What Is Already Known about This Subject?
Genetic polymorphism impacts response after clopidogrel, potentially impacting clinical outcomes.
The associated risks after prasugrel are unclear, especially in the maintenance phase.
What Does This Study Add?
Prasugrel both 5 and 10 mg/day maintenance yielded different antiplatelet potencies depending on the CYP2C19 genetic profile in Korean patients.
This effect is mild, but consistent for both of the approved prasugrel doses potentially affecting long-term outcomes.
How Might This Impact on Clinical Practice?
The clinical utility of these findings is still uncertain, and requires more evidence from larger randomized trials beyond East Asians.
Introduction
Prasugrel is an oral antiplatelet agent which belongs to the class of thienopyridines, and has a mechanism of action similar to clopidogrel. Similarly, the drug is an irreversible antagonist of the CYP2Y12 receptor, leading to inhibition of adenosine diphosphate (ADP)-mediated platelet activation and aggregation []. Just like clopidogrel, prasugrel is a prodrug, which produces the active metabolite. However, the drug is rapidly absorbed in humans and metabolized by hydrolysis followed by methylation and conjugation [, ]. Several mechanisms have been implicated to be responsible for interindividual differences such as drug absorption and variability in drug metabolism, and genetic polymorphisms (GP) of the P2Y12 receptor have been reported to increase ADP-induced platelet reactivity [-]. Since thienopyridines are metabolized by liver enzymes, there is a potential link between CYP2C19 GP and variability of antiplatelet potency in certain carriers [-]. However, while conventional clopidogrel is always a “prime suspect” of such shortcoming [-], prasugrel is mostly overprotected, claiming superiority or at least lack of impact due to fewer metabolization steps required than older thienopyridines. In fact, these issues are tricky and are driven mostly by marketing than scientifically validated evidence. Among the challenges attributed to prasugrel are overdosing, documented response variability, change of myocardial infarction definition in the pivotal TRITON trial, and claiming stent thrombosis benefit despite post hoc event adjudication. In addition, most studies utilized an early assessment of platelet reactivity evaluated before or just after drug loading [-], while the maintenance phase data are scarce. Therefore, whether prasugrel response is consistent or variable in outpatients, and whether the link of GP with platelet reactivity differs over time needs to be explored further. The aim of this prospec tive observational study was to evaluate the impact of CYP2C19 GP on platelet reactivity to determine the link between maintenance responses after two approved doses of prasugrel in Korean outpatients with CYP2C19 genetic profile.
Methods
Study Population
We prospectively included 206 consecutive patients who underwent percutaneous coronary intervention (PCI) with drug-eluting stent implantation in a single center between August 2012 and August 2017. Written informed consent for study participation was obtained by each patient before enrollment. All patients were South Korean passport holders, and were of Korean origin. All patients received a loading dose of prasugrel 60 mg at least 3 h before PCI. Dual antiplatelet therapy was later maintained with 100 mg/day of aspirin, and 10 mg/day (n = 76) or 5 mg/day (n = 130) of prasugrel. Patients weighing less than 60 kg or/and over 75 years of age received prasugrel 5 mg; all others were treated with prasugrel 10 mg daily. Inclusion criteria for acute coronary syndromes were symptomatic documented coronary artery disease with angina, or non-ST elevation myocardial infarction (NSTEMI). Exclusion criteria were as follows: severe renal failure (creatinine level > 2.5 mg/dL), active internal bleeding or bleeding diathesis, hemodynamic instability, malignancies, contraindication to antiplatelet agents, concomitant use of anticoagulants or glycoprotein IIb/IIIa receptor blockers, thrombocytopenia (platelet count < 80,000/µL), or anemia (hemoglobin < 8.0 g/dL). Blood samples for platelet aggregation and genetic tests were obtained from patients after discharge, no earlier than 1 month following coronary intervention, into tubes containing 3.2% sodium citrate (Dynabyte, Munich, Germany). Most sampling occurred between 30 and 90 days (mean 64.7 days) after PCI.
