Introduction
Primary percutaneous coronary intervention (PPCI) of the culprit coronary artery is key to achieve efficient reperfusion in patients with ST-segment elevation myocardial infarction (STEMI) []. Efficient timely reperfusion for acute myocardial infarction (AMI) patients has directly translated into clinical benefit with a dramatic improvement in patient survival over the last 15 years []. Although PPCI is the recommended method to achieve efficient reperfusion, the procedure is not standardized. The referring interventional cardiologist can use a combination of thrombectomy, pre- and post-dilatation of the culprit vessel, and bare-metal or drug-eluting stent with various intracoronary or intravenous vasodilator or antiplatelet drugs to re-establish coronary patency [].
The risk of distal atherothrombotic material microembolization during PPCI has been demonstrated in experimental and small clinical studies [-]. Also, recent revascularization strategy trials testing one single-procedure PCI for all coronary arteries versus culprit-only PCI showed an increase in nonculprit coronary artery myocardial infarction without increasing overall infarct size [, ].
In the DANAMI-3 and PRIMULTI studies in a large group of STEMI patients, nonculprit artery infarcts were rare [], and in this study, multiple scars were significantly associated with a culprit LAD and multiple vessel disease. In this study, factors associated with NCAMIs were not assessed. NCAMIs could also be related to inflammation-induced plaque rupture in nonculprit lesions causing subclinical plaque ruptures and infarctions []. Intracoronary optical coherence tomography (OCT) and intravascular ultrasound studies have shown that there was a global coronary instability or pan-coronary process in acute coronary syndrome patients [-]. Whether nonculprit infarcts are related to inflammation-induced plaque rupture in nonculprit lesions or procedural related remains unclear. Our principal objective was therefore to assess the incidence of nonculprit acute myocardial infarcts (NCAMI) and assess their relationship with PPCI procedures or multivessel coronary instability using coronary angiography and cardiac magnetic resonance (CMR) in a population of anterior STEMI patients treated by PPCI.
Methods
Population
Our study is a substudy from the Does Cyclosporine Improve Clinical Outcome in ST-Elevation Myocardial Infarction Patients (CIRCUS) trial published previously [, ]. In brief, this is an international, multicenter, prospective, double-blinded trial designed to compare the efficacy and safety of intravenous administration of cyclosporine versus placebo, in addition to revascularization by PPCI on adverse clinical outcomes at 1 year in 969 STEMI patients. In this trial, the use of cyclosporine did not result in a smaller infarct size or better clinical outcomes compared to placebo, and it also did not prevent adverse left ventricular remodeling at 1 year.
Ethical Consideration, Consent, and Clinical Trial Registration
This trial was coordinated by the Hospices Civils de Lyon following the principles of the Declaration of Helsinki and the European Guidelines for Good Clinical Practice. Approval was obtained from the ethics committees in the relevant countries. Written informed consent was obtained from all participants before enrollment in the trial. This trial was registered at ClinicalTrials.gov: NCT01502774; and EudraCT number, 2009-013713-99 [, ].
Patients were included in the CIRCUS trial according to the following inclusion criteria: ST-elevation in the anterior leads, admission within 12 h of symptom onset, initial TIMI flow in the left anterior descending culprit coronary artery ≤1, and Killip class <4 at presentation. Patients underwent PCI according to standard guidelines. The use of thrombus aspiration, direct stenting or pre- and post-dilation, bare-metal or drug-eluting stents, and glycoprotein IIb/IIIa inhibition was left to the discretion of the treating interventional physician and was documented. No nonculprit coronary artery complementary PCI was performed, prior to the CMR study.
Our patient population included all patients from the CIRCUS trial who underwent a contrast-enhanced cardiac magnetic resonance (LGE-CMR) study within the first week (7 days) of admission in centers that had access to an MRI scanner []. The database of this study will be made available for analysis upon reasonable request to the sponsor of the study, the Hospices Civils de Lyon.
MRI Study
All CMR studies were performed on 1.5T scanners (multivendor Siemens and Philips) before hospital discharge within the first week of admission. All sequences were performed using vectocardiogram monitoring and 12-element phased-array cardiac receiver coils. After localization, rest left ventricular function was assessed with retrospective electrocardiogram-gated steady-state free precession pulse cine sequences in long- and short-axis views in the true heart axis. The short-axis scans covered the whole left ventricle. T2-weighted triple inversion recovery sequence in 3 (basal, mid-ventricular, and apical) short-axis slices was performed to assess myocardial edema [, ].
