Introduction
Transcatheter aortic valve replacement (TAVR) has emerged as a key treatment option for patients with symptomatic severe aortic stenosis, and its indications have been rapidly expanding over the past few years [,,,,]. Postprocedural thrombocytopenia (TP) is a common occurrence after TAVR and surgical aortic valve replacement. Studies have shown that TP can be caused by hemodilution, platelet activation, bone marrow suppression, and mechanical destruction [,,]. Post-TAVR TP has been compared with TP after other cardiac interventions and surgery, and study results reveal that TP is more common and severe after TAVR than after percutaneous coronary intervention and balloon aortic valvuloplasty, but similar to after surgical aortic valve replacement [,,,,,]. A 2-center, large-scale study proved that severe TP can lead to poor clinical outcomes, thus highlighting its significance in post-TAVR management []. Several studies concerning post-TAVR TP have been conducted, but their conclusions about the predictors and clinical outcomes of TP differ. Data are still limited, particularly on self-expandable valves. We sought to investigate the predictors of post-TAVR TP, clinically significant TP, in particular (also known as major TP).
Methods
Data Collection and Clinical Follow-Up
Data were collected on 123 consecutive patients with symptomatic severe aortic valve stenosis undergoing TAVR at the Heart Center of The Second Affiliated Hospital, Zhejiang University College of Medicine, Hangzhou, China, from March 2013 to March 2016. All patients underwent a risk evaluation for cardiac surgery by our heart team. Procedures were performed as previously reported in detail [,]. Baseline demographics, procedural data, postprocedural data and clinical outcomes were prospectively collected, though the analysis was performed retrospectively. Patients who died periprocedurally were excluded from our study. All patients received dual antiplatelet treatment (DAPT: 100 mg aspirin and 75 mg clopidogrel QD) before the procedure and for 3 months afterwards as routine antithrombotic practice. Antibiotics are regarded as a routine prophylaxis (always cefuroxime) before and after the TAVR procedure. Unfractionated heparin was used in all procedures (50-70 U/kg). General anesthesia (GA) or local anesthesia with sedation was used during the procedure based on the evaluation from the anesthetist. Neither extracorporeal circulation nor platelet transfusion was used in patients. Self-expandable valves, mostly CoreValve (Medtronic, Inc., Minneapolis, MN) were implanted in aortic stenosis patients. Data were entered into a registry at the Heart Center and were approved by the respective ethics committees.
Peri- and postprocedural complications were according to the Valve Academic Research Consortium (VARC-2) standardized end-point definitions for transcatheter aortic valve implantation consensus document. Laboratory evaluation was conducted at least once daily during ICU stay and at the physician's discretion thereafter. Platelet counts within 8 days after the procedure were recorded for data analysis. The nadir platelet count was defined as the minimum platelet count after procedure. TP (a nadir platelet count <150 × 109/L) and major TP (a nadir platelet count <100 × 109/L and a >50% decrease in platelet count) was defined as previously reported [,,]. Bicuspid aortic valve (BAV) malformation was diagnosed by transthoracic echocardiogram []. Patients were stratified into 3 groups according to nadir platelet count as previously described []: no/mild TP (≥100 × 109/L), moderate TP (50-99 × 109/L), or severe (<50 × 109/L). They were also divided into 2 groups according to whether the patient developed major TP after TAVR.
Data Analysis
Continuous variables are summarized as mean ± standard deviation (SD) or as medians and interquartile range (IQR) as appropriate, and were compared using the Student t test or the Mann-Whitney rank-sum test. Categorical variables were compared using the χ2 test or the Fisher exact test. The multivariate model was built by selecting baseline variables of clinical interest and/or satisfaction of the entry criterion of p < 0.050 in the univariate analysis. A two-sided α level of 0.05 was used for all superiority testing. All statistical analyses were performed using SPSS v18 (IBM Corp., Somer, NY, USA).
