Variability is the law of life.
William Osler
In this issue of Cardiology, Mansour et al. [], from the Aortic Institute at Yale-New Haven Hospital, Yale University School of Medicine, New Haven, CT, USA, have suggested that the aortic diameter increases substantially at the time of aortic dissection due to the dissection itself. They studied 55 patients with aortic dissection (29 of type A and 26 of type B, according to the Stanford classification) and compared them to a control group consisting of patients with aortic aneurysm without dissection.
Patients underwent imaging and aortic diameters were determined prior to dissection (average time 1.7 ± 1.9 years) and at the time of dissection. The control group had at least 2 images of the aorta; the time interval between obtaining the last 2 images was 1.9 ± 2.0 years. Mean diameter of the ascending aorta was 54.2 ± 7.0 mm at dissection and 45.1 ± 5.7 mm at predissection, and that of the descending thoracic aorta was 47.1 ± 13.8 mm and 39.5 ± 13.1 mm, respectively. After controlling for aneurysm growth, gender, and age, multivariate analysis revealed that the diameter of the ascending aorta increased by 7.65 mm, due solely to type A dissection, and the diameter of the descending thoracic aorta increased by 6.38 mm, due solely to type B dissection. Rylski et al. [] reported similar findings; however, among others, they did not correct aortic diameter for aneurysmal growth at the time of aortic dissection, as did Mansour et al. []. This observation, even though it was made in a small number of patients, may obviously have important clinical implications, because indications for interventional therapy (surgical or percutaneous) are mostly based on the size of the aorta at the time of dissection, with only limited information being available for the size of the aorta just prior to this catastrophic event [,,,,,,,,,]. However, it appears that, at least in certain patients, the diameter of the aorta prior to dissection is smaller than the size in clinical practice guidelines that recommend therapeutic intervention []. Importantly, the progression of the aortic diameter over time, also taken into consideration for clinical decisions about therapeutic intervention, is not always linear. It is quite plausible that the size of the aorta changes in a linear fashion up to a certain diameter, followed by an acceleration of aortic dilatation in a nonlinear fashion [,].
Factors Determining Aortic Dilatation and Rupture
Structure and Function of the Aortic Wall
There are several factors contributing to instability of the aorta that may lead to dissection. The aorta is a dynamic organ, the function and size of which are largely related to the structure of its wall and also hemodynamic factors; the latter (among other effects) may also alter its wall structure. The normal aortic wall in mammals contains smooth muscle cells, elastin, and collagen. There is normally more elastin than collagen in the wall of the thoracic aorta and more collagen than elastin in that of the abdominal aorta. In patients with heritable disorders of connective tissue prone to aortic dilatation, dissection, and rupture, such as Marfan syndrome, Ehlers-Danlos syndromes, adult polycystic kidney disease, and others, the structure of the aortic wall is mostly genetically determined. Furthermore, approximately 20% of patients with a thoracic aortic aneurysm not related to a heritable connective tissue disorder have an affective family member suggesting that genetic predisposition is present in these patients [,,]. Recently, Brownstein et al. [] published a state of the art article, reporting known gene abnormalities related to aortic dilatation, dissection, and rupture.
There is also a vasa-vasorum (V-V) network with multiple anastomoses among the arterioles in the aortic wall, which plays an important role in aortic structure and function. In the ascending aorta, the V-V originate from the coronary arteries. In the aortic arch, they originate from the great vessels of the neck and their proximal branches, while those of the descending thoracic aorta originate from the intercostal arteries [,]. The flow through the V-V, which provides a large amount of blood to the outer part of the aortic wall, is subject to active neurohumoral regulation. An acute decrease of blood supply to the aortic wall is associated with aortic dysfunction. The senile changes of the media are most frequently seen in places where there is the least V-V supply, and is most likely related to a decrease in nutrition to these areas [,,,]. Studies with experimental animal models indicate that the removal of V-V from the thoracic aorta affects the structure of the outer part, but not the inner part of the aortic wall; these structural changes are also associated with reduced aortic distensibility [,]. The dissimilar structure of the inner and outer part of the aortic wall may predispose and/or precipitate aortic dissection []. V-V flow decreases with age especially when arterial hypertension is present. Patients with coronary artery disease also have reduced aortic distensibility of the ascending aorta compared to matching controls; this phenomenon is at least partially related to a diminished V-V flow []. Importantly, it should be mentioned that during open-heart surgery, a certain number of V-V might be destroyed during the procedure. The effects of this surgery on V-V flow, aortic function, and progression of aortic size are not yet well defined. More recently, it has been shown that in certain heritable diseases associated with aortic aneurysm, V-V flow may be defective [,].
