Introduction
Hyponatremia, usually defined as serum sodium <135 mmol/L, is the most common electrolyte disorder in clinical practice and is associated with increased morbidity and mortality. The severity of symptoms associated with hyponatremia depends on the rapidity and degree of serum sodium concentration reduction. Patients with acute (<48 h) or severe (sodium levels <120 mmol/L) hyponatremia may present with nonspecific symptoms such as nausea, vomiting, and headache up to stupor, coma, seizures, respiratory depression, and death. Chronic moderate (sodium levels 120–129 mmol/L) and mild (sodium levels 130–134 mmol/L) hyponatremia may present with subtle manifestations, such as fatigue, cognitive impairment, gait deficits, falls, impaired bone metabolism, and fractures []. Heart failure (HF) is a relatively frequent cause of hyponatremia []. Conversely, hyponatremia may affect up to one-third of patients with HF [-]. The main underlying mechanism of HF-related hyponatremia is the enhanced non-osmotic release of antidiuretic hormone (ADH) due to effective circulating volume depletion. However, the pathogenesis of hyponatremia in HF is usually multifactorial, something which is frequently overlooked. Indeed, even in the most recent guidelines of the European Society of Cardiology (ESC) for the diagnosis and treatment of HF, it is stated that “hyponatremia in HF patients reflects neurohormonal activation” []. In this context, we aimed to draw the attention to the mechanisms of hyponatremia beyond the neurohormonal activation due to ineffective circulating volume in these patients (Table 1). The treatment of hyponatremia in HF is also discussed.
Methods
A PubMed search was performed up to March 2021 using combinations of the following keywords: hyponatremia, sodium, heart failure, drugs, electrolyte abnormalities, co-morbidities, syndrome of inappropriate antidiuretic hormone secretion (SIAD), alcohol, infections, autoimmune diseases, thyroid diseases, diabetes, depression, cachexia, arthritis, cancer, stroke, chronic kidney disease, and lung diseases. Data from case reports, randomized controlled trials, original papers, and review articles were collected. References of these articles were scrutinized for relevant articles.
Pathophysiology of Hyponatremia
Hyponatremia is attributed either to loss of effective solutes (sodium plus potassium) in excess of water or to water retention. The capacity for water excretion is sufficient in normal states; thus, water retention occurs when renal excretion of water is impaired. An exception to this rule is primary polydipsia, in which the disproportionate water intake (10–15 L) exceeds the normal renal excretory capacity (“acute water intoxication”). High serum levels of ADH are a prerequisite for the development and maintenance of hyponatremia, as the suppression of ADH secretion plays a fundamental role in the renal excretion of any water load. Consequently, most causes of hyponatremia (except for low dietary solute intake, renal failure, primary polydipsia, or beer potomania syndrome) are accompanied by increased ADH, mainly due to the SIAD or to effective circulating volume depletion (true hypovolemia and edematous states) [].
The reduced cardiac output in HF (either due to HF with reduced, mildly reduced, or preserved ejection fraction) decreases the stretch at the carotid and renal baroreceptors, leading to sympathetic nervous system (SNS) and renin-angiotensin-aldosterone system (RAAS) activation along with enhancement of ADH excretion and action []. The role of neurohormonal activation in the development of hyponatremia in patients with HF is well established []. Specifically, angiotensin II (AT II) increases efferent arteriolar tone, promoting sodium and water absorption by way of the accompanying rise in the filtration fraction []. Furthermore, it promotes the release of ADH [, ] and stimulates thirst, increasing free water intake []. ADH causes vasoconstriction and increases water retention in the collecting ducts through vasopressin 2 receptors []. AT II also stimulates the secretion of aldosterone; the latter decreases water and sodium excretion in the distal tubules and collecting ducts []. The activation of the SNS promotes renal vasoconstriction, consequently decreasing glomerular filtration rate. The diminished sodium and water delivery to the distal tubules also reduces renal water excretion []. Importantly, factors that provoke HF may be implicated in the development of hyponatremia. Furthermore, HF patients frequently have comorbidities which contribute to the development or preservation of hyponatremia. Finally, several drugs used in HF may trigger or aggravate hyponatremia.
Etiologies of HF Associated with Hyponatremia
Some causes of HF, as presented in the guidelines of the ESC [], may be potential causes of hyponatremia.
Toxic Damage to the Myocardium
Alcohol
Chronic alcohol abuse has been associated with deleterious effects on the cardiovascular system [] either via increased SNS and RAAS activation [, ] or due to contractile dysfunction [, -]. Hyponatremia is commonly observed in chronic alcoholics. The most important underlying mechanisms are as follows.
Pseudohyponatremia due to alcohol-induced severe hypertriglyceridemia [].
Hypovolemia due to gastrointestinal losses.
Beer potomania syndrome, characterized by excessive hypo-osmolar drinking usually accompanied by low solute intake. Hence, kidneys cannot excrete free water sufficiently and hypo-osmotic hyponatremia occurs [].
Reset osmostat syndrome [], which is characterized by a decrease in plasma osmolality threshold for ADH excretion. Thus, plasma sodium concentration is adjusted to a lower level, typically between 125 and 135 mmol/L [].
Cocaine
Cocaine is a well-known cardiotoxic agent [, ], whereas its use has been associated with dilated [] and Takotsubo cardiomyopathy []. Cocaine blocks the presynaptic reuptake of serotonin and catecholamines, increasing their bioavailability at postsynaptic receptors, subsequently stimulating ADH release []. Hyponatremia develops due to excessive ADH release.
Methamphetamines
Methamphetamine use has been associated both with HF and hyponatremia [, ]. Methamphetamine-associated cardiomyopathy is the consequence of catecholamine excess, direct cardiotoxicity, coronary arterial vasoconstriction, and ischemia []. Methamphetamines may cause severe and life-threatening hyponatremia in the context of increased ADH secretion combined with excessive water intake in an effort to counteract hyperthermia [].
Psychotropic Drugs
The use of antidepressant and antipsychotic drugs has been associated with HF [-]. Among antidepressants, a significant association with dilated cardiomyopathy was found with tricyclic (clomipramine, amitriptyline) and serotoninergic (fluvoxamine) antidepressants []. An acute reversible type of diffusely depressed myocardial contractility has been described with venlafaxine overdose [].
The administration of antidepressant and antipsychotic drugs is a well-recognized cause of SIAD [] and hyponatremia [, ]. These drugs enhance the release of ADH and the renal responsiveness on its action []. Moreover, the sensation of dry mouth caused by psychotropic drugs stimulates water intake, whereas psychogenic polydipsia frequently occurs in psychiatric patients further aggravating hyponatremia [].
Immunomodulating Drugs
Numerous cases of reversible dilated cardiomyopathy have been reported after treatment with interferon-α [, ], while interleukin-2 (IL-2) treatment has been associated with reversible left ventricular dysfunction []. Furthermore, several molecular-targeted therapies, including trastuzumab, sunitinib, sorafenib, and imatinib, appear to impair cardiac mitochondrial function, leading to cardiomyocyte and endothelial cell dysfunction, subsequently deteriorating myocardial contractility []. The aforementioned agents may cause hyponatremia via SIAD [].
Alkylating Agents
Cyclophosphamide. Cyclophosphamide cardiotoxicity ranges from subtle electrocardiographic changes to potentially fatal cardiomyopathy, especially when given in high doses or following treatment with anthracycline and mediastinal radiation []. Myocarditis, and rarely HF, may occur during the first 2 weeks post therapy [, ]. Cyclophosphamide administration may enhance the renal effect of ADH or its central release. Vigorous hydration with hypotonic fluids to prevent hemorrhagic cystitis may also be involved [].
Ifosfamide. Ifosfamide may promote severe left ventricular dysfunction acutely []. Hyponatremia has been reported after ifosfamide administration and is probably attributed to SIAD [].
Nonsteroidal Anti-Inflammatory Drugs
The use of nonsteroidal anti-inflammatory drugs (NSAIDs) may increase the risk for HF hospitalization in patients with HF []. NSAIDs may aggravate HF through sodium and water retention, increased systemic vascular resistance, and blunted response to diuretics []. These drugs occasionally cause hyponatremia by diminishing the normal inhibitory effect of prostaglandins on ADH activity [].
Immune-Mediated and Inflammatory Injury to the Myocardium
Myocardial Injury Associated with Infections
Infections usually aggravate the symptoms of HF, while they may also cause acute HF in a previously healthy myocardium []. In septic patients, the high output along with reduced systemic vascular resistance and low arterial blood pressure leads to activation of SNS and RAAS and increased ADH concentration. The subsequent sodium and water retention may lead to ventricular enlargement, remodeling, and HF [].
Hyponatremia associated with infections is usually multifactorial []. Edematous states including HF may be provoked by an infectious agent [-]. Moreover, infections may deplete the effective circulating volume due to gastrointestinal losses, excessive sweating, systemic vasodilatation, or increased vascular permeability [], subsequently stimulating ADH secretion.
Beyond the appropriate secretion of ADH in response to the decrease in the effective circulating volume, some infections, especially central nervous system and pulmonary infections, may cause hyponatremia via SIAD or cerebral salt-wasting syndrome [, -]. Furthermore, interleukin-6 (IL-6) may be implicated in the non-osmotic release of ADH []. Of note, IL-6 is one of the most important cytokines implicated in the hyper-inflammation syndrome in patients with COVID-19, while IL-6 levels were inversely correlated with serum sodium levels in these patients [, ]. Interestingly, 48 h after the administration of tocilizumab, a humanized monoclonal antibody against IL-6 receptor, sodium levels increased significantly in patients with hyponatremia [].
