Introduction
In health our microbes are in balance – symbiosis – with their host, the human body. This balance can however be disturbed and even lost, leading to a new, disease-associated state – dysbiosis. Frequent metabolism of sugars by oral microbiota leads to acidification of the ecosystem, reduction of microbial diversity and functional flexibility and enhancement of the proportion of highly acid-producing and acid-tolerating microbial taxa [Kilian et al., 2016; Marsh, 2017]. The efforts to recover the healthy balance from this caries-associated dysbiotic state using current broad-spectrum antimicrobial approaches have not been proven successful [Liu et al., 2018]. Another way of treating or preventing caries-related microbial dysbiosis could be by enhancing the growth and survival of health-associated oral microbiota [Twetman, 2018]. In this context, the use of prebiotics and/or probiotic bacteria to modulate the oral ecosystem has gained interest during the last few decades. In this article, current evidence for the role of oral prebiotics (part I) and probiotics (part II) in caries prevention and caries management, as well as future directions in these fields, are discussed.
Prebiotics
The term prebiotics was coined in 1995 by Gibson and Roberfroid as a “non-digestible food ingredient that beneficially affects the host by selectively stimulating the growth and/or activity of one or a limited number of bacteria already resident in the colon” [Gibson and Roberfroid, 1995]. This definition has been adapted multiple times, with the most recent update into a “substrate that is selectively utilized by host microorganisms conferring a health benefit” [Gibson et al., 2017]. With regard to caries management, these would include nutrients for microbial taxa that either inhibit acidogenic and aciduric microbes and/or enhance the pH recovery by generating alkali from these nutrients [Burne and Marquis, 2000; Liu et al., 2012]. The two primary sources of alkali in the oral cavity are urea and arginine, which are metabolized by some oral bacteria, resulting in ammonia production and increase in pH [Liu et al., 2012]. Thus, both might fit the definition of prebiotics, and the existing evidence for acclaiming health-related benefits of urea and arginine in caries management is elaborated below.
Urea as Anti-Caries Prebiotic
Urea, also known as carbamide, serves an important role in the metabolism of nitrogen-containing compounds in the body. It is formed in the liver and functions as nitrogen excretion means by the kidneys. Saliva of healthy individuals contains 3–10 mmol/L urea [Liu et al., 2012], while kidney failure patients may reach much higher levels [Andrade et al., 2015]. Bacteria that possess the enzyme urease can convert urea to ammonia (NH3) or ammonium (NH4+) and bicarbonate (HCO3–) ion. In the oral cavity urease activity has been demonstrated in species such as Streptococcus salivarius, Actinomyces naeslundii and Haemophilus spp. [Liu et al., 2012]. Robert Stephan reported already in 1943 on the role of urea metabolism in neutralization of acids in plaque [Stephan, 1943]. In 1948, Kesel demonstrated the effects of the mouthwash containing 3% urea and 5% dibasic ammonium phosphate; it reduced lactic acid production and lactobacillus counts in saliva of caries active individuals and inhibited the development of new caries lesions [Kesel, 1948]. Consequently, it was shown that physiological salivary urea levels are able to produce a pH rise in fasting plaque [Kleinberg, 1967]. An elegant mathematical model confirmed that normal salivary urea levels are able to increase the pH at the base of a 0.5-mm-thick fasted plaque by up to 1 pH unit and to raise the pH minimum after sucrose exposure by at least half a pH unit [Dibdin and Dawes, 1998]. Several clinical studies have associated reduced levels of urease activity in plaque with caries [Shu et al., 2007; Nascimento et al., 2009; Morou-Bermudez et al., 2011]. Recently, the applicability and feasibility of urea and a urea-sucrose metabolic test as a caries-screening tool in the adult population have been demonstrated [Morou-Bermudez et al., 2017].
Urea and Caries End Points
The use of urea supplementation as a prebiotic anti-caries agent has not been widely studied. Two randomized controlled trials have assessed the caries-inhibitory effects of additional urea to sugar-free chewing gums in children – one in Lithuania [Machiulskiene et al., 2001] and one in Madagascar [Petersen and Razanamihaja, 1999]. In both studies, the urea-containing gum group did not differ in caries increment from the no-gum control group, with the exception of the occlusal surfaces in the 6-year olds in the Madagascar study [Petersen and Razanamihaja, 1999]. The lack of difference among the effects of gum base, sorbitol- and xylitol-containing gums [Machiulskiene et al., 2001] could be explained by the stimulation of saliva through chewing itself [Dodds, 2012]. However, the authors had no explanation for the lack of difference between the sorbitol/urea-supplemented gum and no-gum group.