Platelet Aggregation
In short, citrate-anticoagulated whole blood was centrifuged at 120 g for 10 min at room temperature to obtain platelet-rich plasma. Platelet-poor plasma was obtained from the remaining specimen by second centrifugation at 120 g for 10 min, and used as a reference. Platelet-rich plasma was adjusted to reach a platelet count of 2.5 × 105/μL by adding platelet-poor plasma. Multiplate electrode aggregometry (MEA), light transmission aggregometry (LTA), and VerifyNow (P2Y12 reaction units, PRU) were performed in the whole blood, according to the manufacturer’s recommendations. Platelets were stimulated with 10 µmol ADP (Chronolog, Havertown, PA, USA) and aggregation was assessed as previously described using a Chronolog Lumi-Aggregometer (model 560-Ca) with the AggroLink software package. Aggregation was expressed as the maximal percent change in light transmittance from baseline, using platelet-poor plasma as a reference. Curves were recorded for 6 min, and analyzed according to international standards. We defined VerifyNow PRU ≥235 as high platelet reactivity and VerifyNow PRU values < 85 as low platelet reactivity.
Genetic Testing
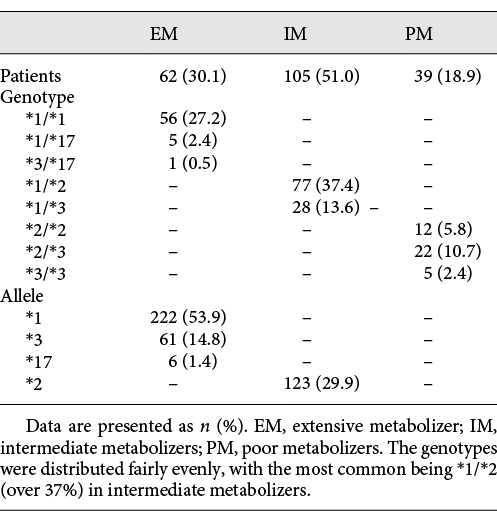
The genotyping of CYP2C19*2 (rs4244285), CYP2C19*3 (rs4986893), and CYP2C19*17 (rs12248560) was performed using the TaqMan fluorogenic 5′ nuclease assay (Spartan Technology, Ottawa, ON, Canada). Allelic discrimination was performed with an ABI Prism 7900 HT Sequence Detection System utilizing allele-specific primers and probes included in the TaqMan Drug Metabolism Assay mix. The polymerase chain reaction (PCR) amplification protocol for the TaqMan assays included denaturation at 94°C for 10 min, followed by 30 cycles at 94°C for 30 s, 63°C for 30 s, and 72°C for 45 s, followed by elongation at 72°C for 10 min. After PCR, fluorescence yield for 2 different dyes was measured and presented as a scatter plot. With regard to the common CYP2C19, genotypes *1/*1 and heterozygous for the *17 allele were assessed as extensive metabolizers (EM), *1/*2 and *1/*3 as intermediate metabolizers (IM), and finally, *2/*2, *2/*3, and *3/*3 as poor metabolizers (PM). Of the 206 Korean patients in the present study, there were no subjects with homozygous *17/*17. Six patients with CYP2C19*1/*17 and 1 with CYP2C19*3/*17 were grouped as CYP2C19 EM in the study.
Statistics
Continuous variables are presented as mean ± standard deviation (SD), and compared using the independent sample t test or ANOVA test and post hoc analysis by Bonferroni or Tamhane. Categorical variables are presented as numbers or percentages, and compared using the χ2 test or Fisher’s exact test. A general linear model univariate analysis was applied to quantify prasugrel responsiveness by entering patients and clinical factors as covariates. A multivariate logistic regression analysis was performed to determine the independent association of gene polymorphisms with high residual platelet reactivity. A 2-sided probability value less than 0.05 was considered statistically significant. Statistical analyses were performed using SPSS version 22.0 software (SPSS Inc, Chicago, IL, USA).