Hyponatremia observed in the course of an infection may be due to infection-induced hyperglycemia (see below) or adrenal insufficiency (primary or secondary) [, -]. Several antibiotics, e.g., trimethoprim, may also be involved in the development of hyponatremia []. Ciprofloxacin rarely causes hyponatremia possibly via SIAD []. Of note, pseudohyponatremia sometimes occurs in patients with underlying infections and hypergammaglobulinemia secondary to β-lymphocyte activation [].
Immune-Mediated HF
Autoimmune diseases may be complicated with the development of HF []. Hyponatremia in these patients may be the consequence of steroid-induced hyperglycemia [], while other commonly prescribed drugs, such as NSAIDs and methotrexate, may lead to or deteriorate hyponatremia. Specifically, methotrexate in high doses may activate natriuretic peptides or change the distribution of body fluid volumes [, ]. Importantly, autoimmune adrenalitis expressed as primary adrenal insufficiency should always be suspected in patients with autoimmune disorders and new-onset hyponatremia []. Pseudohyponatremia due to the existing hypergammaglobulinemia or intravenous immunoglobulin administration should also be considered [, ]. Of note, intravenous immunoglobulin has been used for the treatment of autoimmune disease-related HF [].
Infiltration of the Myocardium
In glycogen storage diseases, abnormal glycogen accumulates in the myocardium, and HF may develop [, ]. Hypertriglyceridemia or mixed dyslipidemia may also occur; thus, pseudohyponatremia may be observed.
Metabolic Derangements (Hormonal)
Diabetes
HF is a major long-term complication of diabetes mellitus (DM). Diabetic subjects are prone to electrolyte disorders []. Hypovolemia is among the most common causes of hyponatremia in diabetic subjects. Osmotic diuresis in uncontrolled DM or DM-associated diarrhea and vomiting, either from the disease itself or antidiabetic therapy (i.e., metformin, glucagon-like peptide-1 receptor analogs), is the usual culprit of hypovolemia [].
Hyponatremia frequently develops secondarily to chronic kidney disease (CKD), a common complication of DM []. Furthermore, hyporeninemic hypoaldosteronism, usually associated with mild to moderate CKD, may be the cause of mild hyponatremia in diabetic individuals [].
Importantly, several drugs used in DM may cause or worsen hyponatremia. First generation sulphonylureas, nowadays seldom prescribed [], and amitriptyline used for diabetic neuropathy [], may induce hyponatremia. However, drug-associated hyponatremia in diabetic individuals usually occurs when several culprit agents are co-administered []. Interestingly, in the Rotterdam study, DM per se was associated with hyponatremia [], probably via induction of aquaporin-2 by insulin [].
In the presence of marked hyperglycemia, the high serum osmolality drives water out of cells and dilutional hyponatremia occurs. The latter should be suspected and corrected sodium for serum glucose levels calculated (Figure 1) [, ]. In DM, pseudohyponatremia may be secondary to hyperglycemia-induced hypertriglyceridemia [].
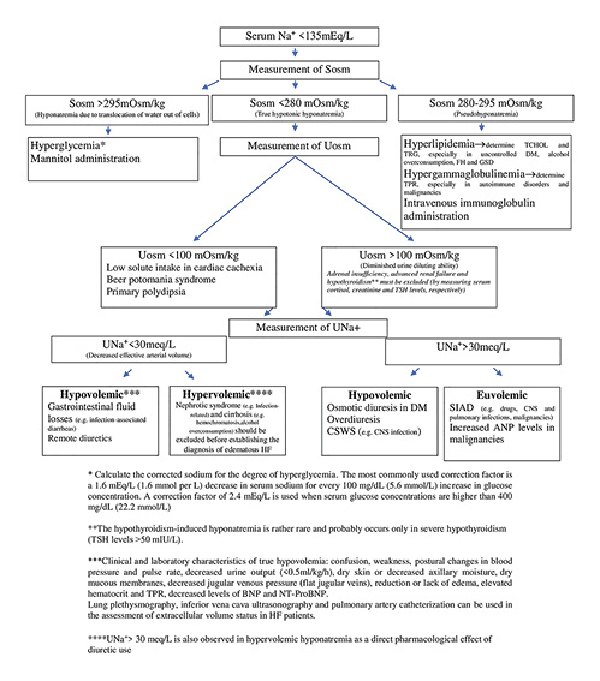
Fig. 1
Diagnostic algorithm of hyponatremia in patients with heart failure (HF). ANP, atrial natriuretic peptide; BNP, brain natriuretic peptide; CNS, central nervous system; CSWS, cerebral salt-wasting syndrome; DM, diabetes mellitus; FH, familial hypercholesterolemia; GSD, glycogen storage disease; NT-ProBNP, N-terminal prohormone of BNP; SIAD, syndrome of inappropriate ADH secretion; Sosm, serum osmolality; TCHOL, total cholesterol; TPR, total serum proteins; TRG, triglycerides; TSH, thyroid-stimulating hormone; UNa+, urinary sodium; Uosm, urine osmolality.
Thyroid Diseases
Hypothyroidism may impair cardiac contractility []. Hypothyroidism-induced hyponatremia is rather rare and occurs only in severe hypothyroidism. The decreased capacity of free water excretion due to elevated ADH levels seems to be the underlying mechanism [].
Addison’s Disease
Among the cardiovascular manifestations of Addison’s disease (primary adrenal insufficiency) is the development of dilated cardiomyopathy and HF []. Hyponatremia in Addison’s disease may be due to systemic blood pressure reduction and enhanced ADH secretion, as cortisol is a tonic inhibitor of ADH secretion []. Furthermore, aldosterone deficiency promotes sodium wasting and hypovolemia, leading to hyponatremia [].
Pregnancy
Preload, afterload, and heart rate variations during gestation result in a 30–50% increase in cardiac output and a prolonged volume overload []. Several complications of pregnancy may cause or increase the risk of developing HF. Namely, peripartum cardiomyopathy is characterized by the presence of signs and symptoms of HF during the last month of pregnancy or within 5 months of delivery [], with echocardiography criteria including ejection fraction less than 45%, end-diastolic diameter greater than 2.7 cm/m2, and/or M-mode fractional shortening less than 30% []. Also, another important complication of pregnancy, preeclampsia, is associated with a 4-fold increased risk of HF after delivery [].
Systemic arterial vasodilatation secondary to hormonal changes leads to RAAS activation and non-osmotic ADH secretion []. Nausea, vomiting, and pain, frequently encountered during pregnancy and labor, enhance ADH secretion []. The latter occurs at a lower serum-sodium concentration threshold in pregnancy, the so-called “reset osmostat syndrome,” leading to inappropriate ADH secretion []. Therefore, plasma osmolality and sodium are lower by 10 mOsm/L and 4–5 mmol/L, respectively [].
Comorbidities of HF Associated with Hyponatremia
Several comorbidities which frequently accompany chronic HF may also be involved in the development or preservation of hyponatremia. These will be presented herein.
Cachexia and Sarcopenia
Cardiac cachexia is a serious and common condition associated with chronic HF []. Anorexia and reduced food intake secondary to tasteless diets with low sodium content, dyspnea, depression, visceral congestion, and intestinal malabsorption contribute to weight loss [-]. Increased tumor necrosis factor (TNF)-α may also be involved [, ].
Reduced protein intake predisposes to the development of hyponatremia due to the low rate of osmole excretion and impaired free water clearance []. In a recent multicenter randomized study, SODIUM-HF, HF patients with tighter diet salt restriction (1,600 mg per day vs. 2,100 mg per day) showed no significant difference in all cause death, HF hospitalizations, or visits to the emergency department for HF. However, the incidence of hyponatremia in the abovementioned study is not known [].
Cancer
HF and cancer share several risk factors [, ] and have some mutual pathophysiologic mechanisms []. HF patients have a higher risk of developing cancer [], whereas cancer survivors are at increased risk of developing CVD and HF []. Furthermore, certain cancer therapies may trigger or exacerbate HF [, , ].
Hyponatremia in oncologic patients is frequent [-]. The main underlying mechanisms are as follows.
Ectopic ADH production by certain tumors, namely, from the lungs (especially small-cell lung cancer) [, ], breast, head, or neck [, ].
Pain and stress, which promote ADH secretion.
Paraneoplastic production of atrial natriuretic peptide (ANP), e.g., in patients with small-cell lung cancer []. ANP suppresses the aldosterone axis [].
Vincristine, vinblastine, cyclophosphamide, cisplatin, melphalan [, , ], brivanib, and sorafenib [, ] have been associated with hyponatremia, possibly through SIAD-like mechanisms [].
Hypovolemic hyponatremia due to chemotherapy-induced diarrhea and vomiting [, ].
Stimulation of ADH release by palliative and pain-relief drugs (e.g., morphine and carbamazepine) []. Opiate-induced nausea or hypotension may also be implicated [].
Hyperglycemia from steroids used for the treatment of nausea, compression syndromes, and lymphomas [].
Adrenocortical insufficiency secondary to adrenal metastases or primary adrenal lymphoma [, ].
Pseudohyponatremia, sometimes encountered in hematologic malignancies due to paraproteinemia [].