Contrast enhancement was evaluated in short-axis orientation covering the whole ventricle 10 min after contrast injection of gadolinium-DOTA (0.2 mmol/kg body weight; Dotarem; Guerbet, Villepinte, France) using 3D-gradient spoiled inversion recovery TurboFLASH sequence covering the left ventricle in the short axis. Additional 2-chamber and 4-chamber long-axis phase sensitive inversion recovery sequences were also performed for better spatial assessment of LGE-enhanced areas.
Image Analysis
Centralized, offline image analysis was performed by 2 experienced observers (M.S. and N.M.) on dedicated workstations for all CMR studies (Argus; Siemens Medical Solution, Malvern, PA, for LV volumes and mass and Osirix; OsiriX Foundation, Geneva, Switzerland, for T2 and contrast enhancement analysis []). Both observers were blinded to clinical characteristics or study status. LV volumes and function were first calculated. The infarct zone was defined semiautomatically on late gadolinium enhancement imaging using the full-width half-maximum technique. Microvascular obstruction (MVO) was defined as areas of hypoenhancement on the late gadolinium enhancement images within the hyperenhanced myocardium. The extent of infarcted myocardium and MVO was expressed in grams of tissue according to the following formula: ∑ [hypoenhanced and/or hyperenhanced area (in cm2) × slice thickness (in cm) × myocardial specific density].
NCAMI Definition
Nonculprit artery acute myocardial infarcts (NCAMIs) were defined as areas of hyperenhancement on the LGE-enhanced studies distinct from the principal territory of the left anterior descending coronary artery and with a hyperenhancement on corresponding T2-weighted images. These areas of hyperenhancement had a sub-endocardial distribution and were situated in a different territory than the LAD: the circumflex artery or right coronary artery territories.
Patients with a previous history of myocardial infarction with local remodeling (reduced myocardial wall thickness) or areas of contrast enhancement without T2-weighted enhancement were not considered as positive for NCAMI. The LGE area of these previous scars was not counted in the total LGE areas of acute myocardial infarction.
Angiographic Analysis
All coronary angiograms from the CIRCUS study were sent to the coordinating center for centralized reading. A single expert observer blinded to any other clinical and biological data read all coronary angiograms on a dedicated workstation and assessed initial and final TIMI flow, thrombus burden, transient angiographic no-reflow, multivessel disease status, and the area at risk extent with the APPROACH angiographic method [].
Each coronary angiogram was also assessed for additional angiographic characteristics of complex atheromatic lesions. The criteria that were used have been previously reported [, ]. Lesions were considered complex if they caused at least 50% stenosis and had 2 or more of the following morphologic features: (a) ulceration (sacciform extraluminal opacification), (b) irregularities or a crenated edge, (c) sharp stenosis angulation in the wall, and (d) endoluminal defects compatible with a thrombus. Multiple complex lesions (MCL) were recorded when there were at least 2 complex lesions within 2 different arteries or within the same artery separated by a 3-cm segment of nonsignificant atheroma artery.
Follow-Up
As described in the CIRCUS study, all patients were prospectively followed up for 1 year, and all-cause death, heart failure worsening during initial hospitalization or re-hospitalization for heart failure, nonfatal myocardial infarct, and stroke were recorded for each patient. All patients also underwent 2 standard echocardiograms at baseline and 1-year follow-up to assess the frequency of adverse remodeling. Adverse remodeling was defined as an increase in LV end-diastolic volume ≥15% between the 1-year follow-up and baseline.
Statistical Analysis
All results for continuous variables are expressed as means ± SDs or as medians and interquartile ranges, depending on the normal distribution as assessed by the Shapiro-Wilk test. Qualitative variables are expressed as absolute numbers and frequencies. Comparisons between groups were performed using the unpaired t test or Wilcoxon’s rank sum test for continuous variables and the χ2 or Fisher’s exact test for categorical variables as appropriate. To limit the alpha risk inflation, we limited the number of statistical comparisons to the variables of interest related to the principal objective of this study.