Urea and Calculus Formation
Reports on the role of urea in calculus formation might have further discouraged supplementation of urea to oral care products. Significantly more calculus, higher salivary pH, buffer capacity and salivary urea concentration were found in hemodialysis patients compared to healthy controls [Andrade et al., 2015]. In vitro mineralization studies showed that calcium phosphate precipitation in plaque is induced by rapid pH rise due to microbial urea metabolism [Sissons et al., 1991; Wong et al., 2002]. Son and Mühlemann demonstrated that the urease inhibitor acetohydroxamic acid can inhibit supragingival calculus formation [Son and Mühlemann, 1971]. A clinical study specifically addressing the role of urea supplementation on calculus formation concluded though that frequent use of chewing gum with or without urea for 3 months neither promoted nor inhibited calculus formation [Fure et al., 1998].
Urea and Plaque pH
The European Food Safety Authority (EFSA) concluded based on the results of five studies that the use of urea-containing sugar-free chewing gum (at least 20 mg urea per piece) for at least 20 min after eating or drinking is able to neutralize plaque acids [EFSA Panel on Dietetic Products, 2011]. Although the EFSA panel concluded that all five studies showed that urea significantly enhanced the pH-rising effect compared to placebo gums, a closer look at the reported studies does not provide this consensus. One of the studies was a meeting abstract and has not been published as a full paper. Another study concluded actually that there were no significant differences in plaque pH between the urea-containing gum and the placebo gum [Smith et al., 2004] and yet another study reported that the additional effects of the urea to chewing a gum base were minimal [Sjögren et al., 2002]. More recent studies with urea applied as oral rinse found no effect on plaque pH [Hassan et al., 2015] or on enamel remineralization in situ [Yu et al., 2017].
Concluding the part on urea, it is clear that dental caries is inversely related with urease activity in dental plaque. However, there is no evidence that the supplementation of urea through means of a chewing gum or a mouth rinse significantly contributes to caries inhibition.
Arginine as Anti-Caries Prebiotic
Protein-rich foods and salivary polypeptides are sources of the amino acid arginine, which is naturally found in parotid saliva at concentrations about 50 µmol/L [Liu et al., 2012]. Arginine is hydrolyzed by the bacterial arginine deiminase system (ADS) pathway and metabolized into ammonia [Kanapka and Kleinberg, 1983; Marquis et al., 1993; Liu et al., 2012]. Among the ADS-positive bacteria are major constituents of oral microbiota such as Streptococcus sanguinis, Streptococcus gordonii, Streptococcus parasanguinis, Streptococcus mitis and certain lactobacilli and Actinomyces strains. There are also some oral spirochetes, microbiota associated with periodontal disease, that possess arginolytic activity [Liu et al., 2012]. Ammonia production from arginine metabolism raises cytoplasmic and environmental pH, hereby delivering both, the ecological (pH increase) and bioenergetic advantages (e.g., synthesis of ATP) to ADS-positive bacteria [Nascimento, 2018]. An elegant experimental work by mixing various combinations and proportions of oral bacteria and incubating those with and without arginine in the presence of glucose showed that either arginolytic or acidogenic bacteria in plaque have to be present in substantial numbers in order to create differences in the pH response observed in caries-active and inactive individuals [Wijeyeweera and Kleinberg, 1989].
The relationship between the ADS activity and caries has been studied both in adults and in children [Nascimento et al., 2009, 2013; Reyes et al., 2014; Bijle et al., 2018]. A recent systematic review based on 7 studies concluded that low ADS activity in saliva and plaque could be a potential caries risk indicator for adults, while this relationship remains inconclusive in children [Bijle et al., 2018].