Iatrogenic hyponatremia as a result of the administration of hypotonic intravenous fluids []
Central Nervous System Disorders
Stroke
Stroke and HF commonly coexist because of shared risk factors. HF is associated with an increased risk of both first [] and recurrent ischemic stroke []
Hyponatremia on stroke admission is mainly ascribed to the presence of comorbidities or medications, including diuretics and antidepressants, whereas hyponatremia during hospitalization may be the consequence of hypotonic solution administration, poor solute intake, or infections (e.g., aspiration pneumonia) and the use of certain drugs in the acute setting []. Specifically, mannitol administered in case of cerebral edema causes dilutional hyponatremia []. Stroke per se may cause hyponatremia due to pituitary ischemia or hemorrhage, secondary adrenal insufficiency, SIAD, and cerebral salt-wasting syndrome [, ].
Depression
Depression is up to 5 times more prevalent in HF patients than in the general population [] and is an independent risk factor for hospitalization and mortality [, ]. As previously mentioned, antidepressant drugs are a common cause of hyponatremia.
Diabetes Mellitus
The close link between DM and HF as well as the possible causes of hyponatremia in diabetic individuals have already been described.
Gout and Arthritis
Hyperuricemia and gout are common in HF patients and may be caused or aggravated by diuretics []. Furthermore, rheumatoid arthritis is associated with an increased risk of HF [], independent of the presence of ischemic heart disease []. Pain and stress, frequently observed in patients with arthritis, are non-osmotic stimuli for ADH secretion. Moreover, these patients frequently take NSAIDs [], which occasionally provoke hyponatremia by enhancing the effect of ADH [].
Chronic Kidney Disease
HF and CKD have several common risk factors and often coexist []. Kidneys are the key organs that maintain water homeostasis. Thus, in CKD, the urinary dilution ability is impaired, and if the volume of ingested fluids exceeds this ability, water retention and hyponatremia develop [].
Lung Diseases
Severe long-standing chronic obstructive pulmonary disease (COPD) may be complicated by pulmonary hypertension and right-sided HF. Conversely, up to 50% of patients with chronic HF have COPD []. Furthermore, respiratory infections are among the most common causes of hospitalization in HF patients [].
Several lung diseases, including lung infections, asthma, COPD, lung tumors, cystic fibrosis, and acute respiratory failure, have been associated with SIAD and, subsequently, hyponatremia []. Furthermore, hypercapnia reduces renal blood flow either by means of direct renal vasoconstriction or indirectly through noradrenaline secretion []. Accordingly, water retention and hyponatremia occur []. The use of diuretics, concomitant renal insufficiency, hypokalemia attributed to bronchodilators or steroids, malnutrition, and poor solute intake during acute COPD exacerbations may also trigger hyponatremia [].
Liver Diseases
Liver diseases and especially cirrhosis are often accompanied by hyponatremia. Importantly, HF and hepatopathies do not infrequently coexist (e.g., hemochromatosis, alcohol overconsumption). Moreover, HF may cause hepatic fibrosis (“cardiac cirrhosis”), whereas liver cirrhosis may induce pulmonary arterial hypertension and deterioration of heart function [].
Sleep Disturbance and Sleep-Disordered Breathing
Sleep disturbance and sleep-disordered breathing are among the most frequent comorbidities in patients with chronic HF []. Sleep disorders are associated with hyponatremia, namely, sleep deprivation reduces cortisol levels during the next day. Taking into consideration that cortisol is a tonic inhibitor of ADH, hypocortisolemia predisposes to hyponatremia [].
Hypertension
Hypertension is among the most significant risk factors for the development of HF [-]. Antihypertensive agents and especially diuretics are commonly associated with the development of hyponatremia (the underlying mechanisms are described in the next section) [].
Hyponatremia Related to Drugs Commonly Used in HF Patients
Diuretics
The relationship between thiazide and thiazide-like diuretics and hyponatremia is well documented [, ]. These drugs block Na+-Cl− cotransporter in the distal convoluted tubule, leading to Na+ and Cl− excretion without concomitant water diuresis since the distal convoluted tubule is impermeable to water []. Therefore, if treated patients ingest large quantities of water, dilutional hyponatremia may develop []. Moreover, the reduction of the extracellular fluid volume due to diuresis stimulates ADH release, increasing water reabsorption []. Importantly, thiazides increase water intake [] and impair water excretion independent of ADH action [, ]. Loop and potassium-sparing diuretics (e.g., furosemide and spironolactone, respectively) have also been associated with the development of hyponatremia [, ]. Spironolactone inhibits sodium reabsorption at the renal collecting duct, causing salt wasting and hyponatremia []. Loop diuretics enhance hypotonic renal losses and thus may cause or worsen hyponatremia [, ].
Αngiotensin-Converting Enzyme (ACE) Inhibitors and Angiotensin Receptor Blockers
Hyponatremia has been rarely reported in patients taking ACE inhibitors or angiotensin receptor blockers []. Hyponatremia may probably be attributed to SIAD caused by these agents [, ].
Sacubitril/Valsartan
Sacubitril/valsartan is the first commercially available angiotensin receptor and neprilysin inhibitor approved for use in HF patients with reduced ejection fraction. Only 1 case of hyponatremia has been described after the initiation of sacubitril/valsartan []. Hyponatremia may be ascribed to neprilysin inhibition, which increases the levels of several endogenous vasoactive and natriuretic peptides [].
Antiarrhythmic Drugs
Hyponatremia attributed to amiodarone is a rare but potentially lethal complication. It occurs during the loading period or the first weeks of treatment initiation. The underlying mechanism of amiodarone-induced hyponatremia is SIAD and has also been described in association with other antiarrhythmic drugs, such as lorcainide and propafenone [].
Anticoagulants
Anticoagulant-related hemorrhage and concomitant reduction in effective circulating volume may be a potential cause of hyponatremia through activation of SNS, RAAS, and ADH secretion. Furthermore, in one study in hospitalized HF patients, the administration of heparins was significantly associated with hospital-acquired hyponatremia []. Both unfractionated and low molecular weight heparins decrease aldosterone levels by reducing the number and affinity of adrenal AT II receptors, thus attenuating aldosterone release from the adrenal cortex []. Moreover, anticoagulants may occasionally cause hyponatremia through intra-adrenal hemorrhage and adrenal failure [].
Clinical Significance, Evaluation, and Treatment Options for Hyponatremia in HF
HF is a clinical syndrome with progressively increasing public health importance associated with considerable morbidity, mortality, and immense healthcare costs []. Although mortality from HF has improved over the past few decades, 5-year mortality remains high, exceeding that of many cancers []. In patients with HF, hyponatremia is frequently encountered and represents an unfavorable prognostic factor [, ]. Of note, the poor prognosis in patients admitted with acute HF and hyponatremia is independent of left ventricular ejection fraction []. Furthermore, in a recent meta-analysis, the improvement of hyponatremia during hospitalization was associated with a lower, particularly short-term, mortality risk at follow-up [].
Strategies aiming at the reduction of the incidence of hospital-acquired hyponatremia are of paramount importance. The administration of hypotonic fluids is common in clinical practice in hospitalized HF patients, especially in the elderly, for the avoidance of volume overload. In the hospital setting, however, patients frequently have multiple stimuli for ADH secretion (e.g., stress, pain, nausea) and thus are at an increased risk for the development of hyponatremia. Isotonic fluids (5% dextrose in a solution of 0.9% saline at a rate of 40–60 mL/h in adults and 40–60% of this amount calculated with the use of the Holliday-Segar formula in children) are considered the most suitable maintenance fluids in patients with compensated HF []. Moreover, the appropriate management of the associated clinical entities (Table 1) is crucial in order to prevent both the development and the deterioration of hyponatremia.
As hyponatremia is frequently multifactorial in patients with HF, it often poses a diagnostic and therapeutic challenge. A step-by-step diagnostic evaluation of hyponatremia in HF is shown in Figure 1. It should be emphasized that the first entity a clinician should exclude in case of hyponatremia is pseudo-hyponatremia. In such cases, sodium should be measured by using direct ion-selective electrodes, as measurement only by indirect ion-selective electrode may lead to spurious hyponatremia. A measured serum osmolality within normal limits (280–295 mOsm/kg) is also suggestive of pseudohyponatremia [, ].
The treatment of hyponatremia should be selected on the basis of its duration, symptoms, and extracellular volume status [, ]. Special attention should be made to correct serum sodium levels at the appropriate rate, especially in chronic hyponatremia, in order to avoid the osmotic demyelination syndrome (ODS) [-]. In chronic hyponatremia, even if it is symptomatic, the correction rate of serum sodium concentration should be restricted at <10 mmol/L/24 h [, ]. Some individuals, including the elderly, the alcoholics, the malnourished patients, and the patients with coexisting hypokalemia, have a higher risk of developing ODS. A tighter safety limit of correction of 8 mmol/L in 24 h and 14 mmol/L in 48 h should be considered for these patients [, ].
In cases of severe neurological symptoms attributed to hyponatremia, an elevation in serum sodium concentration by 4–6 mmol/L within the first 4–6 h is recommended []. The administration of 100–150 mL of hypertonic saline (3% NaCl) over 10–20 min up to 3 times, combined with furosemide to avoid circulatory overload, is plausible until the symptoms subside. However, care should be taken not to exceed the aforementioned targets [, ].