Exploratory statistical analyses were performed with univariate and multivariate logistic regression to assess the relationship of NCAMI with PCI procedures (direct stenting, thrombectomy, pre-post dilation, stenting, and GP2b3a inhibitors use) and angiographic characteristics (multivessel disease, MCL, initial and final TIMI grade, and thrombus burden). These variables were considered as “prespecified” for associations based on general knowledge and prior reports, regardless of univariate (χ2 or Fisher test), and we combined these with all variables that had a significant association upon univariate analysis.
Considering the small sample size of 129 patients and the increased risk of type II error, we did not statistically assess the relationship of NCAMI with adverse outcomes, and we only report the rates of events per group without testing. We however constructed Kaplan-Meier curves for the clinical outcomes of all-cause death and heart failure and survival curves between the NCAMI group and the non-NCAMI group without comparison with the log-rank test.
The results were considered statistically significant at p value <0.05. A Bonferroni correction was applied to correct for multiple comparisons. Statistical analyses were performed using STATA software, version SE 14.2 (StataCorp, College Station, TX, USA).
Results
Population
Our study included 129 patients from the 969 patients in the main trial, and all of these patients completed the full CMR protocol. CMR studies were performed 5 ± 4 days (min 2 days and max 11 days) after admission.
NCAMI was present in 11 (8.5%) patients from our study population. Seven additional (5.5%) patients had chronic infarct scar in a different infarct territory from the LAD.
As shown in Table 1, baseline clinical characteristics were similar between NCAMI patients and patients without NCAMI. Patients with NCAMI had significantly more multivessel disease compared to patients without (64% vs. 28%, respectively; p = 0.03) with a larger number of complex coronary lesions (2.45 ± 0.93 vs. 1.20 ± 0.62, respectively; p < 0.001) and a higher rate of MCL (91% vs. 28%, respectively; p < 0.001).
Figure 1 represents one typical case of our patient population in a patient with NCAMI. There were no differences in terms of procedural interventions with similar rates of thrombectomy and direct stenting in both groups of patients.
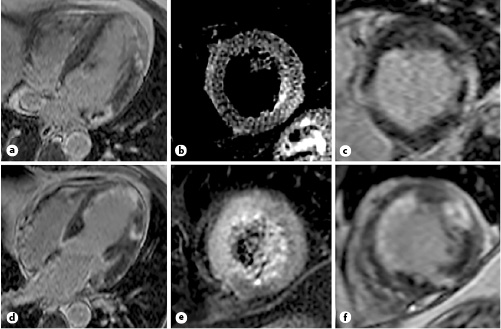
Fig. 1
A patient with acute anterior myocardial infarction and lateral NCAMI. Short-axis late delayed enhancement inversion recovery sequences (a, c, d, f) and short-axis T2w sequences (b, e) of a patient with infarct in the left main coronary artery territory (a–c) and NCAMI in the circumflex artery (d–f) with microvascular obstruction. NCAMI, nonculprit coronary artery myocardial infarction.
MRI Characteristics
All LGE-CMR findings are summarized for both groups in Table 2. NCAMIs were located in the circumflex artery territory in 9 (82%) and the right coronary artery in 2 (18%) cases. There was a significant larger infarct size in NCAMI patients compared to patients without (45.8 ± 20.4 vs. 31.0 ± 15.1% of LV, respectively; p = 0.04), and MVO size was not significantly different (5.4 ± 5.4 vs. 3.1 ± 4.7% of LV; p = 0.1). The LVEF was also significantly reduced in the NCAMI group compared to patients without (34 ± 8 vs. 46 ± 10%, respectively; p < 0.001). There were no significant differences in remodeling indices in patients with NCAMI compared to patients without.
Coronary Characteristics and Percutaneous Coronary Intervention Parameters Related to NCAMI
All results from univariate and multivariate procedural and angiographic parameters associated with NCAMI are presented in Table 3. The only factors that were significantly related to NCAMI were coronary multivessel disease (OR = 4.50; 95% CI [1.24; 16.42], p = 0.02) and MCL (OR = 9.97; 95% CI [2.99; 33.20], p < 0.001) upon univariate logistic regression. There was no significant relationship with thrombus burden, thrombectomy use, or dilatation technique. In a multivariate regression analysis integrating all the important procedural parameters, multiple complex coronary lesions were the only independent factor related to NCAMI (OR = 12.89; 95% CI [3.11; 53.39], p < 0.001).