Results
Platelet Count Changes
A total of 123 patients constituted the study population, 118 (95.9%) treated by transfemoral approach, 4 (3.3%) by transaortic approach, and 1 (0.8%) by transsubclavian approach. Median baseline platelet count was 159 × 109/L (IQR 102-216 × 109/L). Thirteen (10.6%) patients had preexisting moderate-to-severe TP. Seventy-eight (63.4%) patients developed significant postprocedural TP (52.0% moderate and 11.4% severe), with 35 (28.5%) being major TP. Nadir platelet count (median 89 × 109/L) was significantly lower than at baseline (p < 0.001), but recovered quickly (median 142.5 × 109/L on day 7) and remained stable at the follow-ups (median 146 × 109/L at 1 month).
No/Mild TP, Moderate TP, and Severe TP after TAVR
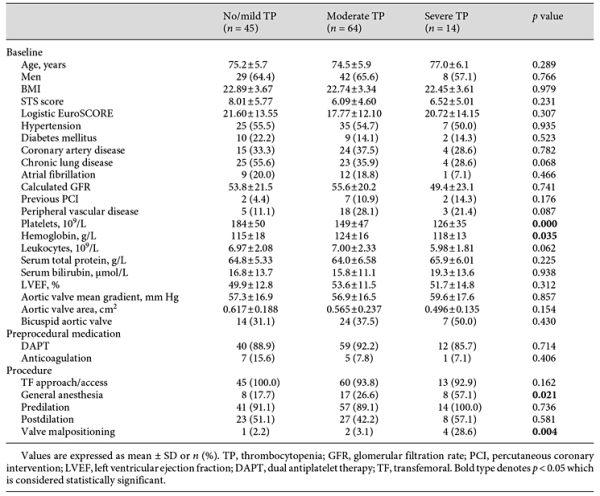
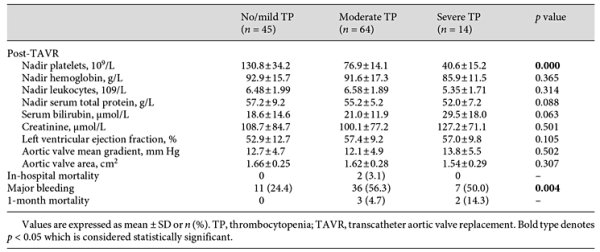
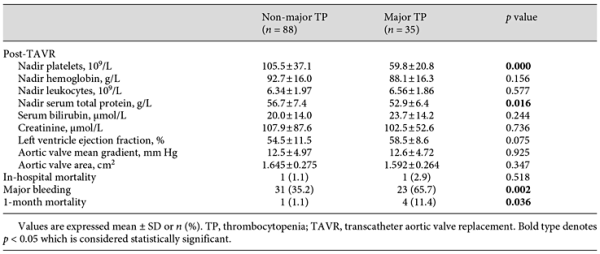
Patients were stratified into 3 groups based on their nadir platelet count: no/mild TP (n = 45), moderate TP (n = 64), and severe TP (n = 14). Baseline and procedural characteristics are shown in Table 1. Age, gender, Society of Thoracic Surgeons (STS) score, and baseline echocardiography data were similar in all groups, yet some procedural factors (GA and valve malpositioning) were more common in the patients with severe TP. Laboratory values, echocardiogram data, and clinical outcomes are shown in Table 2. Patients with moderate and severe TP had more major bleeding during hospitalization (56.3 vs. 24.4% for moderate TP vs. no/mild TP; 50 vs. 24.4% for severe TP vs. no/mild TP; p < 0.01 for both comparisons). No differences were found in other postprocedural complications among groups.