Treatment of the underlying conditions associated with hyponatremia (e.g., infections, hyperglycemia, adrenal insufficiency) is crucial in the case of hypovolemic hyponatremia, where normal saline is usually used to restore the intravascular volume. Potassium deficits should be corrected by adding potassium chloride in hypotonic fluids as its addition to normal saline results in a hypertonic solution; the latter increases the risk of overcorrection of sodium levels as well as of volume overload [].
Fluid restriction remains the first-line therapy in non-hypovolemic hyponatremia. ACE inhibitors or angiotensin receptor blockers and loop diuretics may raise serum sodium concentration. Discontinuation of suspected drugs and treatment of other superimposed factors are warranted [, ]. If this therapeutic approach fails, a vasopressin antagonist (vaptan) may be used to promote water diuresis; these drugs are second-line therapy for hyponatremia related to hyper- or euvolemic HF []. Importantly, vaptans should not be used in hypovolemic HF or in combination with hypertonic saline solution owing to case reports of associated ODS []. Of note, in Japan, tolvaptan is approved for HF patients with inadequate response to conventional diuretics but without coexistent hyponatremia [].
Conclusions
Hyponatremia in patients with HF may be the result of neurohormonal activation due to reduced cardiac output or is associated with several comorbidities or drugs. The rapidity of hyponatremia development, serum sodium concentration per se, the likelihood of pseudohyponatremia, the presence of symptoms, and the extracellular volume status of patients should be assessed carefully. These factors along with the treatment of coexisting conditions will guide therapeutic management accordingly.
Conflict of Interest Statement
This review was written independently; no company or institution supported it financially. George Liamis has given talks and attended conferences sponsored by various pharmaceutical companies, including Bayer, Sanofi, Amgen, Novartis, Vianex, Angelini, and MSD. Drs. Katerina K. Naka, Matilda Florentin, Eliza C. Christopoulou, Panagiotis Touloupis, and Ilias Gkartzonikas have no financial interest or financial ties to disclose.
Funding Sources
The authors received no financial support for the research, authorship, and/or publication of this article.
Author Contributions
The design and conception of the manuscript was made by Dr. George Liamis. The first draft of the manuscript was written by Dr. Eliza Christopoulou under the supervision of Dr. Matilda Florentin. Subsequently, Dr. Katerina Naka, Dr. Panagiotis Touloupis, and Dr. Ilias Gkartzonikas edited the manuscript and approved its final version.
References
- 1. Liamis G, Rodenburg EM, Hofman A, Zietse R, Stricker BH, Hoorn EJ. Electrolyte disorders in community subjects: prevalence and risk factors. Am J Med. 2013 Mar;126(3):256–63. https://doi.org/10.1016/j.amjmed.2012.06.037.
- 2. Liamis G, Mitrogianni Z, Liberopoulos EN, Tsimihodimos V, Elisaf M. Electrolyte disturbances in patients with hyponatremia. Intern Med. 2007;46(11):685–90. https://doi.org/10.2169/internalmedicine.46.6223.
- 3. Milionis HJ, Alexandrides GE, Liberopoulos EN, Bairaktari ET, Goudevenos J, Elisaf MS. Hypomagnesemia and concurrent acid-base and electrolyte abnormalities in patients with congestive heart failure. Eur J Heart Fail. 2002 Mar;4(2):167–73. https://doi.org/10.1016/s1388-9842(01)00234-3.
- 4. Klein L, O’Connor CM, Leimberger JD, Gattis-Stough W, Pina IL, Felker GM, et al. Lower serum sodium is associated with increased short-term mortality in hospitalized patients with worsening heart failure: results from the Outcomes of a Prospective Trial of Intravenous Milrinone for Exacerbations of Chronic Heart Failure (OPTIME-CHF) study. Circulation. 2005 May 17;111(19):2454–60. https://doi.org/10.1161/01.CIR.0000165065.82609.3D.
- 5. Ali K, Workicho A, Gudina EK. Hyponatremia in patients hospitalized with heart failure: a condition often overlooked in low-income settings. Int J Gen Med. 2016;9:267–73. https://doi.org/10.2147/IJGM.S110872.
- 6. McDonagh TA, Metra M, Adamo M, Gardner RS, Baumbach A, Bohm M, et al. 2021 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur Heart J. 2021 Sep 21;42(36):3599–726. https://doi.org/10.1093/eurheartj/ehab368.
- 7. Filippatos TD, Liamis G, Christopoulou F, Elisaf MS. Ten common pitfalls in the evaluation of patients with hyponatremia. Eur J Intern Med. 2016 Apr;29:22–5. https://doi.org/10.1016/j.ejim.2015.11.022.
- 8. Rodriguez M, Hernandez M, Cheungpasitporn W, Kashani KB, Riaz I, Rangaswami J, et al. Hyponatremia in heart failure: pathogenesis and management. Curr Cardiol Rev. 2019;15(4):252–61. https://doi.org/10.2174/1573403X15666190306111812.
- 9. Chatterjee K. Hyponatremia in heart failure. J Intensive Care Med. 2009 Nov–Dec;24(6):347–51. https://doi.org/10.1177/0885066609344941.
- 10. Urso C, Brucculeri S, Caimi G. Acid-base and electrolyte abnormalities in heart failure: pathophysiology and implications. Heart Fail Rev. 2015 Jul;20(4):493–503. https://doi.org/10.1007/s10741-015-9482-y.
- 11. Sica DA. Hyponatremia and heart failure: pathophysiology and implications. Congest Heart Fail. 2005 Sep–Oct;11(5):274–7. https://doi.org/10.1111/j.1527-5299.2005.04180.x.
- 12. Thornton SN. Thirst and hydration: physiology and consequences of dysfunction. Physiol Behav. 2010 Apr 26;100(1):15–21. https://doi.org/10.1016/j.physbeh.2010.02.026.
- 13. Salyer SA, Parks J, Barati MT, Lederer ED, Clark BJ, Klein JD, et al. Aldosterone regulates Na(+), K(+) ATPase activity in human renal proximal tubule cells through mineralocorticoid receptor. Biochim Biophys Acta. 2013 Oct;1833(10):2143–52. https://doi.org/10.1016/j.bbamcr.2013.05.009.
- 14. Filippatos TD, Elisaf MS. Hyponatremia in patients with heart failure. World J Cardiol. 2013 Sep 26;5(9):317–28. https://doi.org/10.4330/wjc.v5.i9.317.
- 15. Gardner JD, Mouton AJ. Alcohol effects on cardiac function. Compr Physiol. 2015 Apr;5(2):791–802.
- 16. Triposkiadis F, Karayannis G, Giamouzis G, Skoularigis J, Louridas G, Butler J. The sympathetic nervous system in heart failure. J Am Coll Cardiol. 2009 Nov 3;54(19):1747–62. https://doi.org/10.1016/j.jacc.2009.05.015.
- 17. Tan Y, Li X, Prabhu SD, Brittian KR, Chen Q, Yin X, et al. Angiotensin II plays a critical role in alcohol-induced cardiac nitrative damage, cell death, remodeling, and cardiomyopathy in a protein kinase C/nicotinamide adenine dinucleotide phosphate oxidase-dependent manner. J Am Coll Cardiol. 2012 Apr 17;59(16):1477–86. https://doi.org/10.1016/j.jacc.2011.12.034.
- 18. Lang CH, Frost RA, Summer AD, Vary TC. Molecular mechanisms responsible for alcohol-induced myopathy in skeletal muscle and heart. Int J Biochem Cell Biol. 2005 Oct;37(10):2180–95. https://doi.org/10.1016/j.biocel.2005.04.013.
- 19. Walker RK, Cousins VM, Umoh NA, Jeffress MA, Taghipour D, Al-Rubaiee M, et al. The good, the bad, and the ugly with alcohol use and abuse on the heart. Alcohol Clin Exp Res. 2013 Aug;37(8):1253–60. https://doi.org/10.1111/acer.12109.
- 20. Rodriguez A, Chawla K, Umoh NA, Cousins VM, Ketegou A, Reddy MG, et al. Alcohol and apoptosis: friends or foes?Biomolecules. 2015 Nov 19;5(4):3193–203. https://doi.org/10.3390/biom5043193.
- 21. Elisaf M, Kalaitzidis R. Metabolic abnormalities in alcoholic patients: focus on acid base and electrolyte disorders. J Alcohol Drug Depend. 2015;3(1). https://doi.org/10.4172/2329-6488.1000185.
- 22. Kujubu DA, Khosraviani A. Beer potomania: an unusual cause of hyponatremia. Perm J. 2015;19(3):74–6. https://doi.org/10.7812/TPP/14-181.
- 23. Kuthiah N, Er C. Reset osmostat: a challenging case of hyponatremia. Case Rep Med. 2018;2018:5670671. https://doi.org/10.1155/2018/5670671.
- 24. Vale BM, Morais S, Mesquita J, Mimoso G. Reset osmostat: a rare cause of hyponatraemia. BMJ Case Rep. 2015 Jun 29;2015:bcr2013009111. https://doi.org/10.1136/bcr-2013-009111.
- 25. Frustaci A, Russo MA, Morgante E, Scopelliti F, Aquilano K, Ciriolo MR, et al. Oxidative myocardial damage in human cocaine-related cardiomyopathy. Eur J Heart Fail. 2015 Mar;17(3):283–90. https://doi.org/10.1002/ejhf.231.