Results
Clinical Variables
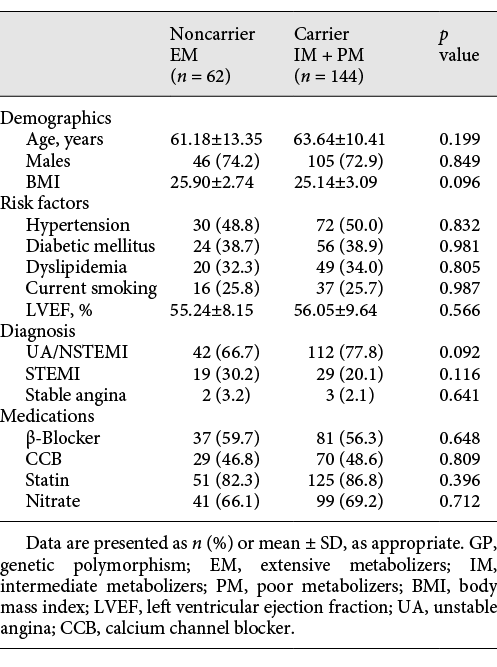
A total pool of 206 patients were screened and provided informed consent. Based on genetic testing 62 patients were triaged into the noncarrier group and 144 into the carrier group. The details of baseline clinical characteristics of noncarriers and carriers are presented in Table 1.
Genetic Polymorphism and Platelet Function
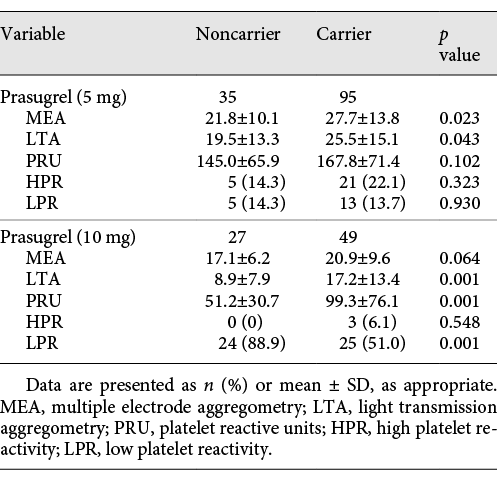
As expected, residual platelet reactivity was lower after the full (10 mg) prasugrel dose, when compared to prasugrel 5 mg/day, and there was a consistent trend towards higher aggregation in CYP2C19 carriers independently from maintenance dosing, reaching significance for prasugrel 10 mg. Interestingly, the frequency of the 0-carrier of CYP2C19*2 or *2 allele was as high as 51.0% and that of the 1-, 2-carrier of CYP2C19*2 or *2 allele was 18.9%. Subgroup analysis in the 5- or 10-mg maintenance dose group shows similar results for gene distribution. For the prasugrel 5-mg group, LTA (p = 0.043) and MEA (p = 0.023) had significant differences in residual platelet reactivity. For the prasugrel 10-mg group, the differences in residual platelet reactivity reached statistical difference for LTA (p = 0.001) and VerifyNow analyzer (p = 0.001). The details are outlined in Table 2.
The results of multivariate logistic regression for high platelet reactivity in the entire study population are shown in Figure 1. Accordingly, the VerifyNow, LTA assays, and MEA assays were capable of distinguishing post-prasugrel platelet reactivity between noncarriers and carriers (VerifyNow: 104.1 ± 70.8 vs. 144.5 ± 79.7, p = 0.001; LTA: 14.9 ± 12.3 vs. 22.7 ± 15.0, p = 0.001; and MEA: 19.7 ± 8.9 vs. 25.4 ± 12.9, p = 0.023).
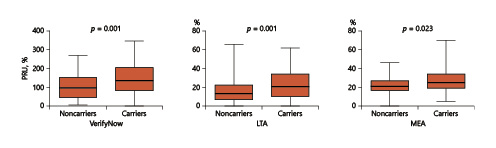
Fig. 1
Platelet activity analysis: postprasugrel platelet reactivity assessed by different techniques in carriers versus noncarriers. According to the VerifyNow, light transmission aggregometry (LTA), and multiplate electrode aggregometry (MEA) assays, higher platelet reactivity was identified in carrier patients compared to noncarrier patients: VerifyNow (noncarriers vs. carriers: 104.1 ± 70.8 vs.144.5 ± 79.7, p = 0.001), LTA (noncarriers vs. carriers: 14.9 ± 12.3l vs. 22.7 ± 15.0, p = 0.001), and MEA (noncarriers vs. carriers: 19.7 ± 8.9l vs. 25.4 ± 12.9, p = 0.023). PRU, P2Y12 reaction units.
