carpentier-Edwards Perimount bioprosthesis in Japanese patients according to age. Circ J 2014;78:2688-2695.
- 21. Prasongsukarn K, Jamieson WR, Lichtenstein SV: Performance of bioprostheses and mechanical prostheses in age group 61-70 years. J Heart Valve Dis 2005;14:501-508, 510-511; discussion 509.
- 22. Hanania G: Which heart valve prosthesis for patients aged between 60 and 70 years? Heart 2003;89:481-482.