- 26. Hantson P. Mechanisms of toxic cardiomyopathy. Clin Toxicol. 2019 Jan;57(1):1–9. https://doi.org/10.1080/15563650.2018.1497172.
- 27. Arora S, Alfayoumi F, Srinivasan V. Transient left ventricular apical ballooning after cocaine use: is catecholamine cardiotoxicity the pathologic link?Mayo Clin Proc. 2006 Jun;81(6):829–32. https://doi.org/10.4065/81.6.829.
- 28. McDonald B, Walton M, Idowu O, Barratt J, Vakilgilani T. A case of cocaine-induced acute symptomatic hyponatraemia. Endocr Abstr. 2019;62. https://doi.org/10.1530/endoabs.62.wh7.
- 29. Neeki MM, Kulczycki M, Toy J, Dong F, Lee C, Borger R, et al. Frequency of methamphetamine use as a major contributor toward the severity of cardiomyopathy in adults ≤50 years. Am J Cardiol. 2016 Aug 15;118(4):585–9. https://doi.org/10.1016/j.amjcard.2016.05.057.
- 30. Schurer S, Klingel K, Sandri M, Majunke N, Besler C, Kandolf R, et al. Clinical characteristics, histopathological features, and clinical outcome of methamphetamine-associated cardiomyopathy. JACC Heart Fail. 2017 Jun;5(6):435–45. https://doi.org/10.1016/j.jchf.2017.02.017.
- 31. Richards JR, Harms BN, Kelly A, Turnipseed SD. Methamphetamine use and heart failure: prevalence, risk factors, and predictors. Am J Emerg Med. 2018 Aug;36(8):1423–8. https://doi.org/10.1016/j.ajem.2018.01.001.
- 32. Liamis G, Megapanou E, Elisaf M, Milionis H. Hyponatremia-inducing drugs. Front Horm Res. 2019;52:167–77. https://doi.org/10.1159/000493246.
- 33. Puttegowda B, Theodore J, Basappa R, Nanjappa MC. Olanzapine induced dilated cardiomyopathy. Malays J Med Sci. 2016 Mar;23(2):82–4.
- 34. Patuszynski D, Applegate PM. Suspected clozapine-induced cardiomyopathy and heart failure with reduced ejection fraction. Fed Pract. 2017 Apr;34(4):20–2.
- 35. Smolders DME, Smolders WAP. Case report and review of the literature: cardiomyopathy in a young woman on high-dose quetiapine. Cardiovasc Toxicol. 2017 Oct;17(4):478–81. https://doi.org/10.1007/s12012-016-9390-y.
- 36. Montastruc G, Favreliere S, Sommet A, Pathak A, Lapeyre-Mestre M, Perault-Pochat MC, et al. Drugs and dilated cardiomyopathies: a case/noncase study in the French PharmacoVigilance database. Br J Clin Pharmacol. 2010 Mar;69(3):287–94. https://doi.org/10.1111/j.1365-2125.2009.03596.x.
- 37. Vinetti M, Haufroid V, Capron A, Classen JF, Marchandise S, Hantson P. Severe acute cardiomyopathy associated with venlafaxine overdose and possible role of CYP2D6 and CYP2C19 polymorphisms. Clin Toxicol. 2011 Nov;49(9):865–9. https://doi.org/10.3109/15563650.2011.626421.
- 38. Shepshelovich D, Schechter A, Calvarysky B, Diker-Cohen T, Rozen-Zvi B, Gafter-Gvili A. Medication-induced SIADH: distribution and characterization according to medication class. Br J Clin Pharmacol. 2017 Aug;83(8):1801–7. https://doi.org/10.1111/bcp.13256.
- 39. Liamis G, Milionis H, Elisaf M. A review of drug-induced hyponatremia. Am J Kidney Dis. 2008 Jul;52(1):144–53. https://doi.org/10.1053/j.ajkd.2008.03.004.
- 40. Viramontes TS, Truong H, Linnebur SA. Antidepressant-induced hyponatremia in older adults. Consult Pharm. 2016 Mar;31(3):139–50. https://doi.org/10.4140/TCP.n.2016.139.
- 41. Jacob S, Spinler SA. Hyponatremia associated with selective serotonin-reuptake inhibitors in older adults. Ann Pharmacother. 2006 Sep;40(9):1618–22. https://doi.org/10.1345/aph.1G293.
- 42. Kuwata A, Ohashi M, Sugiyama M, Ueda R, Dohi Y. A case of reversible dilated cardiomyopathy after alpha-interferon therapy in a patient with renal cell carcinoma. Am J Med Sci. 2002 Dec;324(6):331–4. https://doi.org/10.1097/00000441-200212000-00008.
- 43. Khakoo AY, Halushka MK, Rame JE, Rodriguez ER, Kasper EK, Judge DP. Reversible cardiomyopathy caused by administration of interferon alpha. Nat Clin Pract Cardiovasc Med. 2005 Jan;2(1):53–7. https://doi.org/10.1038/ncpcardio0069.
- 44. Feenstra J, Grobbee DE, Remme WJ, Stricker BH. Drug-induced heart failure. J Am Coll Cardiol. 1999 Apr;33(5):1152–62. https://doi.org/10.1016/s0735-1097(99)00006-6.
- 45. Varga ZV, Ferdinandy P, Liaudet L, Pacher P. Drug-induced mitochondrial dysfunction and cardiotoxicity. Am J Physiol Heart Circ Physiol. 2015 Nov;309(9):H1453–67. https://doi.org/10.1152/ajpheart.00554.2015.
- 46. Liamis G, Filippatos TD, Elisaf MS. Electrolyte disorders associated with the use of anticancer drugs. Eur J Pharmacol. 2016 Apr 15;777:78–87. https://doi.org/10.1016/j.ejphar.2016.02.064.
- 47. Yeh ET, Tong AT, Lenihan DJ, Yusuf SW, Swafford J, Champion C, et al. Cardiovascular complications of cancer therapy: diagnosis, pathogenesis, and management. Circulation. 2004 Jun 29;109(25):3122–31. https://doi.org/10.1161/01.cir.0000133187.74800.b9.
- 48. Quezado ZM, Wilson WH, Cunnion RE, Parker MM, Reda D, Bryant G, et al. High-dose ifosfamide is associated with severe, reversible cardiac dysfunction. Ann Intern Med. 1993 Jan 1;118(1):31–6. https://doi.org/10.7326/0003-4819-118-1-199301010-00006.
- 49. Huerta C, Varas-Lorenzo C, Castellsague J, Garcia Rodriguez LA. Non-steroidal anti-inflammatory drugs and risk of first hospital admission for heart failure in the general population. Heart. 2006 Nov;92(11):1610–5. https://doi.org/10.1136/hrt.2005.082388.
- 50. Page RL 2nd, O’Bryant CL, Cheng D, Dow TJ, Ky B, Stein CM, et al. Drugs that may cause or exacerbate heart failure: a scientific statement from the American heart association. Circulation. 2016 Aug 9;134(6):e32–69. https://doi.org/10.1161/cir.0000000000000426.
- 51. Ponikowski P, Voors AA, Anker SD, Bueno H, Cleland JGF, Coats AJS, et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: the task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Europ Heart J. 2016;37(27):2129–200. https://doi.org/10.1093/eurheartj/ehw128.
- 52. Koniari K, Parissis J, Paraskevaidis I, Anastasiou-Nana M. Treating volume overload in acutely decompensated heart failure: established and novel therapeutic approaches. Eur Heart J Acute Cardiovasc Care. 2012 Sep;1(3):256–68. https://doi.org/10.1177/2048872612457044.
- 53. Liamis G, Milionis HJ, Elisaf M. Hyponatremia in patients with infectious diseases. J Infect. 2011 Nov;63(5):327–35. https://doi.org/10.1016/j.jinf.2011.07.013.
- 54. Massilamany C, Gangaplara A, Reddy J. Intricacies of cardiac damage in coxsackievirus B3 infection: implications for therapy. Int J Cardiol. 2014 Dec 15;177(2):330–9. https://doi.org/10.1016/j.ijcard.2014.09.136.
- 55. Kodner C. Diagnosis and management of nephrotic syndrome in adults. Am Fam Physician. 2016 Mar 15;93(6):479–85.
- 56. Zhang L, Chen Y, Zhang LJ, Wang M, Chang DL, Wan WW, et al. HBV induces different responses of the hepatocytes and oval cells during HBV-related hepatic cirrhosis. Cancer Lett. 2019 Feb 28;443:47–55. https://doi.org/10.1016/j.canlet.2018.11.020.
- 57. Inamdar P, Masavkar S, Shanbag P. Hyponatremia in children with tuberculous meningitis: a hospital-based cohort study. J Pediatr Neurosci. 2016 Jul–Sep;11(3):182–7. https://doi.org/10.4103/1817-1745.193376.
- 58. More A, Verma R, Garg RK, Malhotra HS, Sharma PK, Uniyal R, et al. A study of neuroendocrine dysfunction in patients of tuberculous meningitis. J Neurol Sci. 2017 Aug 15;379:198–206. https://doi.org/10.1016/j.jns.2017.06.015.
- 59. Cui H, He G, Yang S, Lv Y, Jiang Z, Gang X, et al. Inappropriate antidiuretic hormone secretion and cerebral salt-wasting syndromes in neurological patients. Front Neurosci. 2019;13:1170. https://doi.org/10.3389/fnins.2019.01170.