Clinical Outcomes
The median time to follow-up was 365 days, and no patients were lost to follow-up. In our study population, there were 15 (12%) all-cause death or heart failure events at 1-year follow-up. One (9%) death occurred in the NCAMI group versus none in the control group, and 3 (27%) heart failure events occurred in the NCAMI group compared to 12 (10%) in patients without NCAMI. The Kaplan-Meier survival curves at 1 year are shown in Figure 2.
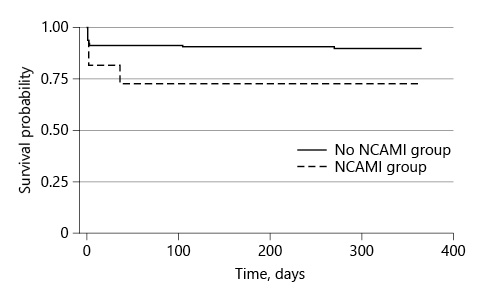
Fig. 2
Kaplan-Meier survival estimates for all-cause death and heart failure events between patients with NCAMI and patients without (control). There was no significant difference at 1-year follow-up between patients with NCAMI and patients without NCAMI by log-rank test, p = 0.22. NCAMI, nonculprit coronary artery myocardial infarction.
Discussion
This is one of the few studies with predischarge CMR focusing on NCAMI, plausible causative associated factors, and their relationship to infarct characteristics in the setting of acute anterior STEMI patients treated by PPCI. Our study shows that NCAMIs are rare and mainly occur in multivessel disease patients where there is a strong relationship with multiple complex coronary lesions. They do not seem to be related to any specific revascularization procedure. However, their presence is related to increased infarct and MVO size and worse LVEF without significant impact on LV remodeling.
There are few reports in the literature on the incidence of NCAMI, also referred to as “noninfarct related artery myocardial infarction,” in STEMI patients treated by PPCI [, ]. Recently, McCann et al. [] reported up to 4% of patients presenting new CMR-detected infarcts in a different territory from the culprit coronary artery, in STEMI patients treated by traditional culprit lesion-only PPCI. In another report from the DANAMI-3 and PRIMULTI studies in a large group of STEMI patients, spontaneous nonculprit artery infarct frequency was reported around 1–2% [].
In their report, McCann et al. [] found that there was a significant 2-fold increase in NCAMI incidence related to additional revascularization procedures to treat nonculprit lesions compared to a culprit-only PPCI. In this report, they also found a significant relationship with coronary lesion complexity as assessed with the SYNTAX score. In our study, we found a greater rate of “spontaneous” NCAMI as the culprit-only PCI group in the report by McCann et al. [] but no significant relationship with any PCI procedure. This might be related to the fact that no complementary revascularizations were performed prior to the CMR studies in our study population. In the setting of PCI for non-AMI patients, reports have shown that up to one-third of patients undergoing elective PCI have evidence of new infarction as assessed by LGE-CMR or troponin elevation [, , ]. These well-described type 4a myocardial infarctions according to the universal definition of MI have been associated with increased adverse clinical outcomes following AMI [, ].
Another interesting finding in our study is the significant relationship between coronary artery multivessel disease, multiple complex coronary lesions, and NCAMI incidence. The first logical explanation for this relationship would be the prior existence of unknown MI either asymptomatic or related to previous revascularization procedures. In our analysis, however, the recent onset of infarct was confirmed by T2-weighted imaging studies showing edema in the area of the NCAMI. T2w CMR images are associated with recent MI with high levels of sensitivity and specificity []. The accuracy for differentiating acute from chronic MI with T2w CMR has recently been shown to be within the first 6 months following infarction []. Seven patients from our study population had positive delayed enhancement outside the LAD territory, associated with features of chronic infarct scar (dense sub-endocardial delayed enhancement, no T2w hyperintensity, and myocardial wall thinning) and were not classified in the NCAMI group. Finally, no patients in the NCAMI group had a prior history of MI, and only 1 patient had a prior history of PCI. Therefore, the probability of prior myocardial infarction in the NCAMI group is very low.