The aortic wall structure also changes with age. In addition to changes in the V-V flow and the accumulative effect in the elderly of risk factors that may also affect aortic wall structure, significant changes in the wall of the aorta occur regardless of other risk factors []. The proximal aorta in young individuals expands by approximately 10% during left ventricular systole. Due to repetitive stretch of the aortic wall over time, fatigue and fracture of the elastic lamellae occur. This results in an increase in the collagen content and a decrease in the elastic properties of the aortic wall. This is in addition to and independent of, the changes that occur due to risk factors and the atherosclerotic process []. Furthermore, in certain diseases, and especially in the elderly, an inflammatory process may be present in the wall of the aorta that also contributes to its structural and functional abnormalities [,,]. All these alterations of the aortic wall over time can eventually lead to aortic dilatation, dissection, and rupture (Fig. 1) [].
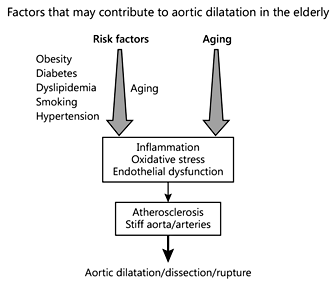
Fig. 1
Significant changes of the aortic wall occur with age regardless of the presence of risk factors; these changes may initiate or precipitate aortic dilatation and rupture. In addition, the long-term effects of coexisting common risk factors, especially in the elderly, may also affect the aorta (modified from []).
Hemodynamic Factors
In addition to aortic wall structural abnormalities, hemodynamic factors play an important role in the development of aortic dilatation and dissection. According to Laplace's Law, the stress in the aortic wall is directly related to aortic pressure and aortic diameter, and inversely related to aortic wall thickness (stress = pressure × radius/wall thickness). Triggers that may acutely increase aortic pressure, such as weight-lifting, may result in aortic dissection. Furthermore, frequent stress, which is related to heart rate, may also affect the progression of aortic size [,,].
The Timing of Surgery in Patients with Aortic Dilatation
Life is short, the art long, opportunity fleeting, experience fallacious, judgment difficult.
Hippocrates
How often a relatively small size aorta dissects or ruptures in relation to the entire patient population with aortic dissection/rupture is not well known. Based on the information available, therefore, what approach should be used today for patients with aortic dilatation prone to aortic dissection or rupture? In the past, most of the recognizable aortic diseases were diagnosed at end-stage or at autopsy, with the result that therapeutic interventions were limited.
A series of events propelled the aorta into clinical consciousness. The imaging revolution, including molecular imaging in cardiovascular medicine, has had a profound effect on diagnostic precision in aortic disease states. In addition, innovative surgical techniques and percutaneous approaches have changed the prognosis for many patients who were previously considered untreatable []. At present, a mosaic approach is necessary since the population in which aortic dissection occurs is nonhomogeneous, and so several parameters have to be taken into consideration. To make a rational clinical decision, the size at which the aorta dissects has to be defined in each subgroup in which dissections commonly occur, and the indications for therapeutic intervention should be based on this information [,]. Family history, arterial survey, arterial hypertension, aortic size and progression of aortic size, aortic function, inflammatory processes of the aortic wall that play an important role in the progression of aortic size, gene mutations, genetic biomarkers, V-V flow, hemodynamic factors, and triggers of aortic dissection must all be considered for each individual patient, as it is obvious that one aortic size does not apply to all (Fig. 2) [,,].