- 60. Swart RM, Hoorn EJ, Betjes MG, Zietse R. Hyponatremia and inflammation: the emerging role of interleukin-6 in osmoregulation. Nephron Physiol. 2011;118(2):45–51. https://doi.org/10.1159/000322238.
- 61. Berni A, Malandrino D, Parenti G, Maggi M, Poggesi L, Peri A. Hyponatremia, IL-6, and SARS-CoV-2 (COVID-19) infection: may all fit together?J Endocrinol Invest. 2020 May 25;43(8):1137–9. https://doi.org/10.1007/s40618-020-01301-w.
- 62. Gubernatorova EO, Gorshkova EA, Polinova AI, Drutskaya MS. IL-6: relevance for immunopathology of SARS-CoV-2. Cytokine Growth Factor Rev. 2020 Jun;53:13–24. https://doi.org/10.1016/j.cytogfr.2020.05.009.
- 63. Quinkler M, Beuschlein F, Hahner S, Meyer G, Schofl C, Stalla GK. Adrenal cortical insufficiency: a life threatening illness with multiple etiologies. Dtsch Arztebl Int. 2013 Dec 23;110(51–52):882–8. https://doi.org/10.3238/arztebl.2013.0882.
- 64. Rushworth RL, Torpy DJ. A descriptive study of adrenal crises in adults with adrenal insufficiency: increased risk with age and in those with bacterial infections. BMC Endocr Disord. 2014 Oct 1;14:79. https://doi.org/10.1186/1472-6823-14-79.
- 65. Afreen B, Khan KA, Riaz A. Adrenal insufficiency in Pakistani Hiv infected patients. J Ayub Med Coll Abbottabad. 2017 Jul–Sep;29(3):428–31.
- 66. Babayev R, Terner S, Chandra S, Radhakrishnan J, Mohan S. Trimethoprim-associated hyponatremia. Am J Kidney Dis. 2013 Dec;62(6):1188–92. https://doi.org/10.1053/j.ajkd.2013.06.007.
- 67. Vasilios GL. Hyponatremia in diabetes mellitus: clues to diagnosis and treatment. J Diabetes Metab. 2015;6(6). https://doi.org/10.4172/2155-6156.1000560.
- 68. Brandao Neto RA, de Carvalho JF. Diagnosis and classification of Addison’s disease (autoimmune adrenalitis). Autoimmun Rev. 2014 Apr–May;13(4–5):408–11. https://doi.org/10.1016/j.autrev.2014.01.025.
- 69. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Spurious electrolyte disorders: a diagnostic challenge for clinicians. Am J Nephrol. 2013;38(1):50–7. https://doi.org/10.1159/000351804.
- 70. Pecoraro A, Crescenzi L, Carucci L, Genovese A, Spadaro G. Heart failure not responsive to standard immunosuppressive therapy is successfully treated with high dose intravenous immunoglobulin therapy in a patient with Eosinophilic Granulomatosis with Polyangiitis (EGPA). Int Immunopharmacol. 2017 Apr;45:13–5. https://doi.org/10.1016/j.intimp.2017.01.025.
- 71. Dagli A, Sentner CP, Weinstein DA. Glycogen storage disease type III. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Stephens K, editors. GeneReviews((R)). Seattle, WA; 1993.
- 72. Wang S, Hou X, Liu Y, Lu H, Wei L, Bao Y, et al. Serum electrolyte levels in relation to macrovascular complications in Chinese patients with diabetes mellitus. Cardiovasc Diabetol. 2013 Oct 10;12:146. https://doi.org/10.1186/1475-2840-12-146.
- 73. Thomas MC, Cooper ME, Zimmet P. Changing epidemiology of type 2 diabetes mellitus and associated chronic kidney disease. Nat Rev Nephrol. 2016 Feb;12(2):73–81. https://doi.org/10.1038/nrneph.2015.173.
- 74. Sousa AG, Cabral JV, El-Feghaly WB, de Sousa LS, Nunes AB. Hyporeninemic hypoaldosteronism and diabetes mellitus: pathophysiology assumptions, clinical aspects and implications for management. World J Diabetes. 2016 Mar 10;7(5):101–11. https://doi.org/10.4239/wjd.v7.i5.101.
- 75. Snyder MJ, Gibbs LM, Lindsay TJ. Treating painful diabetic peripheral neuropathy: an update. Am Fam Physician. 2016 Aug 1;94(3):227–34.
- 76. Bustamante M, Hasler U, Kotova O, Chibalin AV, Mordasini D, Rousselot M, et al. Insulin potentiates AVP-induced AQP2 expression in cultured renal collecting duct principal cells. Am J Physiol Renal Physiol. 2005 Feb;288(2):F334–44. https://doi.org/10.1152/ajprenal.00180.2004.
- 77. Liamis G, Liberopoulos E, Barkas F, Elisaf M. Diabetes mellitus and electrolyte disorders. World J Clin Cases. 2014 Oct 16;2(10):488–96. https://doi.org/10.12998/wjcc.v2.i10.488.
- 78. Pang J, Chan DC, Watts GF. Origin and therapy for hypertriglyceridaemia in type 2 diabetes. World J Diabetes. 2014 Apr 15;5(2):165–75. https://doi.org/10.4239/wjd.v5.i2.165.
- 79. Hernando VU. Role of thyroid hormones in different aspects of cardiovascular system. Endocrinol Metab Syndr. 2015;04(02). https://doi.org/10.4172/2161-1017.1000166.
- 80. Liamis G, Filippatos TD, Liontos A, Elisaf MS. Management of endrocine disease: hypothyroidism-associated hyponatremia: mechanisms, implications and treatment. Eur J Endocrinol. 2017 Jan;176(1):R15–R20. https://doi.org/10.1530/EJE-16-0493.
- 81. Mozolevska V, Schwartz A, Cheung D, Shaikh B, Bhagirath KM, Jassal DS. Addison’s disease and dilated cardiomyopathy: a case report and review of the literature. Case Rep Cardiol. 2016;2016:4362514. https://doi.org/10.1155/2016/4362514.
- 82. Liamis G, Milionis HJ, Elisaf M. Endocrine disorders: causes of hyponatremia not to neglect. Ann Med. 2011 May;43(3):179–87. https://doi.org/10.3109/07853890.2010.530680.
- 83. Limongelli G, Rubino M, Esposito A, Russo M, Pacileo G. The challenge of cardiomyopathies and heart failure in pregnancy. Curr Opin Obstet Gynecol. 2018 Dec;30(6):378–84. https://doi.org/10.1097/GCO.0000000000000496.
- 84. Hibbard JU, Lindheimer M, Lang RM. A modified definition for peripartum cardiomyopathy and prognosis based on echocardiography. Obstet Gynecol. 1999 Aug;94(2):311–6. https://doi.org/10.1016/s0029-7844(99)00293-8.
- 85. Orabona R, Sciatti E, Prefumo F, Vizzardi E, Bonadei I, Valcamonico A, et al. Pre-eclampsia and heart failure: a close relationship. Ultrasound Obstet Gynecol. 2018 Sep;52(3):297–301. https://doi.org/10.1002/uog.18987.
- 86. Belzile M, Pouliot A, Cumyn A, Cote AM. Renal physiology and fluid and electrolyte disorders in pregnancy. Best Pract Res Clin Obstet Gynaecol. 2019 May;57:1–14. https://doi.org/10.1016/j.bpobgyn.2018.11.008.
- 87. Pazhayattil GS, Rastegar A, Brewster UC. Approach to the diagnosis and treatment of hyponatremia in pregnancy. Am J Kidney Dis. 2015 Apr;65(4):623–7. https://doi.org/10.1053/j.ajkd.2014.09.027.
- 88. Harris K, Shankar R, Black K, Rochelson B. Reset osmostat in pregnancy: a case report. J Matern Fetal Neonatal Med. 2014 Mar;27(5):530–3. https://doi.org/10.3109/14767058.2013.819333.
- 89. Christensen HM, Kistorp C, Schou M, Keller N, Zerahn B, Frystyk J, et al. Prevalence of cachexia in chronic heart failure and characteristics of body composition and metabolic status. Endocrine. 2013 Jun;43(3):626–34. https://doi.org/10.1007/s12020-012-9836-3.
- 90. Sandek A, Bauditz J, Swidsinski A, Buhner S, Weber-Eibel J, von Haehling S, et al. Altered intestinal function in patients with chronic heart failure. J Am Coll Cardiol. 2007 Oct 16;50(16):1561–9. https://doi.org/10.1016/j.jacc.2007.07.016.
- 91. Rahman A, Jafry S, Jeejeebhoy K, Nagpal AD, Pisani B, Agarwala R. Malnutrition and cachexia in heart failure. JPEN J Parenter Enteral Nutr. 2016 May;40(4):475–86. https://doi.org/10.1177/0148607114566854.
- 92. Okoshi MP, Capalbo RV, Romeiro FG, Okoshi K. Cardiac cachexia: perspectives for prevention and treatment. Arq Bras Cardiol. 2017 Jan;108(1):74–80. https://doi.org/10.5935/abc.20160142.
- 93. Levine B, Kalman J, Mayer L, Fillit HM, Packer M. Elevated circulating levels of tumor necrosis factor in severe chronic heart failure. N Engl J Med. 1990 Jul 26;323(4):236–41. https://doi.org/10.1056/NEJM199007263230405.