Arginine and Microbiological End Points
Clinical studies with 8% arginine and fluoride-containing toothpaste used for 4 [Zheng et al., 2017] or 8 weeks [Koopman et al., 2017] enhanced the arginolytic potential and reduced acidogenicity, while 1.5% arginine toothpaste without fluoride resulted in increased ADS activity and ecologically beneficial microbial shifts in caries-active individuals [Nascimento et al., 2014]. These effects were in agreement with the findings from studies using different in vitro models [Koopman et al., 2015; Agnello et al., 2017; Huang et al., 2017; Ledder et al., 2017]. It was shown that the continuous presence of 1.6% arginine in the growth medium inhibited outgrowth of Candida in oral microcosms [Koopman et al., 2015].
There are several reports that indicate that arginine may affect oral microbiota directly. In a 3-species biofilm model with Streptococcus mutans, A. naeslundii and S. gordonii, arginine reduced biofilm formation, promoted the growth of A. naeslundii and S. gordonii, reduced the formation of insoluble exopolysaccharides, reduced the bacteriocin expression by S. mutans and increased H2O2 production by S. gordonii [He et al., 2016]. Arginine detrimentally affected adhesion of S. mutans biofilm and exopolysaccharides [Sharma et al., 2014] and had direct adverse effects on multiple virulence-related properties of S. mutans [Chakraborty and Burne, 2017]; it inhibited growth and downregulated the genes encoding virulence factors required for attachment/accumulation (gtfB and spaP), bacteriocins (nlmA, nlmB, nlmD and cipB) and the sigma factor required for competence development (comX).
Arginine and Caries End Points
In 1999, Israel Kleinberg presented a new “saliva-based anti-caries composition” named CaviStat, consisting of arginine bicarbonate and calcium carbonate complex [Kleinberg, 1999]. A toothpaste containing CaviStat (produced by Ortek Therapeutics) was compared with 1,100 ppm fluoride toothpaste in 11- to 12-year-old Venezuelan schoolchildren [Acevedo et al., 2005]. This 2-year clinical study found that the experimental group had significantly lower total DMFS scores than the control group after 1 year but not at the 2-year examination. However, the caries increment was significantly lower in the experimental group compared to the control at both examinations, when only caries in premolars and second molars was accounted for. This was mainly due to high (91%) caries prevalence in the first molars at the baseline of the study in both groups and to the fact that most premolars and second molars erupted after the start of the study. Another clinical study, comparing the effects of a CaviStat-containing mint confection with a placebo on caries increment in 10- to 11-year-old Venezuelan schoolchildren for 1 year, concluded that CaviStat mints were able to inhibit both caries onset and caries progression in these children [Acevedo et al., 2008].
In 2007, the multinational company Colgate-Palmolive purchased the rights for using the arginine bicarbonate/calcium carbonate technology in toothpastes [Panagakos et al., 2009], originally aimed at relieving sensitive teeth [Kleinberg, 2002]. In 2009 their product containing Pro-Argin technology (toothpaste with 8% arginine) against dentin hypersensitivity was launched [Cummins, 2009, 2011]. More recently the company launched their 1.5% arginine-containing anti-caries product [Cummins, 2013]. The caries-preventive effects of this toothpaste were assessed in several studies with the company closely involved in the entire research line and in presenting the study results: (i) 3 studies on remineralization of enamel lesions in Thai [Srisilapanan et al., 2013] and Chinese [Yin et al., 2013a, b] schoolchildren after 6 months, assessed by quantitative light fluorescence; (ii) two 5-month studies on remineralization of root caries lesions in Brazilian adults, assessed by measuring lesion hardness [Hu et al., 2013; Souza et al., 2013]; (iii) two 2-year studies on caries prevention in Thai [Kraivaphan et al., 2013] and Chinese [Li et al., 2015b] children, assessed by visual-tactile caries assessment; and (iv) 1 community-based intervention study in Thai schoolchildren, assessing caries incidence after 2 years [Petersen et al., 2015]. The common conclusion of all these studies was that fluoride toothpaste with arginine-calcium base was superior to fluoride toothpaste in remineralization of lesions or in caries prevention.