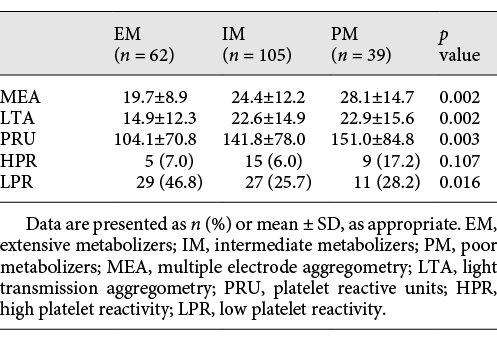
When the entire patient cohort was divided into groups of 62 patients (30.1%, EM group), 105 patients (50.1%, IM group), and 39 patients (18.9%, PM group) (see Table 3), residual post-prasugrel platelet reactivity exhibited statistical differences among the 3 groups, respectively, by LTA (EM, IM, and PM: 14.9 ± 12.3, 22.6 ± 14.9, and 22.9 ± 15.6, p = 0.002), VerifyNow test (EM, IM, and PM: 104.1 ± 70.8, 141.8 ± 78.0, and 151.0 ± 84.8, p = 0.003), and MEA (EM, IM, and PM: 19.7 ± 8.9, 24.4 ± 12.2, and 28.1 ± 14.7, p = 0.002). In a subgroup dose analysis, 130 patients (63.1%) were in the prasugrel 5-mg group and 76 patients (36.9%) were in the prasugrel 10-mg group (Table 4).
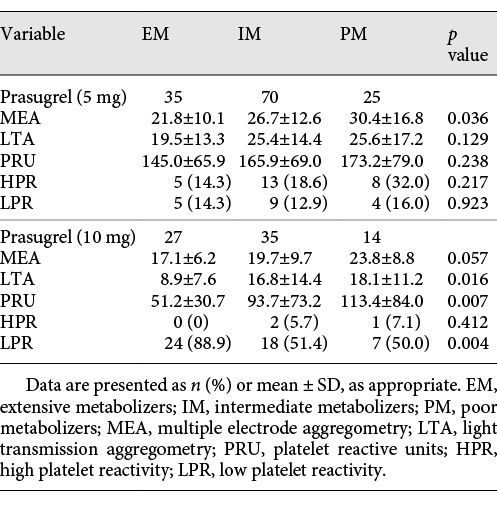
In the prasugrel 5-mg group, there were 35 patients in the EM group, 70 patients in the IM group, and 25 patients in the PM group. MEA exhibited significant differences in the residual platelet reactivity (EM, IM, and PM: 21.8 ± 10.1, 26.7 ± 12.6, and 30.4 ± 16.8, p = 0.036). Within the prasugrel 10-mg group, 27 patients were in the EM group, 35 patients were in the IM group, and 14 patients belonged to the PM group. There was a significant difference in the LTA and PRU readings in the prasugrel 10-mg group (LTA: EM, IM, and PM: 8.9 ± 7.6, 16.8 ± 14.4, and 18.1 ± 11.2, p = 0.016; PRU: EM, IM, and PM: 51.2 ± 30.7, 93.7 ± 73.2, and 113.4 ± 84.0, p = 0.007). The genotype distributions and the calculated allelic frequencies of each variant are exhibited in Table 5.