- 94. Thaler SM, Teitelbaum I, Berl T. “Beer potomania” in non-beer drinkers: effect of low dietary solute intake. Am J Kidney Dis. 1998 Jun;31(6):1028–31. https://doi.org/10.1053/ajkd.1998.v31.pm9631849.
- 95. Ezekowitz JA, Colin-Ramirez E, Ross H, Escobedo J, Macdonald P, Troughton R, et al. Reduction of dietary sodium to less than 100 mmol in heart failure (SODIUM-HF): an international, open-label, randomised, controlled trial. Lancet. 2022 Apr 9;399(10333):1391–400. https://doi.org/10.1016/S0140-6736(22)00369-5.
- 96. Koene RJ, Prizment AE, Blaes A, Konety SH. Shared risk factors in cardiovascular disease and cancer. Circulation. 2016 Mar 15;133(11):1104–14. https://doi.org/10.1161/circulationaha.115.020406.
- 97. Siegel RL, Miller KD, Jemal A. Cancer statistics, 2018. CA Cancer J Clin. 2018 Jan;68(1):7–30. https://doi.org/10.3322/caac.21442.
- 98. Hasin T, Gerber Y, McNallan SM, Weston SA, Kushwaha SS, Nelson TJ, et al. Patients with heart failure have an increased risk of incident cancer. J Am Coll Cardiol. 2013 Sep 3;62(10):881–6. https://doi.org/10.1016/j.jacc.2013.04.088.
- 99. Tashakkor AY, Moghaddamjou A, Chen L, Cheung WY. Predicting the risk of cardiovascular comorbidities in adult cancer survivors. Curr Oncol. 2013 Oct;20(5):e360–70. https://doi.org/10.3747/co.20.1470.
- 100. Bloom MW, Hamo CE, Cardinale D, Ky B, Nohria A, Baer L, et al. Cancer therapy-related cardiac dysfunction and heart failure: Part 1: definitions, pathophysiology, risk factors, and imaging. Circ Heart Fail. 2016 Jan;9(1):e002661. https://doi.org/10.1161/CIRCHEARTFAILURE.115.002661.
- 101. Finet JE. Management of heart failure in cancer patients and cancer survivors. Heart Fail Clin. 2017 Apr;13(2):253–88. https://doi.org/10.1016/j.hfc.2016.12.004.
- 102. Berghmans T, Paesmans M, Body JJ. A prospective study on hyponatraemia in medical cancer patients: epidemiology, aetiology and differential diagnosis. Support Care Cancer. 2000 May;8(3):192–7. https://doi.org/10.1007/s005200050284.
- 103. Castillo JJ, Vincent M, Justice E. Diagnosis and management of hyponatremia in cancer patients. Oncologist. 2012;17(6):756–65. https://doi.org/10.1634/theoncologist.2011-0400.
- 104. Doshi SM, Shah P, Lei X, Lahoti A, Salahudeen AK. Hyponatremia in hospitalized cancer patients and its impact on clinical outcomes. Am J Kidney Dis. 2012 Feb;59(2):222–8. https://doi.org/10.1053/j.ajkd.2011.08.029.
- 105. Castillo JJ, Glezerman IG, Boklage SH, Chiodo J 3rd, Tidwell BA, Lamerato LE, et al. The occurrence of hyponatremia and its importance as a prognostic factor in a cross-section of cancer patients. BMC Cancer. 2016 Jul 29;16(1):564. https://doi.org/10.1186/s12885-016-2610-9.
- 106. Onitilo AA, Kio E, Doi SA. Tumor-related hyponatremia. Clin Med Res. 2007 Dec;5(4):228–37. https://doi.org/10.3121/cmr.2007.762.
- 107. Chute JP, Taylor E, Williams J, Kaye F, Venzon D, Johnson BE. A metabolic study of patients with lung cancer and hyponatremia of malignancy. Clin Cancer Res. 2006 Feb 1;12(3):888–96. https://doi.org/10.1158/1078-0432.ccr-05-1536.
- 108. Ezoe Y, Mizusawa J, Katayama H, Kataoka K, Muto M. An integrated analysis of hyponatremia in cancer patients receiving platinum-based or nonplatinum-based chemotherapy in clinical trials (JCOG1405-A). Oncotarget. 2018 Jan 19;9(5):6595–606. https://doi.org/10.18632/oncotarget.23536.
- 109. Berardi R, Santoni M, Rinaldi S, Nunzi E, Smerilli A, Caramanti M, et al. Risk of hyponatraemia in cancer patients treated with targeted therapies: a systematic review and meta-analysis of clinical trials. PLoS One. 2016;11(5):e0152079. https://doi.org/10.1371/journal.pone.0152079.
- 110. Berardi R, Santoni M, Newsom-Davis T, Caramanti M, Rinaldi S, Tiberi M, et al. Hyponatremia normalization as an independent prognostic factor in patients with advanced non-small cell lung cancer treated with first-line therapy. Oncotarget. 2017 Apr 4;8(14):23871–9. https://doi.org/10.18632/oncotarget.13372.
- 111. Raftopoulos H. Diagnosis and management of hyponatremia in cancer patients. Support Care Cancer. 2007 Dec;15(12):1341–7. https://doi.org/10.1007/s00520-007-0309-9.
- 112. Ekhzaimy A, Mujamammi A. Bilateral primary adrenal lymphoma with adrenal insufficiency. BMJ Case Rep. 2016 Oct 26;2016.
- 113. Doleschal B, Petzer A, Aichberger KJ. Adrenal crisis in metastatic breast cancer. BMJ Case Rep. 2017 Jul 6;2017:bcr2017220284. https://doi.org/10.1136/bcr-2017-220284.
- 114. Hoorn EJ, Lindemans J, Zietse R. Development of severe hyponatraemia in hospitalized patients: treatment-related risk factors and inadequate management. Nephrol Dial Transplant. 2006 Jan;21(1):70–6. https://doi.org/10.1093/ndt/gfi082.
- 115. Cuadrado-Godia E, Ois A, Roquer J. Heart failure in acute ischemic stroke. Curr Cardiol Rev. 2010 Aug;6(3):202–13. https://doi.org/10.2174/157340310791658776.
- 116. Katsanos AH, Parissis J, Frogoudaki A, Vrettou AR, Ikonomidis I, Paraskevaidis I, et al. Heart failure and the risk of ischemic stroke recurrence: a systematic review and meta-analysis. J Neurol Sci. 2016 Mar 15;362:182–7. https://doi.org/10.1016/j.jns.2016.01.053.
- 117. Liamis G, Barkas F, Megapanou E, Christopoulou E, Makri A, Makaritsis K, et al. Hyponatremia in acute stroke patients: pathophysiology, clinical significance, and management options. Eur Neurol. 2019;82(1–3):32–40. https://doi.org/10.1159/000504475.
- 118. Kalita J, Singh RK, Misra UK. Cerebral salt wasting is the most common cause of hyponatremia in stroke. J Stroke Cerebrovasc Dis. 2017 May;26(5):1026–32. https://doi.org/10.1016/j.jstrokecerebrovasdis.2016.12.011.
- 119. Parissis JT, Fountoulaki K, Paraskevaidis I, Kremastinos D. Depression in chronic heart failure: novel pathophysiological mechanisms and therapeutic approaches. Expert Opin Investig Drugs. 2005 May;14(5):567–77. https://doi.org/10.1517/13543784.14.5.567.
- 120. Moraska AR, Chamberlain AM, Shah ND, Vickers KS, Rummans TA, Dunlay SM, et al. Depression, healthcare utilization, and death in heart failure: a community study. Circ Heart Fail. 2013 May;6(3):387–94. https://doi.org/10.1161/CIRCHEARTFAILURE.112.000118.
- 121. Doehner W, Rauchhaus M, Florea VG, Sharma R, Bolger AP, Davos CH, et al. Uric acid in cachectic and noncachectic patients with chronic heart failure: relationship to leg vascular resistance. Am Heart J. 2001 May;141(5):792–9. https://doi.org/10.1067/mhj.2001.114367.
- 122. Khalid U, Egeberg A, Ahlehoff O, Lane D, Gislason GH, Lip GYH, et al. Incident heart failure in patients with rheumatoid arthritis: a nationwide cohort study. J Am Heart Assoc. 2018 Jan 19;7(2):e007227. https://doi.org/10.1161/JAHA.117.007227.
- 123. Mantel A, Holmqvist M, Andersson DC, Lund LH, Askling J. Association between rheumatoid arthritis and risk of ischemic and nonischemic heart failure. J Am Coll Cardiol. 2017 Mar 14;69(10):1275–85. https://doi.org/10.1016/j.jacc.2016.12.033.
- 124. Bondugulapati LNR, Shandilya S. Chronic kidney disease and cardiovascular disease. Curr Opin Lipidol. 2015 Aug;26(4):353–4. https://doi.org/10.1097/mol.0000000000000202.
- 125. Combs S, Berl T. Dysnatremias in patients with kidney disease. Am J Kidney Dis. 2014 Feb;63(2):294–303. https://doi.org/10.1053/j.ajkd.2013.09.017.
- 126. van der Wal HH, van Deursen VM, van der Meer P, Voors AA. Comorbidities in heart failure. Handb Exp Pharmacol. 2017;243:35–66. https://doi.org/10.1007/164_2017_27.