An alternative explanation for the presence of NCAMI and the link with coronary artery multivessel disease and multiple complex coronary lesions is multiple plaque destabilization. In case reports, it was suggested that NCAMI was related to multiple plaque destabilization. More generally, several angiographic studies have reported worsening and destabilization of coronary atherosclerosis in the months after an acute coronary event. In the acute stage of a coronary syndrome, a worsening of other lesions initially deemed insignificant in other coronary branches distinct from the culprit coronary artery has been reported; this pattern appears in 20% of cases, as compared with 5% in cases of stable angina [, ]. This rapid development and destabilization of atherosclerotic lesions can be diffuse, leading to the concept of “pancoronaritis” [, , ]. In a coronary intravascular ultrasound study performed in STEMI patients referred to PPCI, Rioufol et al. [] showed the presence of ruptured atherosclerotic plaques in the nonculprit coronary artery in up to 79% of patients. This report was also confirmed in an OCT study by Kubo et al. [] comparing nonculprit coronary artery OCT between acute MI patients and stable angina patients. In this study, OCT examination demonstrated multiple lesion instability in the presence of AMI. These plaque ruptures cannot always be diagnosed by coronary angiography [] and could explain the presence of MI in nonculprit coronary arteries. Also, studies in larger cohorts of STEMI patients have shown that multiple complex coronary lesions are present in one-third of patients and were independently associated with subsequent death events []. The significantly higher proportion of patients with multiple complex coronary lesions in patients with NCAMI in our study adds further pathophysiology explanation to this finding. Multiple coronary complex lesions are associated with further myocardial damage at the time of acute MI and in turn are associated with adverse events [].
Study Limitation
Although our study is based on prospective data, the post hoc nature of our analysis as well as the realization of CMR studies only in a small subgroup of patients from the original study exposes us to a high risk of selection bias and limits the clinical significance of our findings. This kind of risk is also present in previous reports where only up to 47% of all eligible patients undergo CMR [, ]. As such, all findings from this exploratory analysis should be considered as hypothesis generating.
Only 13% of the original CIRCUS study patient population underwent a CMR study within the first week following reperfusion. Only centers who had access to a CMR scanner in the setting of their routine practice participated in this substudy. But, the patient population that participated in this substudy was not significantly different from the original cohort, although there was a trend for being younger. However, CMR studies were carried out for research purposes only and none were clinically indicated.
Also, because of the small sample size, there is a statistical power limitation and an increased risk of type I error for all statistical tests. We tried to control for these risks by limiting the number of statistical tests and correcting for multiple comparisons. The association between specific coronary interventions or the prognostic impact of NCAMI with adverse outcomes should be assessed in accurately powered studies including larger groups of patients. The recent report by Ekstrom et al. [] in a group of 762 patients clearly shows the negative impact of multiple scars following STEMI on adverse clinical outcomes.
Another limitation comes from our CMR imaging protocol. We utilized T2-weighted short-tau inversion recovery (STIR) sequences in order to evaluate myocardial edema. However, T1 and T2 mapping has demonstrated a better performance than STIR sequences in myocardial edema identification []. Moreover, STIR sequences were acquired in 3 short-axis slices only. Therefore, in this respect, a full coverage of the left ventricle was not obtained, and small areas of myocardial edema might have been missed.
Conclusion
Our study shows that NCAMI is present in 8.5% of acute anterior STEMI and mainly occurs in multivessel disease patients with multiple complex coronary lesions. They do not seem to be related to any specific revascularization procedure; however, their presence could be related with increased infarct and MVO size and worse functional LV indices. These findings remain to be confirmed in larger clinical studies.
Acknowledgments
This work was supported and funded by the RHU MARVELOUS (ANR-16-RHUS-0009) of Université de Lyon, within the program “Investissements d’Avenir” operated by the French National Research Agency (ANR). The authors would like to thank the Clinical Investigation Center for all the work that has been performed in data collection and management for this study.
Statement of Ethics
This trial was coordinated by the Hospices Civils de Lyon following the principles of the Declaration of Helsinki and the European Guidelines for Good Clinical Practice. Approval was obtained from the ethics committees in the relevant countries (assigned by the CPP SUD EST IV on April 14, 2010, CPP 10/024). Written informed consent was obtained from all participants before enrollment in the trial. This trial was registered at ClinicalTrials.gov: NCT01502774; and EudraCT number, 2009-013713-99 [, ].
Conflict of Interest Statement
The authors have no conflicts of interest to declare related to this manuscript.