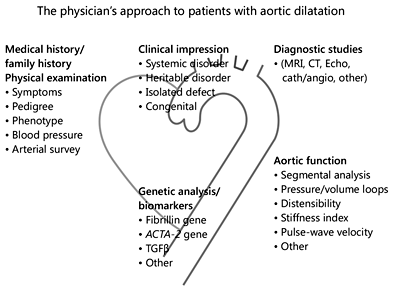
Fig. 2
The physician's approach to patients with aortic dilatation begins with examining the patient's history and a physical examination, which leads to the first clinical impression regarding etiology. Diagnostic studies should include imaging and other techniques to precisely define the anatomy, and determine aortic function and genetic analysis (modified from []).
Future advances in the understanding of aortic aneurysm formation and the natural history of the disease will assist in better management of patients and result in better outcomes. Clinical investigators, pathologists, surgeons, imagers, and geneticists should be involved in this process by developing new approaches to genotype-phenotype correlations in clinical practice. At present, the approach should be based on current knowledge, common sense, and good clinical judgment. Recommendations and advice should be balanced in order to avoid early and unnecessary surgery, but also to prevent aortic dissection.
Since antiquity, physicians have had to make important decisions about the well-being of their patients, often based on incomplete information and under conditions of uncertainty []. The father of medicine, Hippocrates (460-370 B.C.), stated this nicely: “Life is short, the art long, opportunity fleeting, experience fallacious, judgment difficult.”
Conflict of Interest
There were no conflicts of interest.
References
- 1. Mansour AM, Peters S, Mohammad A, et al: Prevention of aortic dissection suggests a diameter shift to a lower aortic size threshold for intervention. Cardiology 2018, DOI: 10.1159/ 000481930.
- 2. Rylski B, Branchetti E, Bavaria IE, et al: Modeling of pre-dissection aortic size in acute type A dissection: more than 90% fail to meet the guidelines for elective assending replacement. J Thorac Cardiovasc Surg 2014;148:944-948.
- 3. Davies RR, Goldstein LJ, Coady MA, et al: Yearly rupture or dissection rates for thoracic aortic aneurysms: simple prediction based on size. Ann Thorac Surg 2002;73:17-27.
- 4. Elefteriades JA, Ziganshin BA, Rizzo JA, et al: Indications and imaging for aortic surgery: size and other matters. J Thorac Cardiovasc Surg 2015;149:S10-S13.
- 5. Erbel R, Aboyans V, Boileau C, et al: 2014 ESC Guidelines on the diagnosis and treatment of aortic diseases: document covering acute and chronic aortic disease of the thoracic and abdominal aorta of the adult. Eur Heart J 2014;35:2873-2896.
- 6. Hiratzka LF, Bakris GL, Beckman JA, et al: 2010 Guidelines for the diagnosis and management of patients with thoracic aortic disease. J Am Coll Cardiol 2010;55:e27-e129.
- 7. Hagan PG, Nienaber CA, Isselbacher EM, et al: The International Registry of Acute Aortic Dissection (IRAD): new insights into an old disease. JAMA 2000; 283:897-903.
- 8. Tsai TT, Trimarchi S, Nienaber CA: Acute aortic dissection: perspectives from the International Registry of Acute Aortic Dissection (IRAD). Eur J Vasc Endovasc Surg 2009;37:149-159.
- 9. Parish LM, Gorman JH 3rd, Kahn S, et al: Aortic size in acute type A dissection: implications for preventive ascending aortic replacement. Eur J Cadiothorac Surg 2009;35:941-945.
- 10. Paruchuri V, Salhab KF, Kuzmik G, et al: Aortic size distribution in the general population: explaining the size paradox in aortic dissection. Cardiology 2015;131:265-272.