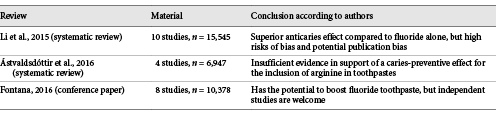
The company’s involvement in the studies mentioned above was not appreciated by the academic society. Within a couple of years, three reviews on this topic appeared (Table 1), all mentioning high risk of bias due to involvement of the industry and a need for independent studies [Li et al., 2015a; Ástvaldsdóttir et al., 2016; Fontana, 2016]. Li and colleagues from China and New Zealand evaluated 10 studies and concluded that arginine in combination with calcium base and fluoride provides a superior effect compared to fluoride alone, but a potential publication bias due to the involvement of the industry could not be ignored [Li et al., 2015a]. A conference on “Beyond 1,000 ppm: High Fluorides and Boosters” held in Boston in March 2015 resulted in another review on alternatives to fluoride for caries management by Fontana [2016]. Here, 8 papers on arginine were included, and the conclusion was similar to that of Li and coauthors. Just a few months later, a systematic review by Ástvaldsdóttir et al. [2016] from Sweden evaluated 7 studies, of which 3 were subsequently excluded due to high risk of bias [Acevedo et al., 2008; Hu et al., 2013; Souza et al., 2013]. The remaining 4 studies [Kraivaphan et al., 2013; Srisilapanan et al., 2013; Yin et al., 2013a, b] led to a conclusion that due to conflicts of interest and weak transferability to Swedish conditions no conclusions could be drawn by the authors of the review [Ástvaldsdóttir et al., 2016].
Large clinical randomized trials as in the studies above are very expensive to perform. Therefore, such studies are frequently performed by independent academic researchers subcontracted by an industrial partner with interest in the tested product. A key difference between an industry-sponsored but independently performed and reported study and a study where a senior author of the publication is from the company that sponsored the study is in the trust the academic society will have in research ethics while assessing these two types of studies. To avoid a perception of low trust even without a breach in research ethics, the industry should limit their involvement solely to financial support. The studies should be performed according to the principles of good clinical practice [ICH, 2016], where the quality management system, e.g. involving an external clinical study monitor, for guarding the independence of study design, data collection, analysis and reporting is implemented.
General Remarks on Prebiotics
A healthy oral ecosystem is expected to be at equilibrium regarding acid-alkali balance [Marsh et al., 2011]. A recent study on integrating salivary microbiome, metabolome and biochemical properties of saliva of clinically healthy young adults categorized these individuals in five ecological types [Zaura et al., 2017]. Three of the five types were termed “adaptive” (able to adapt to both, proteolytic and saccharolytic conditions, and thus at a healthy balance with the host), while two others were termed “specialized” – either a saccharolytic or a proteolytic ecological type [Zaura et al., 2017]. High salivary pH and protein-breakdown metabolites correlated with high microbial diversity and complexity, involving proteolytic taxa, associated with gingival or periodontal inflammation, suggesting an early pro-inflammatory dysbiosis. On the other side, saliva high in lipid metabolites and with low resting pH was associated with low microbial diversity and enrichment of saccharolytic taxa, suggesting an early pro-caries dysbiosis in these individuals. Enhancing pH recovery by generating alkali from prebiotic supplements such as urea or arginine would be highly beneficial in the latter group. However, additional arginine supplement might lead to unwanted effects in individuals already suffering from a highly proteolytic microbiome. Dental plaque of individuals who brushed with 8% arginine-containing toothpaste for either 1 or 2 months showed a significant increase in proteolytic taxa such as Treponema, Porphyromonas and Prevotella [Koopman et al., 2017; Zheng et al., 2017]. This calls for individually tailored or “one size does not fit all” preventive strategies. Another issue to consider is the role of nitric oxide generated from arginine metabolism in the pathogenesis of periodontitis [Parwani and Parwani, 2015].
Summarizing part I, arginine seems to fulfill the criteria for oral prebiotics; it is selectively utilized by oral microbiota, and the conferred health benefit to the host – inhibitory effect on the caries process – has been shown in multiple studies. The issues with the research ethics of the studies addressing arginine effects on caries however undermine the above statement.