Funding Sources
This work was supported and funded by the RHU MARVELOUS (ANR-16-RHUS-0009) of Université de Lyon, within the program “Investissements d’Avenir” operated by the French National Research Agency (ANR), and the CIRCUS trial was funded by a research grant from the French Ministry of Health (PHRC). N.M. and T.B. received a grant from the French Federation of Cardiology to support research in the field of acute myocardial infarction (Bourse René Foudon). None of the funding sources participated in the preparation of the data and writing of the manuscript.
Author Contributions
M.S., P.C., A.P., F.R., L.B., T.B., T.P., L.B., F.D.P., T.H., O.L., I.B., G.R., F.P., M.O., and N.M. shared a critical part in the making of this manuscript with substantial contributions to the conception or design of the work; acquisition, analysis, or interpretation of data for the work; drafting the work or revising it critically for important intellectual content; final approval of the version to be published; and agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of any part of the work are appropriately investigated and resolved.
Data Availability Statement
Under the European RGPD Act (personal data protection), which came into effect in the last 4 years, we do not have permission to liberate the access of any of the patient data to a third party outside of the original study. We did not request authorization in the consent form of the study, thus data are not made available.
References
- 1. Ibanez B, James S, Agewall S, Antunes MJ, Bucciarelli-Ducci C, Bueno H, et al. 2017 ESC Guidelines for the management of acute myocardial infarction in patients presenting with ST-segment elevation: the task force for the management of acute myocardial infarction in patients presenting with ST-segment elevation of the European Society of Cardiology (ESC). Eur Heart J. 2018;39:119–77. http://dx.doi.org/10.1093/eurheartj/ehx393.
- 2. Puymirat E, Simon T, Steg PG, Schiele F, Guéret P, Blanchard D, et alUSIK USIC 2000 Investigators, FAST MI Investigators. Association of changes in clinical characteristics and management with improvement in survival among patients with ST-elevation myocardial infarction. JAMA. 2012;308:998–1006. http://dx.doi.org/10.1001/2012.jama.11348.
- 3. Heusch G, Kleinbongard P, Böse D, Levkau B, Haude M, Schulz R, et al. Coronary microembolization: from bedside to bench and back to bedside. Circulation. 2009;120:1822–36. http://dx.doi.org/10.1161/CIRCULATIONAHA.109.888784.
- 4. Napodano M, Ramondo A, Tarantini G, Peluso D, Compagno S, Fraccaro C, et al. Predictors and time-related impact of distal embolization during primary angioplasty. Eur Heart J. 2009;30:305–13. http://dx.doi.org/10.1093/eurheartj/ehn594.
- 5. Niccoli G, Cosentino N, Minelli S, Cataneo L, Crea F. Microvascular obstruction after primary percutaneous coronary intervention: pathogenesis, diagnosis and prognostic significance. Curr Vasc Pharmacol. 2013;11:245–62. http://dx.doi.org/10.2174/1570161111311020013.
- 6. Porto I, Selvanayagam JB, Van Gaal WJ, Prati F, Cheng A, Channon K, et al. Plaque volume and occurrence and location of periprocedural myocardial necrosis after percutaneous coronary intervention: insights from delayed-enhancement magnetic resonance imaging, thrombolysis in myocardial infarction myocardial perfusion grade analysis, and intravascular ultrasound. Circulation. 2006;114:662–9. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.593210.
- 7. Watabe H, Sato A, Nishina H, Hoshi T, Sugano A, Kakefuda Y, et al. Enhancement patterns detected by multidetector computed tomography are associated with microvascular obstruction and left ventricular remodelling in patients with acute myocardial infarction. Eur Heart J. 2016;37:684–92. http://dx.doi.org/10.1093/eurheartj/ehv467.
- 8. Mangion K, Carrick D, Hennigan BW, Payne AR, McClure J, Mason M, et al. Infarct size and left ventricular remodelling after preventive percutaneous coronary intervention. Heart. 2016;102:1980–7. http://dx.doi.org/10.1136/heartjnl-2015-308660.
- 9. McCann GP, Khan JN, Greenwood JP, Nazir SA, Dalby M, Curzen N, et al. The randomised complete versus lesion-only primary percutaneous coronary intervention trial: cardiovascular magnetic resonance imaging substudy (CvLPRIT-CMR). Efficacy Mech Eval. 2016;3(1):1. http://dx.doi.org/10.3310/eme03010.