- 127. Cardoso JN, Del Carlo CH, Oliveira Junior MT, Ochiai ME, Kalil Filho R, Barretto ACP. Infection in patients with decompensated heart failure: in-hospital mortality and outcome. Arq Bras Cardiol. 2018 Apr;110(4):364–70. https://doi.org/10.5935/abc.20180037.
- 128. Martín Guerra JM, Martín Asenjo M, Sánchez Muñoz LÁ, Dueñas Gutiérrez CJ. Hyponatremia in COPD: a little known complication. Arch Bronconeumol. 2018;54(7):391–3. https://doi.org/10.1016/j.arbres.2017.11.006.
- 129. Valli G, Fedeli A, Antonucci R, Paoletti P, Palange P. Water and sodium imbalance in COPD patients. Monaldi Arch Chest Dis. 2004 Apr–Jun;61(2):112–6. https://doi.org/10.4081/monaldi.2004.708.
- 130. Smith HD, Kelly CW, DiCocco M. Syndrome of inappropriate antidiuretic hormone production caused by multiple factors. J Am Acad Phys Assist. 2015;28(12):1. https://doi.org/10.1097/01.jaa.0000473194.49650.1d.
- 131. Bauer FK, Telfer N, Herbst HH, Austin RC, Hetter B. Hyponatremia and increased exchangeable sodium in chronic obstructive lung disease. Am J Med Sci. 1965 Sep;250(3):245–53. https://doi.org/10.1097/00000441-196509000-00001.
- 132. Liamis G, Filippatos TD, Liontos A, Elisaf MS. Hyponatremia in patients with liver diseases: not just a cirrhosis-induced hemodynamic compromise. Hepatol Int. 2016 Sep;10(5):762–72. https://doi.org/10.1007/s12072-016-9746-1.
- 133. Levy D, Larson MG, Vasan RS, Kannel WB, Ho KK. The progression from hypertension to congestive heart failure. JAMA. 1996 May 22–29;275(20):1557–62. https://doi.org/10.1001/jama.1996.03530440037034.
- 134. Messerli FH, Rimoldi SF, Bangalore S. The transition from hypertension to heart failure: contemporary update. JACC Heart Fail. 2017 Aug;5(8):543–51. https://doi.org/10.1016/j.jchf.2017.04.012.
- 135. Pfeffer MA. Heart failure and hypertension: importance of prevention. Med Clin North Am. 2017 Jan;101(1):19–28. https://doi.org/10.1016/j.mcna.2016.08.012.
- 136. Tsioufis C, Georgiopoulos G, Oikonomou D, Thomopoulos C, Katsiki N, Kasiakogias A, et al. Hypertension and heart failure with preserved ejection fraction: connecting the dots. Curr Vasc Pharmacol. 2017;16(1):15–22. https://doi.org/10.2174/1570161115666170414120532.
- 137. Liamis G, Milionis H, Elisaf M. Blood pressure drug therapy and electrolyte disturbances. Int J Clin Pract. 2008 Oct;62(10):1572–80. https://doi.org/10.1111/j.1742-1241.2008.01860.x.
- 138. Liamis G, Filippatos TD, Elisaf MS. Thiazide-associated hyponatremia in the elderly: what the clinician needs to know. J Geriatr Cardiol. 2016 Feb;13(2):175–82.
- 139. Tamargo J, Segura J, Ruilope LM. Diuretics in the treatment of hypertension. Part 1: thiazide and thiazide-like diuretics. Expert Opin Pharmacother. 2014 Mar;15(4):527–47. https://doi.org/10.1517/14656566.2014.879118.
- 140. Frenkel NJ, Vogt L, De Rooij SE, Trimpert C, Levi MM, Deen PM, et al. Thiazide-induced hyponatraemia is associated with increased water intake and impaired urea-mediated water excretion at low plasma antidiuretic hormone and urine aquaporin-2. J Hypertens. 2015 Mar;33(3):627–33. https://doi.org/10.1097/HJH.0000000000000423.
- 141. Sica DA. Diuretic-related side effects: development and treatment. J Clin Hypertens. 2004 Sep;6(9):532–40. https://doi.org/10.1111/j.1524-6175.2004.03789.x.
- 142. Sligl W, McAlister FA, Ezekowitz J, Armstrong PW. Usefulness of spironolactone in a specialized heart failure clinic. Am J Cardiol. 2004 Aug 15;94(4):443–7. https://doi.org/10.1016/j.amjcard.2004.04.059.
- 143. Filippatos T, Elisaf M, Liamis G. Pharmacological management of hyponatremia. Expert Opin Pharmacother. 2018 Aug;19(12):1337–44. https://doi.org/10.1080/14656566.2018.1504920.
- 144. Murakami T, Horibata Y, Morimoto Y, Tateno S, Kawasoe Y, Niwa K. Syndrome of inappropriate secretion of antidiuretic hormone associated with angiotensin-converting enzyme inhibitor administration. Pediatr Cardiol. 2013 Jun;34(5):1261–3. https://doi.org/10.1007/s00246-012-0373-x.
- 145. Fuzaylova I, Lam C, Talreja O, Makaryus AN, Ahern D, Cassagnol M. Sacubitril/valsartan (entresto(R))-Induced hyponatremia. J Pharm Pract. 2020 Oct;33(5):696–9. https://doi.org/10.1177/089719001982891.
- 146. Myhre PL, Vaduganathan M, Claggett B, Packer M, Desai AS, Rouleau JL, et al. B-type natriuretic peptide during treatment with sacubitril/valsartan: the PARADIGM-HF trial. J Am Coll Cardiol. 2019 Mar 26;73(11):1264–72. https://doi.org/10.1016/j.jacc.2019.01.018.
- 147. Saepudin S, Ball PA, Morrissey H. Hyponatremia during hospitalization and in-hospital mortality in patients hospitalized from heart failure. BMC Cardiovasc Disord. 2015 Aug 14;15(1):88. https://doi.org/10.1186/s12872-015-0082-5.
- 148. Norman NE, Sneed AM, Brown C, Ellis CA, Minard G, Brown RO. Heparin-induced hyponatremia. Ann Pharmacother. 2004 Mar;38(3):404–7. https://doi.org/10.1345/aph.1C442.
- 149. Zampetti B, Attanasio R, Cozzi R. Hyponatremia after anticoagulant treatment: a rare cause of adrenal failure. Endo Diab Metab Case Rep. 2018 Sep 25;2018:18–0101. https://doi.org/10.1530/EDM-18-0101.
- 150. Bui AL, Horwich TB, Fonarow GC. Epidemiology and risk profile of heart failure. Nat Rev Cardiol. 2011 Jan;8(1):30–41. https://doi.org/10.1038/nrcardio.2010.165.
- 151. Balling L, Schou M, Videbaek L, Hildebrandt P, Wiggers H, Gustafsson F, et al. Prevalence and prognostic significance of hyponatraemia in outpatients with chronic heart failure. Eur J Heart Fail. 2011 Sep;13(9):968–73. https://doi.org/10.1093/eurjhf/hfr086.
- 152. Vicent L, Alvarez-Garcia J, Gonzalez-Juanatey JR, Rivera M, Segovia J, Worner F, et al. Prognostic impact of hyponatremia and hypernatremia at admission and discharge in heart failure patients with preserved, mid-range, and reduced ejection fraction. Intern Med J. 2021 Jun;51(6):930–8. https://doi.org/10.1111/imj.14836.
- 153. Wang J, Zhou W, Yin X. Improvement of hyponatremia is associated with lower mortality risk in patients with acute decompensated heart failure: a meta-analysis of cohort studies. Heart Fail Rev. 2019 Mar;24(2):209–17. https://doi.org/10.1007/s10741-018-9753-5.
- 154. Moritz ML, Ayus JC. Maintenance intravenous fluids in acutely ill patients. N Engl J Med. 2015 Oct;373(14):1350–60. https://doi.org/10.1056/nejmra1412877.
- 155. Grant P, Ayuk J, Bouloux PM, Cohen M, Cranston I, Murray RD, et al. The diagnosis and management of inpatient hyponatraemia and SIADH. Eur J Clin Invest. 2015 Aug;45(8):888–94. https://doi.org/10.1111/eci.12465.
- 156. Filippatos TD, Makri A, Elisaf MS, Liamis G. Hyponatremia in the elderly: challenges and solutions. Clin Interv Aging. 2017;12:1957–65. https://doi.org/10.2147/CIA.S138535.
- 157. Seay NW, Lehrich RW, Greenberg A. Diagnosis and management of disorders of body tonicity-hyponatremia and hypernatremia: core curriculum 2020. Am J Kidney Dis. 2020;75(2):272–86. https://doi.org/10.1053/j.ajkd.2019.07.014.
- 158. Liamis G, Filippatos TD, Elisaf MS. Correction of hypovolemia with crystalloid fluids: Individualizing infusion therapy. Postgrad Med. 2015 May;127(4):405–12. https://doi.org/10.1080/00325481.2015.1029421.
- 159. Kinugawa K, Sato N, Inomata T, Yasuda M, Shimakawa T, Fukuta Y. Real-world effectiveness and tolerability of tolvaptan in patients with heart failure-final results of the samsca post-marketing surveillance in heart failure (SMILE) study. Circ J. 2019 Jun 25;83(7):1520–7. https://doi.org/10.1253/circj.CJ-19-0158.
The authors take responsibility for all aspects of the reliability and freedom from bias of the data presented and their discussed interpretation.