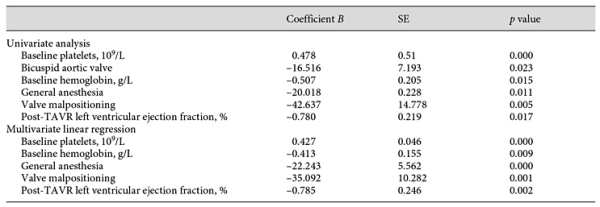
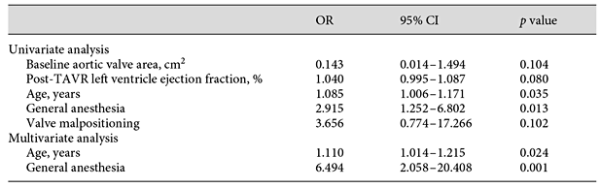
Univariate analysis was performed in order to detect factors that were statistically significant in their relationship to the nadir platelet count post-TAVR. Baseline platelet, BAV, baseline hemoglobin, GA, valve malpositioning, and post-TAVR left ventricular ejection fraction (LVEF) showed significant relationships with nadir platelet count in the univariate analysis. Data are shown in Table 3. In the multivariable analysis, all factors remained significant, except for BAV (R2 = 0.622, adjusted R2 = 0.599). The model suggests: for each 1 × 109/L decrease in baseline platelet count, there was a 0.427 × 109/L drop in post-TAVR nadir platelet count; for each 1 g/L increase in baseline hemoglobin, there was a 0.413 × 109/L drop in nadir platelet count post-TAVR; GA and valve malpositioning were inversely associated with nadir platelet count post-TAVR; for each 1% increase in post-TAVR LVEF, there was a 0.785 × 109/L drop in nadir platelet count.
Major TP Post-TAVR
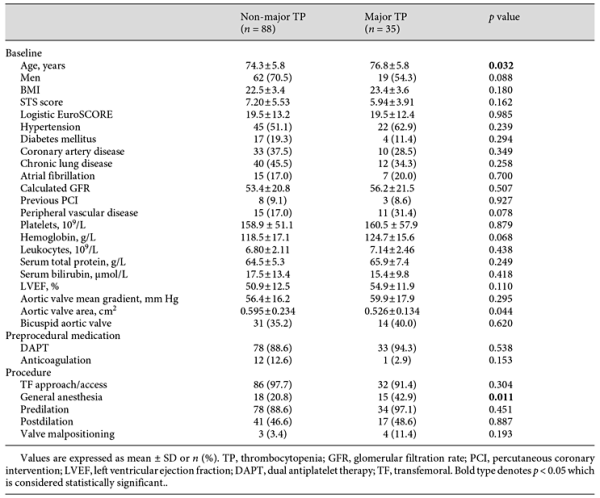
Of the 123 patients, 35 (28.5%) developed major TP post-TAVR; their baseline platelet counts seemed no different from the patients that did not have major TP (161 × 109/L vs. 159 × 109/L, p > 0.05). These patients were older (77 vs. 74 years, p < 0.05) and had a smaller baseline aortic valve area (0.526 vs. 0.595 cm2, p < 0.05). As for the procedural characteristics, GA was performed in more of these patients (42.9 vs. 20.8%, p < 0.05). They were deemed to have more major bleeding events (65.7 vs. 35.2%, p < 0.05) and a higher 1-month mortality rate (11.4 vs. 1.1%, p < 0.05). Data are shown in Tables 4, 5.
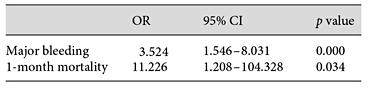
Logistic regression revealed an association between major TP and worse clinical outcomes. Major TP predicted a higher risk of major bleeding (OR 3.524, 95% CI 1.546-8.031) and 1-month mortality (OR 11.226, 95% CI 1.208-104.328). We also performed a multivariate analysis to predict major TP, although it turned out that the correlation only exists with age (OR 1.110, 95% CI 1.014-1.215) and GA (OR 6.494, 95% CI 2.058-20.408). Data are shown in Tables 6, 7.
Discussion
This study demonstrated the importance of clinically significant TP (major TP) in TAVR, and we analyzed major TP and its predictors. Moreover, it is the first study reporting post-TAVR TP in an Asian population.
In our cohort, patients who developed major TP seemed to have a normal baseline platelet count when compared to the group that did not have major TP, which implies that there might be a subgroup prone to clinically significant TP. Our findings suggest that TP is associated with a higher risk of postprocedural mortality. Further studies on clinically significant TP in TAVR patients are warranted.