- 10. Ekström K, Nepper-Christensen L, Ahtarovski KA, Kyhl K, Göransson C, Bertelsen L, et al. Impact of multiple myocardial scars detected by CMR in patients following STEMI. JACC Cardiovasc Imaging. 2019;12:2168–78. http://dx.doi.org/10.1016/j.jcmg.2019.01.032.
- 11. Kubo T, Imanishi T, Kashiwagi M, Ikejima H, Tsujioka H, Kuroi A, et al. Multiple coronary lesion instability in patients with acute myocardial infarction as determined by optical coherence tomography. Am J Cardiol. 2010;105:318–22. http://dx.doi.org/10.1016/j.amjcard.2009.09.032.
- 12. Rioufol G, Finet G, André-Fouët X, Rossi R, Vialle E, Desjoyaux E, et al. [Multiple ruptures of atherosclerotic plaques in acute coronary syndrome. Endocoronary ultrasonography study of three arteries]. Arch Mal Coeur Vaiss. 2002;95:157–65.
- 13. Rioufol G, Gilard M, Finet G, Ginon I, Boschat J, André-Fouët X. Evolution of spontaneous atherosclerotic plaque rupture with medical therapy: long-term follow-up with intravascular ultrasound. Circulation. 2004;110:2875–80. http://dx.doi.org/10.1161/01.CIR.0000146337.05073.22.
- 14. Cung TT, Morel O, Cayla G, Rioufol G, Garcia-Dorado D, Angoulvant D, et al. Cyclosporine before PCI in patients with acute myocardial infarction. N Engl J Med. 2015;373:1021–31. http://dx.doi.org/10.1056/NEJMoa1505489.
- 15. Mewton N, Cung TT, Morel O, Cayla G, Bonnefoy-Cudraz E, Rioufol G, et al. Rationale and design of the cyclosporine to improve clinical outcome in ST-elevation myocardial infarction patients (the CIRCUS trial). Am Heart J. 2015;169:758–66.e6. http://dx.doi.org/10.1016/j.ahj.2015.02.020.
- 16. Aletras AH, Tilak GS, Natanzon A, Hsu LY, Gonzalez FM, Hoyt RF, et al. Retrospective determination of the area at risk for reperfused acute myocardial infarction with T2-weighted cardiac magnetic resonance imaging: histopathological and displacement encoding with stimulated echoes (DENSE) functional validations. Circulation. 2006;113:1865–70. http://dx.doi.org/10.1161/CIRCULATIONAHA.105.576025.
- 17. Friedrich MG, Abdel-Aty H, Taylor A, Schulz-Menger J, Messroghli D, Dietz R. The salvaged area at risk in reperfused acute myocardial infarction as visualized by cardiovascular magnetic resonance. J Am Coll Cardiol. 2008;51:1581–7. http://dx.doi.org/10.1016/j.jacc.2008.01.019.
- 18. Moulin K, Viallon M, Romero W, Chazot A, Mewton N, Isaaz K, et al. MRI of reperfused acute myocardial infarction edema: ADC quantification versus T1 and T2 mapping. Radiology. 2020 Jun;295(3):542–9.
- 19. Vauchot F, Ben Bouallègue F, Hedon C, Piot C, Roubille F, Mariano-Goulart D. Assessment of the area at risk after acute myocardial infarction using 123I-MIBG SPECT: comparison with the angiographic APPROACH-score. J Nucl Cardiol. 2018;25:572–80. http://dx.doi.org/10.1007/s12350-016-0644-7.
- 20. Goldstein JA, Demetriou D, Grines CL, Pica M, Shoukfeh M, O’Neill WW. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2000;343:915–22. http://dx.doi.org/10.1056/NEJM200009283431303.
- 21. Rioufol G, Zeller M, Dentan G, Laurent Y, L’Huillier I, Ravisy J, et al. Predictors and prognosis for complex coronary lesions in patients with acute myocardial infarction: data from RICO survey. Am Heart J. 2007;154:330–5. http://dx.doi.org/10.1016/j.ahj.2007.04.013.
- 22. Gerbaud E, De Clermont-Galleran H, Erickson M, Coste P, Montaudon M. Unexpected coexisting myocardial infarction detected by delayed enhancement MRI. Case Rep Med. 2009;2009:370542. http://dx.doi.org/10.1155/2009/370542.