- 11. Trimarchi S, Jonker FH, Hutchison S, et al: Descending aortic diameter of 5.5 cm or greater is not an accurate predictor of acute type B aortic dissection. J Thorac Cardiovasc Surg 2011;142:e101-e107.
- 12. Kim JB, Kim K, Lindsay ME, et al: Risk of rupture or dissection in descending thoracic aortic aneurysm. Circulation 2015;132:1620-1629.
- 13. Boudoulas H, Toutouzas PK, Wooley CF (eds): Functional Abnormalities of the Aorta. Armonk, Futura, 1996.
- 14. Boudoulas H, Stefanadis CH (eds): The Aorta: Structure, Function, Dysfunction and Diseases. New York, Informa Healthcare, 2009.
- 15. Brownstein AJ, Ziganshin BA, Kaivaniemi H, et al: Genes associated with thoracic aortic aneurysm and dissection: an update and clinical implications. Aorta 2017;5:11020.
- 16. Boudoulas KD, Boudoulas H: The troika of aortopathy: size, function and etiology. Cardiology 2015;131:260-264.
- 17. Boudoulas KD, Vlachopoulos C, Raman SV, Sparks EA, Triposkiadis F, Stefanadis C, Boudoulas H: Aortic function: from the research laboratory to the clinic. Cardiology 2012;121:31-42.
- 18. Stefanadis C, Karayannacos PE, Boudoulas H, et al: Medial necrosis and acute alterations in aortic distensibility following removal of vasa vasorum of canine ascending aorta. Cardiovasc Res 1993;27:951-956.
- 19. Stefanadis C, Vlachopoulos C, Karayannacoa PA, Boudoulas H, et al: Effects of vasa vasorum flow on structure and function of the aorta in experimental animals. Circulation 1995;91:2669-2678.
- 20. Angouras D, Sokolis DP, Dosios D, et al: Effect of impaired vasa vasorum flow on the structure and mechanics of the thoracic aorta: implications for the pathogenesis of aortic dissection. Eur J Cardiothorac Surg 2000;17:468-473.
- 21. Stefanadis C, Wooley CF, Bush CA, Kolibash AJ, Boudoulas H: Aortic distensibility abnormalities in coronary artery disease. Am J Cardiol 1987;59:1300-1304.
- 22. O'Rourke MF, Safar ME, Dzau V: The cardiovascular continuum extended: aging effects of the aorta and mictovasculature. Vasc Med 2010;15:461-468.
- 23. Boudoulas KD, Triposkiadis F, Parissis J, Butler J, Boudoulas H: The cardiorenal relationship. Progr Cardiovasc Dis 2017;54:636-648.
- 24. Joly L, Djaballah W, Koehl G, Mandry D, Marie PV, Benetos A: Aortic inflammation assessed by hybrid FDG-PET/CT images is associated with enhanced aortic stiffness in addition to concurrent calcification. Eur J Nucl Med Mol Imaging 2009;36:979-985.
- 25. Tarkin JM, Joshi FR, Rudd JHF: PET imaging of inflammation in atherosclerosis. Nat Rev Cardiol 2014;11:443-457.
- 26. Singh P, Almarzooq Z, Salata B, Devereux RB: Role of molecular imaging with positron emission tomography in aortic aneurysms. J Thoracic Dis 2017;9:supplement 4.
- 27. Thubrikar MJ, Agali P, Robicsek F: Wall stress as a possible mechanism for the development of transverse intimal tears in aortic dissections. J Med Eng Technol 1999;23:127-134.
- 28. Elefteriadis J (ed): Acute aortic disease; in: Fundamental and Clinical Cardiology. London, Informa Healhtcare 2007.
- 29. El-Hamamsy D, Yacoub MH: Towards more personalized surgical indications for thoracic aortic dilatations: are we there yet? Can J Cardiol 2016;32:4-7.
- 30. Boudoulas KD, Leier CV, Geleris P, Boudoulas H: The shortcomings of clinical practice guidelines. Cardiology 2015;130:187-200.