Discussion
The most important message from the current study is the fact that CYP2C19 genotype carriers still have some impact on residual post-prasugrel platelet activity during the maintenance phase while on dual antiplatelet therapy. Almost all work on prasugrel response genetics was done early after, or even before, intervention, around the loading phase, making long-term maintenance response somewhat “terra incognita.” Moreover, the data on low-dose prasugrel are very seldom, but especially important now, after the successful relaunch of 3.75 mg/day prasugrel in Japan. Therefore, the index evidence yielded from East Asian patients is indeed important and new. In contrast to the negative findings at the acute in-hospital setting, it seems that there is still a delayed impact of GP on platelet reactivity in outpatients. These data have important practical implications focusing on the potential possibility that certain genotypes may impact both ischemic and bleeding adverse events while on prasugrel. It is well established that the CYP2C19*2 genotype influences platelet reactivity in patients treated with clopidogrel. It is known that PM among Asian patients may experience better efficacy with a higher clopidogrel dose via the potential reduction of the incidence of major adverse cardiovascular events (MACE) []. Indeed, the elevated risks of MACE may be attributed to the specific CYP2C19 genotypes causing higher residual platelet reactivity []. The current guidelines recommend that dual antiplatelet therapy should apply for at least 1 year after stenting in patients with documented coronary artery disease []. Clopidogrel is still a number one antiplatelet agent, but with obvious limitations including delayed metabolization requiring 2-step processes: 85% hydrolyzed inactive metabolite and 15% metabolized by cytochrome P450 (CYP450) pathway. Clopidogrel causes wide interpatient variability in the platelet response, and multiple drug-drug interactions [-]. Importantly, the link between GP and drug-related responses are especially valid in Asians since about 50% of the Asian population are loss-of-function allele carriers. The patients carrying at least 1 loss-of-function allele can experience decreased active metabolism, diminished platelet inhibition, and an increase of thrombotic events. Therefore, nearly half of patients with acute coronary syndromes who underwent percutaneous intervention are genotype carriers associated with increased risk of MACE while on standard doses of clopidogrel []. In short, there is a mutual agreement that genetic biomarkers may serve as an independent predictor of MACE after stenting in patients treated with clopidogrel [-].
Being a next-generation thienopyridine, prasugrel provides faster, more potent platelet inhibition compared with clopidogrel in post-stent patients [, ]. As an advantage to clopidogrel, prasugrel requires single-step activation, and irreversibly blocks the P2Y12 platelet receptor after hepatic transformation, while CYP2C19 plays a minimal role in metabolism []. Importantly, there have been numerous studies suggesting that prasugrel did not affect active metabolism and platelet inhibition by CYP2C19 genotype [-]. These pharmacogenetic findings were in contrast to observations with clopidogrel, which might partly explain different pharmacological and clinical responses to these medications []. Many studies showed that CYP genetic variants had a significant influence on the pharmacokinetic and pharmacodynamic responses to clopidogrel, causing diminished antiplatelet response []. However, prasugrel is believed to be less affected by genotypic variability [-]. Moreover, one study found no significant difference in pharmacodynamic response for prasugrel 5 or 10 mg [], what is in disagreement with the index data. Another study revealed that prasugrel, in contrast to clopidogrel, rapidly and independently inhibited platelet aggregation of CYPC19 carriers in healthy Japanese subjects []. However, a strong impact of PM carrying the *2 genotype on platelet function after prasugrel has also been reported []. Importantly, the maintenance phase platelet-genetic data for prasugrel are very limited. A single report suggested that a loading dose of prasugrel 15 mg followed by a maintenance dose of 3.75 mg or a regimen of prasugrel 20 mg/5 mg were well tolerated and achieved a rapid, higher, and consistent antiplatelet effect in Japanese coronary artery disease patients []. There are some data indicative that the impact of the CYP2C19 genotype would be less on antiplatelet activity by prasugrel compared to clopidogrel; however, the CYP2C19 contribution to active metabolite formation of prasugrel is not negligent. In fact, 8–11% of CYP2C19 was still involved in the active metabolite metabolism of prasugrel []. Another elegant prospective, randomized, single-blind, crossover study of platelet inhibition by prasugrel 10 mg/day versus high-dose 150 mg/day clopidogrel in 71 (of 210 screened; 33.8%) post-PCI patients suggested lower residual platelet aggregation (RPA) rates for prasugrel than for clopidogrel in all patients (7.5 vs. 35.8%, p < 0.001), in carriers (5.3 vs. 47.4%, p = 0.007), and in noncarriers (8.8 vs. 29.4%, p = 0.005), respectively [].