- 23. Kawecki D, Morawiec B, Monney P, Pellaton C, Wojciechowska C, Jojko J, et al. Diagnostic contribution of cardiac magnetic resonance in patients with acute coronary syndrome and culprit-free angiograms. Med Sci Monit. 2015;21:171–80. http://dx.doi.org/10.12659/MSM.892296.
- 24. Alcock RF, Roy P, Adorini K, Lau GT, Kritharides L, Lowe HC, et al. Incidence and determinants of myocardial infarction following percutaneous coronary interventions according to the revised joint task force definition of troponin T elevation. Int J Cardiol. 2010;140:66–72. http://dx.doi.org/10.1016/j.ijcard.2008.11.005.
- 25. Selvanayagam JB, Porto I, Channon K, Petersen SE, Francis JM, Neubauer S, et al. Troponin elevation after percutaneous coronary intervention directly represents the extent of irreversible myocardial injury: insights from cardiovascular magnetic resonance imaging. Circulation. 2005;111:1027–32. http://dx.doi.org/10.1161/01.CIR.0000156328.28485.AD.
- 26. Rahimi K, Banning AP, Cheng AS, Pegg TJ, Karamitsos TD, Channon KM, et al. Prognostic value of coronary revascularisation-related myocardial injury: a cardiac magnetic resonance imaging study. Heart. 2009;95:1937–43. http://dx.doi.org/10.1136/hrt.2009.173302.
- 27. Thygesen K, Alpert JS, Jaffe AS, Chaitman BR, Bax JJ, Morrow DA, et al. Fourth universal definition of myocardial infarction (2018). Eur Heart J. 2019;40(3):237–69.
- 28. Smulders MW, Bekkers SC, Kim HW, Van Assche LM, Parker MA, Kim RJ. Performance of CMR methods for differentiating acute from chronic MI. JACC Cardiovasc Imaging. 2015;8:669–79. http://dx.doi.org/10.1016/j.jcmg.2014.12.030.
- 29. Guazzi MD, Bussotti M, Grancini L, De Cesare N, Guazzi M, Pera IL, et al. Evidence of multifocal activity of coronary disease in patients with acute myocardial infarction. Circulation. 1997;96:1145–51. http://dx.doi.org/10.1161/01.cir.96.4.1145.
- 30. Kaski JC, Chen L, Crook R, Cox I, Tousoulis D, Chester MR. Coronary stenosis progression differs in patients with stable angina pectoris with and without a previous history of unstable angina. Eur Heart J. 1996;17:1488–94. http://dx.doi.org/10.1093/oxfordjournals.eurheartj.a014711.
- 31. Asakura M, Ueda Y, Yamaguchi O, Adachi T, Hirayama A, Hori M, et al. Extensive development of vulnerable plaques as a pan-coronary process in patients with myocardial infarction: an angioscopic study. J Am Coll Cardiol. 2001;37:1284–8. http://dx.doi.org/10.1016/s0735-1097(01)01135-4.
- 32. Pasterkamp G, Vink A, Borst C. Multiple complex coronary plaques in patients with acute myocardial infarction. N Engl J Med. 2001;344:527. author reply 528. http://dx.doi.org/10.1056/nejm200102153440713.
- 33. Gilard M, Rioufol G, Zeller M, Cottin Y, Rochette L, Finet G. Reliability and limitations of angiography in the diagnosis of coronary plaque rupture: an intravascular ultrasound study. Arch Cardiovasc Dis. 2008;101:114–20. http://dx.doi.org/10.1016/s1875-2136(08)70268-7.
- 34. Eitel I, de Waha S, Wöhrle J, Fuernau G, Lurz P, Pauschinger M, et al. Comprehensive prognosis assessment by CMR imaging after ST-segment elevation myocardial infarction. J Am Coll Cardiol. 2014;64:1217–26. http://dx.doi.org/10.1016/j.jacc.2014.06.1194.
- 35. Verhaert D, Thavendiranathan P, Giri S, Mihai G, Rajagopalan S, Simonetti OP, et al. Direct T2 quantification of myocardial edema in acute ischemic injury. JACC Cardiovasc Imaging. 2011;4:269–78. http://dx.doi.org/10.1016/j.jcmg.2010.09.023.