Probiotics
Probiotics are defined as “live microorganisms which when administered in adequate amounts confer a health benefit on the host” [Sanders, 2008]. The concept “defense by diversity” with the aid of beneficial bacteria to maintain health or alleviate symptoms has a long tradition in veterinary and gastrointestinal medicine. In dentistry, most attention has been paid to gingivitis and periodontal diseases, but this article is limited to clinical trials related to caries. The mechanisms of action of probiotics are thought to combine local and systemic events including adhesion, co-aggregation, growth inhibition, bacteriocin production and immunomodulation [Twetman et al., 2017]. Thus, probiotics may have both preventive and therapeutic effects. In the context of oral health, probiotic supplements may prevent the oral biofilm from being environmentally “stressed” and maintain a stable symbiosis associated with health. Beneficial bacteria may however also “repair” a dysbiotic biofilm associated with diseases. An obvious example of the former is that regular intake of live probiotic bacteria can reduce caries risk and prevent caries development in preschool children [Jørgensen et al., 2016]; the latter is illustrated by patients with periodontal disease that can benefit from probiotic supplements when combined with traditional treatment and reduce the need for periodontal surgery [Martin-Cabezas et al., 2016].
Probiotics and Microbial End Points
A large number of studies from all around the world have addressed the short-term effect of probiotic supplements on the counts of salivary mutans streptococci in plaque and whole saliva, and the findings have been summarized in systematic reviews [Cagetti et al., 2013; Laleman et al., 2014; Gruner et al., 2016]. Graded as moderate, the evidence has consistently suggested reduced levels of salivary mutans streptococci following probiotic interventions, independently of the probiotic strain used, mode of delivery and daily dose. However, with the current concept of caries being a result of an unbalanced dysbiotic biofilm, the reduction of a single group of bacteria, even if regarded as pathogens, makes little sense. Few studies have so far investigated the effect of probiotic supplements on the composition of the oral microbiome with DNA/RNA-based methods. In two studies, no impact was found after short-term interventions with lozenges containing probiotic strains from the Bifidobacterium and Lactobacillus genera [Toiviainen et al., 2015] or candidate strains of L. rhamnosus and L. curvatus [Keller et al., 2018]. Contrasting findings were however reported in 2 other studies: Dassi et al. [2014] showed an increased diversity of the salivary microbiome after short-term intake of fermented milk with a mix of probiotic bacteria (Streptococcus thermophilus, Lactobacillus delbrueckii ssp. bulgaricus, Lactobacillus paracasei). Likewise, a Swedish study [Romani Vestman et al., 2015] found a shift in the composition of the oral microbiota after 12 weeks of daily L. reuteri DSM 17938 and PTA 5289. The intake correlated with increased levels of common commensals, such as S. oralis and S. mitis, and reduced levels of S. mutans, S. anginosus and Fusobacterium spp. This shift was however transient and disappeared 1 month after the probiotic exposure. Obviously, there is yet insufficient data to answer the question whether or not the function of the oral microbiome can be modulated with the aid of beneficial bacteria.
Probiotics and Caries End Points
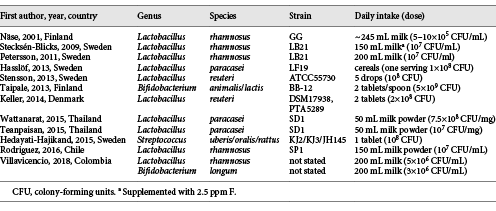
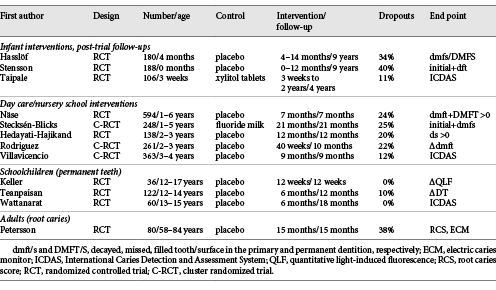
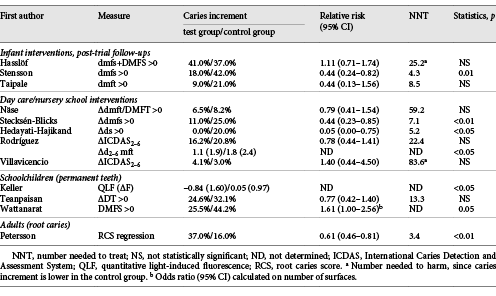