Currently, no clear correlation exists between TP and anesthesia techniques, partially due to the prudent use of local anesthesia with sedation. In our study, the severity of TP was strongly associated with anesthesia techniques. The choice of GA or local anesthesia with sedation is influenced by numerous factors, such as procedural difficulty, cardiopulmonary function, etc. For example, in our center, if the patient is in a state of debility, especially with poor cardiopulmonary function, GA is preferred for the TAVR procedure. In our study, baseline platelet count, STS score, and other preprocedural data were similar in the GA and non-GA (local anesthesia with sedation) groups (data not shown). Anesthetic agents, such as fentanyl, propofol, dexmedetomidine, and midazolam, have never been reported to influence blood platelets. Recent data, using propensity score-matching analysis, showed less procedural duration, less fluoroscopic time, and more postprocedural complications in a non-GA cohort [,]. Longer X-ray exposure, longer procedural time, and larger contrast amounts have been reported to be associated with TP, which might help to elucidate this correlation between TP and anesthesia techniques []. More complications (such as bleeding events), due to procedural difficulty, could also lead to more severe TP in patients who undergo GA.
Recently, a multicenter, large-scale TAVR study revealed that Asian populations had lower STS scores (5.2%), body mass index, 30-day and 1-year mortality (2.5 and 10.8%), but similar post-TAVR complications when compared to Western populations []. Our sample is a typical Asian population, albeit with a slightly higher STS score. We found that more than half of the patients developed moderate-to-severe post-TAVR TP, consistent with previous studies [,,], implying that post-TAVR TP might be a common phenomenon across races.
The concept of major TP primarily described in percutaneous coronary intervention is similar to the one raised in a 2-center, large-scale TAVR study [,]. The definition of major TP (a nadir platelet count <100 × 109/L and a >50% decrease in platelet count) was formally described in 2 studies on postprocedural complications and mortality [,]. However, no studies have tried to investigate the predictors of major TP after TAVR. In aagreement with a previous study, our major TP population had a significantly smaller aortic valve area in the univariate analysis [], although no significance was found in the multivariate logistic regression analysis. Our multivariate analysis suggested that old age and GA are risk factors for major TP. The association between age and TP, mentioned recently in another study investigating post-TAVR TP [], could be explained by bone marrow dysfunction in the elderly.
A multivariate linear regression model was created to predict post-TAVR nadir platelet count, indicating baseline platelet count, baseline hemoglobin, GA, valve malpositioning, and post-TAVR LVEF as independent predictors. A similar model with different variables (such as STS score) was built in another study []. We also found a marginal significant correlation (p = 0.083) between STS score and nadir platelet count, which means that severely ill patients tend to have a higher post-TAVR nadir platelet count. Recent data also show that a thromboinflammatory state might be related to post-TAVR TP, which we did not measure []. An uncertain relationship was mentioned between valve malpositioning and TP in previously studies [,]. Our study suggests that valve malpositioning is a strong predictor of a post-TAVR platelet count decrease, which can be explained by vascular endothelial injury and platelet activation []. Interestingly, pre- and postdilatation could also cause tissue injury, but these were not associated with TP in our analysis.
We also attempted to understand TP in a BAV population, since BAV are more common in the Chinese population, and were found to have an association with post-TAVR platelet count in our study. Both baseline and postprocedural platelet count were lower in the BAV group (n = 45) than in the tricuspid aortic valve group (147 × 109/L vs. 166 × 109/L for baseline, p < 0.05; 82 × 109/L vs. 98 × 109/L for postprocedure, p < 0.05). Interestingly, the decrease in platelet count was similar in these 2 groups (65 × 109/L vs. 68 × 109/L, p > 0.05), suggesting that BAV patients might have the same mechanism of platelet count decrease as patients with tricuspid aortic valve. BAV abnormality used to be a relative contraindication in TAVR [,,] but, in our study, mortality and complications were similar in these 2 groups. Platelet activation was found in the BAV population, which might explain why the baseline platelet count was lower in our BAV patients []. The association between BAV and TP needs to be corroborated by further studies [].
Obviously, our study has some limitations. Data were extracted from a single medical center, so our conclusions may not be universal. Our sample size was not big enough to do further analyses, especially on the clinical outcomes of the study population, e.g., mortality (only 5 patients died during 1 month of follow-up). Additionally, it was a retrospective study and some undetectable uncertainties may exist that may have influenced our analysis results.