Therefore, it will be critical in the future to compare similar properties of prasugrel with those of clopidogrel in a direct, better-randomized fashion. Platelet inhibition was mildly affected by the CYP2C19 phenotype in both prasugrel groups. We are pretty confident in the index data since we applied 3 different methods for platelet activity assessment, and all trends were similar, suggesting that CYP2C19 carriers exhibit various responses to thienopyridines in general, and prasugrel in particular. Some of the disagreements with the previous evidence may be attributed to the timing of the platelet tests which was truly delayed from the acute phase. We utilized MEA, LTA, and PRU to reflect the residual post-prasugrel platelet activity to make sure that the data are consistent. Indeed, MEA and LTA exhibit significant differences between carriers and noncarriers, while the VerifyNow assay shows a strong trend as well. With regard to the extent of metabolization, all 3 platelet activity methods exhibit a significant impact, linking residual activity and genotype. Importantly, no clear dose-allele effect was found (Table 3), especially for LTA measures, in contrast to what was reported by Kim et al. [] for clopidogrel in a similar Korean cohort. It is, therefore, possible that other gene polymorphisms are involved that are not explored here. For instance, CYP2B6 and 2C9 have been proposed [] as more reliable markers of thienopyridine response depending on their frequency in the Korean population.
A few limitations of this study should be mentioned. First, the study was not randomized, and the practical utility of our findings is still quite limited. This is especially true since the study was implemented exclusively in Korean patients, making any general extrapolations premature. Another confounding issue is related to the complexity of applying 3 different methods to assess platelet activity. Therefore, some of the data show different patterns which are difficult, if possible, to explain. Indeed, dichotomizing the data between 2 prasugrel doses, and among 3 types of metabolization, makes it difficult to comprehend the evidence. Finally, for plasma preparations, we commonly use centrifugation at 120 g as a conventional operation procedure. In general, higher-speed centrifugation may be required for plasma preparation to avoid cell contamination, which was not assessed in the current study. With regard to the methodology for assessing platelet activity, we applied 3 conventional methods; however, further studies may add the VASP assay to avoid the impact of aspirin, and measure the true potency of the P2Y12 inhibitor []. Nonetheless, the strengths of our study were the relatively large sample size, monogenetic population, uniformed enrollment rules, single-center setup, and considerably delayed assessment in the maintenance phase.
We conclude that CYP2C19 polymorphism impacts residual platelet reactivity during maintenance with prasugrel in Korean outpatients. This effect is consistent for both of the approved prasugrel doses potentially affecting long-term outcomes. The other important message to highlight is that noncarriers of loss-of-function alleles have a higher frequency of low platelet reactivity, and could therefore be at a higher risk factor of bleeding. However, the clinical utility of these findings is still uncertain, and requires more evidence from larger randomized trials beyond East Asians.
Acknowledgments
This research was supported by a grant from the Korea Health Technology R&D Project through the Korea Clinical Trials Global Initiative, funded by the Ministry of Health and Welfare (No. HI14C1731), and by the National Research Foundation of Korea (No. 2015R1D1A1A09057025). Part of this work was supported by the “Brain Pool” program, funded by the Korea Ministry of Science and Technology, to Dr. Serebruany.
Disclosure Statement
The authors have no conflicts of interest other than those indicated in the Acknowledgments.
References
- 1. Winter MP, Koziński M, Kubica J, et al: Personalized antiplatelet therapy with P2Y12 receptor inhibitors: benefits and pitfalls. Postepy Kardiol Interwencyjnej 2015; 11: 259–280.
- 2. Wiviott SD, Antman EM, Winters KJ, et al: Randomized comparison of prasugrel (CS-747, LY640315), a novel thienopyridine P2Y12 antagonist, with clopidogrel in percutaneous coronary intervention: results of the Joint Utilization of Medications to Block Platelets Optimally (JUMBO)-TIMI 26 trial. Circulation 2005; 111: 3366–3373.
- 3. Wiviott SD, Braunwald E, McCabe CH, et al: Prasugrel versus clopidogrel in patients with acute coronary syndromes. N EngI J Med 2007; 357: 2001–2015.
- 4. Kubica A, Kozinski M, Grzesk G, et al: Genetic determinants of platelet response to clopidogrel. J Thromb Thrombolysis 2011; 32: 459–466.