An updated search (June 2018) in PubMed, Cochrane Library and Trip database for randomized controlled trials with probiotic supplements and caries retrieved 11 unique hits (Table 2). The PICO was (i) population: all ages, (ii) intervention: any form of administration with live probiotic bacteria, (iii) control: placebo, good clinical practice or no treatment, and (iv) outcome: caries prevalence, increment, regression or reversal. The search terms were “probiotics,” “beneficial bacteria,” “lactobacilli,” “dental caries,” “dental decay,” “root caries” and “oral health” in various combinations. A hand search of reference lists from recent systematic and narrative reviews gave 1 additional paper [Teanpaisan et al., 2015]. Of the 12 publications included, 3 were post-trial follow-ups of interventions during infancy with medical end points [Hasslöf et al., 2013; Taipale et al., 2013; Stensson et al., 2014;], 5 were day care/nursery school projects [Näse et al., 2001; Stecksén-Blicks et al., 2009; Hedayati-Hajikand et al., 2015; Rodríguez et al., 2016; Villavicencio et al., 2018] and 3 were conducted in schoolchildren [Keller et al., 2014; Teanpaisan et al., 2015; Wattanarat et al., 2015]. Only 1 study in adults, focusing on root caries, was found [Petersson et al., 2011]. An overview of the probiotic bacteria employed together with the mode of administration and doses are shown in Table 2. The main characteristics and the outcome of the included studies are summarized in Tables 3 and 4. Statistically significant differences between the test and placebo groups in favor of the probiotic intervention were evident in 6 of the 12 studies. Due to the heterogeneity in age, probiotic strains used, daily doses, duration of intervention and end points, a formal meta-analysis could not be conducted. However, when the data on caries increment from the 5 preschool/nursery school projects [Näse et al., 2001; Stecksén-Blicks et al., 2009; Hedayati-Hajikand et al., 2015; Rodríguez et al., 2016; Villavicencio et al., 2018] were pooled, a study population of 1,273 children, 1–6 years of age, was formed. For this group, the calculated relative risk reduction (RR) for caries development was 0.65 (95% CI 0.47–0.90), and this was statistically significant (p < 0.05). The number-needed-to-treat was 22.7. The mean absolute RR for caries increment was 4.6% with a preventive fraction of 35.6%. When data on the caries prevalence (dmfs/DMFS > 0) for all children (n = 296) in the post-trial evaluations [Hasslöf et al., 2013; Taipale et al., 2013; Stensson et al., 2014] were merged and compared, no significant difference was found between the test and control groups (RR 0.68, 95% CI 0.42–1.09). The 3 studies conducted in schoolchildren could not be added together due to disparities in the reporting of end points. One single study addressed the reversal of primary root caries lesions after a 14-month daily intake of milk supplemented with probiotic lactobacilli [Petersson et al., 2011]. The test group showed significantly more reversals, verified by visual scoring and electric resistance, when compared with the control group consuming milk without probiotic bacteria, but further and larger trials are needed to confirm this finding among older adults.
The risk of bias is summarized in Figure 1. There was no study with a low risk of bias, while 4 were assessed with a moderate risk of bias. The most frequent shortcoming was attrition bias (7 out of 12 studies). Notably, no adverse side effects were reported from any of the studies.
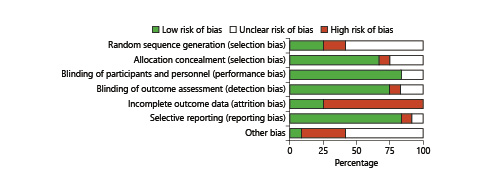
Fig. 1
Risk of bias graph (RevMan 5.3): authors’ judgments about each risk of bias item presented as percentages across all included studies.
General Remarks on Probiotics
The main finding from part II of this review was that probiotic supplements administered in school-based settings seemed to reduce caries incidence in preschool children and in schoolchildren with a high caries risk. However, due to the risk of bias that was found in the trials, the confidence in the effect estimate was limited. Notably, all studies but 2 [Hasslöf et al., 2013; Villavicencio et al., 2018] pointed in the same direction, even though the infant probiotic interventions clearly preceded the eruption of the primary teeth. Most projects were conservatively evaluated per protocol, but using subgroup analyses and selective reporting, the picture could be more in favor of probiotics, as elaborated below.