In conclusion, the post-TAVR nadir platelet count could be predicted based on baseline and procedural data, and we created a linear model consisting of baseline platelet count, baseline hemoglobin, GA, valve malpositioning, and post-TAVR LVEF values to predict it. Clinically significant TP has a detrimental effect on TAVR patients and seems to be difficult to detect. We also found that old age and the use of GA can contribute to clinically significant TP.
Acknowledgment
This work was supported by the Zhejiang Province Science and Technology Department Major Scientific and Technological Projects of major social programs (2015C03028).
Conflict of Interest
All authors declared they have no conflicts of interest to disclose.
References
- 1. Praz F, Windecker S, Huber C, Carrel T, Wenaweser P: Expanding indications of transcatheter heart valve interventions. JACC Cardiovasc Interv 2015;8:1777-1796.
- 2. Leon MB, Smith CR, Mack MJ, Makkar RR, Svensson LG, Kodali SK, et al: Transcatheter or surgical aortic-valve replacement in intermediate-risk patients. N Engl J Med 2016;374:1609-1620.
- 3. Leon MB, Smith CR, Mack M, Miller DC, Moses JW, Svensson LG, et al; PARTNER Trial Investigators: Transcatheter aortic-valve implantation for aortic stenosis in patients who cannot undergo surgery. N Engl J Med 2010;363:1597-1607.
- 4. Beohar N, Kirtane AJ, Blackstone E, Waksman R, Holmes D, Jr., Minha S, Alli O, Suri RM, Svensson LG, Leon M, Kodali S: Trends in complications and outcomes of patients undergoing transfemoral transcatheter aortic valve replacement: experience from the PARTNER Continued Access Registry. JACC Cardiovasc Interv 2016;9:355-363.
- 5. Vahl TP, Kodali SK, Leon MB: Transcatheter aortic valve replacement 2016: a modern-day “Through the Looking-Glass” adventure. J Am Coll Cardiol 2016;67:1472-1487.
- 6. Wang TY, Ou FS, Roe MT, Harrington RA, Ohman EM, Gibler WB, Peterson ED: Incidence and prognostic significance of thrombocytopenia developed during acute coronary syndrome in contemporary clinical practice. Circulation 2009;119:2454-2462.
- 7. Piccardo A, Rusinaru D, Petitprez B, Marticho P, Vaida I, Tribouilloy C, Caus T: Thrombocytopenia after aortic valve replacement with freedom solo bioprosthesis: a propensity study. Ann Thorac Surg 2010;89:1425-1431.
- 8. van Straten AHM, Hamad MAS, Berreklouw E, ter Woorst JF, Martens EJ, Tan M: Thrombocytopenia after aortic valve replacement: comparison between mechanical and biological valves. J Heart Valve Dis 2010;19:394-399.
- 9. Gallet R, Seemann A, Yamamoto M, Hayat D, Mouillet G, Monin JL, Gueret P, Couetil JP, Dubois-Rande JL, Teiger E, Lim P: Effect of transcatheter (via femoral artery) aortic valve implantation on the platelet count and its consequences. Am J Cardiol 2013;111:1619-1624.
- 10. Dvir D, Genereux P, Barbash IM, Kodali S, Ben-Dor I, Williams M, Torguson R, Kirtane AJ, Minha S, Badr S, Pendyala LK, Loh JP, Okubagzi PG, Fields JN, Xu K, Chen F, Hahn RT, Satler LF, Smith C, Pichard AD, Leon MB, Waksman R: Acquired thrombocytopenia after transcatheter aortic valve replacement: clinical correlates and association with outcomes. Eur Heart J 2014;35:2663-2671.
- 11. McCabe JM, Huang PH, Riedl LA, Devireddy SR, Grondell J, Connors AC, Davidson MJ, Eisenhauer AC, Welt FG: Incidence and implications of idiopathic thrombocytopenia following transcatheter aortic valve replacement with the Edwards Sapien© valves: a single-center experience. Catheter Cardiovasc Interv 2014;83:633-641.