- 5. Shuldiner AR, O’Connell JR, Bliden KP, et al: Association of cytochrome P450 genotype with antiplatelet effect and clinical efficacy of clopidogrel therapy. JAMA 2009; 302: 849–857.
- 6. Lev EI, Patel RT, Guthikonda S, et al: Genetic polymorphisms of the platelet receptors P2Y12, P2Y1 and GP IIb/IIIa and response to aspirin and clopidogrel. Thromb Res 2007; 119: 355–360.
- 7. Myrand SP, Sekiguchi K, Man MZ, et al: Pharmacokinetics/genotype associations for major cytochrome P450 enzymes in native and first- and third-generation Japanese populations: comparison with Korean, Chinese, and Caucasian populations. Clin Pharmacol Ther 2008; 84: 347–361.
- 8. Viviani Anselmi C, Briguori C, Roncarati R, et al: Routine assessment of on-clopidogrel platelet reactivity and gene polymorphisms in predicting clinical outcome following drug-eluting stent implantation in patients with stable coronary artery disease. JACC Cardiovasc Interv 2013; 6: 1166–1175.
- 9. Mega JL, Close SL, Wiviott SD, et al: Cytochrome p-450 polymorphisms and response to clopidogrel. N EngI J Med 2009; 360: 354–362.
- 10. Mega JL, Simon T, Collet JP, et al: Reduced-function CYP2C19 genotype and risk of adverse clinical outcomes among patients treated with clopidogrel predominantly for PCI: a meta-analysis. JAMA 2010; 304: 1821–1830.
- 11. Kazui M, Nishiya Y, Ishizuka T, et al: Identification of the human cytochrome P450 enzymes involved in the two oxidative steps in the bioactivation of clopidogrel to its pharmacologically active metabolite. Drug Metab Dispos 2010; 38: 92–99.
- 12. Gurbel PA, Bliden KP, Hiatt BL, O’Connor CM: Clopidogrel for coronary stenting: response variability, drug resistance, and the effect of pretreatment platelet reactivity. Circulation 2003; 107: 2908–2913.
- 13. Ang L, Palakodeti V, Khalid A, et al: Elevated plasma fibrinogen and diabetes mellitus are associated with lower inhibition of platelet reactivity with clopidogrel. J Am Coll Cardiol 2008; 52: 1052–1059.
- 14. Patti G, Nusca A, Manqiacapra F, et al: Point-of-care measurement of clopidogrel responsiveness predicts clinical outcome in patients undergoing percutaneous coronary intervention results of the ARMYDA-PRO (Antiplatelet therapy for reduction of Myocardial Damage during Angioplasty-Platelet Reactivity Predicts Outcome) study. J Am Coll Cardiol 2008; 30: 1128–1133.
- 15. Gurbel PA, Bliden KP, Zaman KA, et al: Clopidogrel loading with eptifibatide to arrest the reactivity of platelets: results of the Clopidogrel Loading with Eptifibatide to Arrest the Reactivity of Platelets (CLEAR PLATELETS) study. Circulation 2005; 111: 1153–1159.
- 16. Bonello L, Tantry US, Marcucci R, et al: Consensus and future directions on the definition of high on-treatment platelet reactivity to adenosine diphosphate. J Am Coll Cardiol 2010; 56: 919–933.
- 17. Sun H, Qu Q, Chen ZF, et al: Impact of CYP2C19 variants on clinical efficacy of clopidogrel and 1-year clinical outcomes in coronary heart patients undergoing percutaneous coronary intervention. Front Pharmacol 2016; 7: 453.
- 18. Yamamoto K, Hokimoto S, Chitose T, et al: Impact of CYP2C19 polymorphism on residual platelet reactivity in patients with coronary heart disease during antiplatelet therapy. J Cardiol 2011; 57: 194–201.
- 19. Levine,GN, Bates ER, Bittl JA, et al: 2016 ACC/AHA guideline focused update on duration of dual antiplatelet therapy in patients with coronary artery disease: a report of the American College of Cardiology/American Heart Association Task Force on Clinical Practice Guidelines. J Am Coll Cardiol 2016; 68: 1082–1115.