Selective Reporting
In the Helsinki kindergarten study [Näse et al., 2001], marked differences in caries increment between test and placebo were found when the 3- to 4-year-olds were analyzed separately. The motive to exclude the youngest children was that they did not have a fully erupted primary dentition during the time of intervention. Another example can be found in the study of Teanpaisan et al. [2015]. When the study group was split according to caries risk, the effect of the probiotic intervention was significant in the high caries risk group when compared with placebo (ΔDT 0.3 vs. 0.9; p < 0.05), but not among those with low caries risk (ΔDT 0.3 vs. 0.4). A nursery school-based intervention has also presented mixed results for various caries measures at follow-up [Rodríguez et al., 2016]. The difference in caries prevalence, expressed as percent, between the study groups was statistically insignificant while the caries increment during the study period differed significantly (Δd2–6 mft 1.1 vs. 1.8; p < 0.05). Another study from Thailand displayed significantly reduced caries increment in pits and fissures of permanent teeth but not on smooth surfaces [Wattanarat et al., 2015]. An interesting observation from the included trials was that the probiotic intervention seemed to be more effective in preschool children categorized with a high caries risk at baseline [Hedayati-Hajikand et al., 2015; Rodríguez et al., 2016] and in high-risk schoolchildren [Teanpaisan et al., 2015]. Theoretically, this could indicate that an established dysbiosis in the oral microbiome was actually influenced by the exposure to beneficial bacteria, but this needs to be established in a controlled clinical trial.
Risk of Bias
The fact that all included studies were conducted with a double-blind randomized placebo-controlled design does not certainly mean that they were free from bias or confounders. Hereby, the post-trial evaluations present specific problems with blinding and attrition. All of them were originally designed and power-calculated with respect to medical end points, and in two of them [Hasslöf et al., 2013; Stensson et al., 2014], the dental check-ups were not included in the original plan. Thus, the dropout rate was large, and most importantly, the group assignment was unveiled for the patients long before the dental examinations. One infant study had also selection bias as the participants were selected from families with a history of allergies [Stensson et al., 2014]. Among the nursery school/preschool projects, the study of Näse et al. [2001] had a short duration and follow-up (7 months) while the study of Stecksén-Blicks et al. [2009] was confounded by the supplementation of fluoride (2.5 ppm) to the milk in both the test and the control group. Also, the study from Chile may have been influenced by a short follow-up period and elevated fluoride content in the drinking water [Rodríguez et al., 2016]. Furthermore, in the recent study of Villavicencio et al. [2018], all children underwent a daily supervised tooth brushing program with 1,450 ppm fluoride during the 9-month intervention period.
Doses and Compliance
The included studies differed in intervention time and were performed with a blend of different species and strains. As the potential effects of probiotic bacteria are strain specific [Reid, 2016], this heterogeneity hampers any firm conclusions. The most common species under study were lactobacillus-derived strains of L. rhamnosus, L. paracasei and L. reuteri, but only two seemed to be of oral origin. In addition, the daily doses ranged considerably from 1 × 108 colony-forming units in infancy up to 3.7 × 1010 colony-forming units among the schoolchildren. Obviously, there is a need for dose response evaluation in order to gain knowledge concerning questions around the strain, dosage and mode of delivery. As with all interventions, compliance is a crucial factor. In the included papers, the compliance was mainly checked through self-reported logbooks by parents or by the school staff, rather than by returned pills or verified by oral and/or fecal colonization. In general, the compliance was rated as “good” or “high” (> 80%) in 9 of the 11 studies and “acceptable” or “satisfactory” (60%) in 3 studies. The study of Taipale et al. [2013], faced another compliance problem. The administration of the probiotic tablets was intended through pacifiers, which however were rejected by many children. By increasing age, the pacifiers were abandoned in favor for crushed tablets given by spoon. For the preschool- and school-based projects, it should be underlined that the active and placebo interventions were only distributed on week-days (4–5 days per week) and not during school holidays. Thus, a strict daily exposure of probiotics may not be necessary to influence caries development in children.
Cost-Effectiveness
Beneficial results from clinical trials, even from those with a low risk of bias, must be kept apart from guidelines and marketing. For this, health-economic evaluations are required on community level as well as estimating the value for individual patients. Although post-trial estimations of direct and indirect costs can be carried out retrospectively, no such information is currently available. In the present review, the number needed to treat varied strongly between 3 and 59, and the interventions were in most cases sponsored by the industry. In theory, school-based interventions in high-risk areas with a probiotic milk powder suspended in water, distributed and monitored by school staff, have the potential of being most cost efficient.