- 12. Flaherty MP, Mohsen A, Moore JBt, Bartoli CR, Schneibel E, Rawasia W, Williams ML, Grubb KJ, Hirsch GA: Predictors and clinical impact of pre-existing and acquired thrombocytopenia following transcatheter aortic valve replacement. Catheter Cardiovasc Interv 2015;85:118-129.
- 13. Grube E, Laborde JC, Gerckens U, Felderhoff T, Sauren B, Buellesfeld L, Mueller R, Menichelli M, Schmidt T, Zickmann B, Iversen S, Stone GW: Percutaneous implantation of the CoreValve self-expanding valve prosthesis in high-risk patients with aortic valve disease: the Siegburg first-in-man study. Circulation 2006;114:1616-1624.
- 14. Jilaihawi H, Doctor N, Chakravarty T, Kashif M, Mirocha J, Cheng W, Lill M, Nakamura M, Gheorghiu M, Makkar RR: Major thrombocytopenia after balloon-expandable transcatheter aortic valve replacement: prognostic implications and comparison to surgical aortic valve replacement. Catheter Cardiovasc Interv 2015;85:130-137.
- 15. Adams DH, Popma JJ, Reardon MJ, Yakubov SJ, Coselli JS, Deeb GM, et al: Transcatheter aortic-valve replacement with a self-expanding prosthesis. N Engl J Med 2014;370:1790-1798.
- 16. Kappetein AP, Head SJ, Genereux P, Piazza N, van Mieghem NM, Blackstone EH, et al: Updated standardized endpoint definitions for transcatheter aortic valve implantation: the Valve Academic Research Consortium-2 consensus document. J Thorac Cardiovasc Surg 2013;145:6-23.
- 17. Goren O, Finkelstein A, Gluch A, Sheinberg N, Dery E, Matot I: Sedation or general anesthesia for patients undergoing transcatheter aortic valve implantation - does it affect outcome? An observational single-center study. J Clin Anesth 2015;27:385-390.
- 18. Brecker SJ, Bleiziffer S, Bosmans J, Gerckens U, Tamburino C, Wenaweser P, Linke A: Impact of anesthesia type on outcomes of transcatheter aortic valve implantation (from the Multicenter ADVANCE Study). Am J Cardiol 2016;117:1332-1338.
- 19. Yoon S-H, Ahn J-M, Hayashida K, Watanabe Y, Shirai S, Kao H-L, et al: Clinical outcomes following transcatheter aortic valve replacement in an Asian population. JACC Cardiovasc Interv 2016;9:926-933.
- 20. Sedaghat A, Falkenberg N, Sinning JM, Kulka H, Hammerstingl C, Nickenig G, Oldenburg J, Potzsch B, Werner N: TAVI induces an elevation of hemostasis-related biomarkers, which is not causative for post-TAVI thrombocytopenia. Int J Cardiol 2016;221:719-725.
- 21. Sexton TR, Wallace EL, Chen A, Charnigo RJ, Reda HK, Ziada KM, Gurley JC, Smyth SS: Thromboinflammatory response and predictors of outcomes in patients undergoing transcatheter aortic valve replacement. J Thromb Thrombolysis 2016;41:384-393.
- 22. Smith CR, Leon MB, Mack MJ, Miller C, Moses JW, Svensson LG, et al; PARTNER Trial Investigators: Transcatheter versus surgical aortic-valve replacement in high-risk patients. N Engl J Med 2011;364:2187-2198.
- 23. Vahanian A, Alfieri O, Andreotti F, Antunes MJ, Baron-Esquivias G, Baumgartner H, et al: Guidelines on the management of valvular heart disease (version 2012). Eur Heart J 2012;33:2451-2496.
- 24. Bilen E, Tanboga IH, Kurt M, Kocak U, Ayhan H, Durmaz T, Bozkurt E: Mean platelet volume is increased in patients with bicuspid aortic valve. Clin Appl Thromb Hemost 2012;18:351-355.
- 25. Zhao Z-G, Jilaihawi H, Feng Y, Chen M: Transcatheter aortic valve implantation in bicuspid anatomy. Nat Rev Cardiol 2015;12:123-128.
Drs. Zhu and Liu contributed equally to the manuscript.