- 20. Mega JL, Close SL, Wiviott SD, et al: Genetic variants in ABCB1 and CYP2C19 and cardiovascular outcomes after treatment with clopidogrel and prasugrel in the TRITON-TIMI 38 trial: a pharmacogenetic analysis. Lancet 2010; 376: 1312–1319.
- 21. Brandt JT, Close SL, Iturria SJ, et al: Common polymorphisms of CYP2C19 and CYP2C9 affect the pharmacokinetic and pharmacodynamic response to clopidogrel but not prasugrel. J Thromb Haemost 2007; 5: 2429–2436.
- 22. Gurbel PA, Bergmeijer TO, Tantry US, et al: The effect of CYP2C19 gene polymorphisms on the pharmacokinetics and pharmacodynamics of prasugrel 5-mg, prasugrel 10-mg and clopidogrel 75-mg in patients with coronary artery disease. Thromb Haemost 2014; 112: 589–597.
- 23. Mega JL, Close SL, Wiviott SD, et al: Cytochrome P450 genetic polymorphisms and the response to prasugrel: relationship to pharmacokinetic, pharmacodynamic, and clinical outcomes. Circulation 2009; 119: 2553–2560.
- 24. Serebruany VL, Midei MG, Meilman H, et al: Rebound platelet activation after termination of prasugrel and aspirin therapy due to confirmed non-compliance in patient enrolled in the JUMBO Trial. Intern J Clin Pract 2006; 60: 83–86.
- 25. Serebruany VL, Hanley DF, Atar D, Ferguson JJ: Noncompliance in antiplatelet trials: the AGATE trial perspective. Stroke 2004; 35: 143–144.
- 26. Bin Sayeed MS, Hasan Apu MN, Munir MT, et al: Prevalence of CYP2C19 alleles, pharmacokinetic and pharmacodynamic variation of clopidogrel and prasugrel in Bangladeshi population. Clin Exp Pharmacol Physiol 2015; 42: 451–457.
- 27. Kelly RP, Close SL, Farid NA, et al: Pharmacokinetics and pharmacodynamics following maintenance doses of prasugrel and clopidogrel in Chinese carriers of CYP2C19 variants. Br J Clin Pharmacol 2012; 73: 93–105.
- 28. Umemura K, Iwaki T: The pharmacokinetics and pharmacodynamics of prasugrel and clopidogrel in healthy Japanese volunteers. Clin Pharmacol Drug Dev 2016; 5: 480–487.
- 29. Cuisset T, Loosveld M, Morange PE, et al: CYP2C19*2 and *17 alleles have a significant impact on platelet response and bleeding risk in patients treated with prasugrel after acute coronary syndrome. JACC Cardiovasc Interv 2012; 5: 1280–1287.
- 30. Yokoi H, Kimura T, Isshiki T, et al: Pharmacodynamic assessment of a novel P2Y12 receptor antagonist in Japanese patients with coronary artery disease undergoing elective percutaneous coronary intervention. Thromb Res 2012; 129: 623–628.
- 31. Rehmel JL, Eckstein JA, Farid NA, et al: Interactions of two major metabolites of prasugrel, a thienopyridine antiplatelet agent, with the cytochromes P450. Drug Metab Dispos 2006; 34: 600–607.
- 32. Alexopoulos D, Dimitropoulos G, Davlouros P, et al: Prasugrel overcomes high on-clopidogrel platelet reactivity post-stenting more effectively than high-dose (150-mg) clopidogrel: the importance of CYP2C19*2 genotyping. JACC Cardiovasc Interv 2011; 4: 403–410.
- 33. Kim HS, Cho DY, Park BM, et al: The effect of CYP2C19 genotype on the time course of platelet aggregation inhibition after clopidogrel administration. J Clin Pharmacol 2014; 54: 850–857.
- 34. Franken CC, Kaiser AF, Krüger JC, et al: Cytochrome P450 2B6 and 2C9 genotype polymorphism – a possible cause of prasugrel low responsiveness. Thromb Haemost 2013; 110: 131–140.
- 35. Bagoly Z, Homoródi N, Kovács EG, et al: How to test the effect of aspirin and clopidogrel in patients on dual antiplatelet therapy? Platelets 2016; 27: 59–65.