Clinical Recommendations
A pertinent question is how much evidence is needed for a clinical recommendation. There is no definite answer, but a suggestion is that at least two independent studies with a low risk of bias and nothing talking against, or one very large well-performed multicenter study should be present [Ryan and Hill, 2016]. The GRADE system [Guyatt et al., 2008] offers two grades of recommendations; when the desirable effects of an action clearly outweigh the undesirable effects or clearly do not, a “strong” recommendation can be given. When the balance of desirable versus undesirable effects is less certain, because of low-quality evidence or because evidence suggests that desirable and undesirable effects are more evenly balanced, the recommendation is described as “weak.” Thus, considering the limited confidence, partly conflicting results and lack of health-economic analyses, it would be premature to discuss probiotic treatment recommendations on the community level. However, as the benefits are likely greater than any potential harm, probiotic therapy may very well be considered for individual patients as additive to established preventive measures.
Future Directions
One factor that may be a barrier for using pre- and probiotics is the lack of knowledge concerning the exact mechanisms of action. It is anticipated that probiotic bacteria exert a mix of local and systemic events [Reid, 2016] with good and reliable evidence from systematic reviews for relieving diarrhea, atopic diseases and respiratory infections [Zuccotti et al., 2015; Blaabjerg et al., 2017; Goldenberg et al., 2017; Laursen and Hojsak, 2018]. In this context, it is important to underline that in 4 of the caries trials identified above, significant reductions of respiratory tract infections [Hatakka et al., 2001; Taipale et al., 2016], prescription of antibiotics and days with sick leave [Hatakka et al., 2001; Stecksén-Blicks et al., 2009], and reduced incidence of IgE-associated eczema [Abrahamsson et al., 2007] were reported. Such significant general health and economic gains for the society should also be taken into account in future trials with pre- and probiotic interventions in dentistry.
The search for and validation of new oral pre- and probiotics belongs also to the future [Ohshima et al., 2016; Slomka et al., 2017; Keller et al., 2018; Slomka et al., 2018]. For instance, Slomka and colleagues systematically screened over 700 nutritional sources together with the cultures of individual commensal either with caries- or periodontal-disease-associated bacteria, followed by dual [Slomka et al., 2017] and multispecies biofilm experiments [Slomka et al., 2018] with those compounds that promoted health-associated and inhibited disease-associated taxa. This way they identified N-acetyl-D-mannosamine, succinic acid and β-methyl-D-galactoside as potential prebiotics [Slomka et al., 2018]. Their impact on caries prevention though still needs to be assessed. As mentioned above, most probiotic strains used for oral health are of gastrointestinal origin, which may not be optimal for the unique and complex environment in the oral cavity. Recently, two commensal oral streptococcus strains – Streptococcus dentisani [López-López et al., 2017] and Streptococcus A12 [Huang et al., 2016] – were isolated from supragingival plaque of healthy individuals. Due to their inhibitory action against S. mutans and their arginolytic activity they both hold a potential as anti-caries probiotics. The effect of combining oral prebiotics, such as arginine, with oral probiotic strains into one synbiotic product is also a challenge for the future. As an example, it has recently been suggested [Nascimento, 2018] that arginine supplementation in combination with ADS-positive probiotics may constitute a nonpharmaceutical advancement in caries prevention. Both pre- and probiotics are natural and inexpensive food additives with a small or even negligible risk of side effects. Thus, the concept may have the potential to be accepted by consumers and play a role in the causal management of caries.
Conclusion
There is evidence from multiple studies that prebiotic arginine can inhibit caries development and probiotic supplements seem to reduce caries incidence in preschool children and schoolchildren with a high caries risk. Due to ethical research issues for arginine and the risk of bias for both the pre- and probiotic studies, the confidence in effect estimate is limited. More trials are needed to gain better knowledge of pre- and probiotic supplements and to confirm that their use is beneficial and cost-effective in caries care. Combining prebiotic arginine with arginolytic probiotics may be a future synbiotic for the prevention and management of dental caries.
Statement of Ethics
The authors have no ethical conflicts to disclose.
Disclosure Statement
E.Z. has received funding for clinical and in vitro studies on arginine from Colgate Palmolive. S.T. has received funding for two PhD students from BioGaia AB, Sweden, and research support from Bifodan A/S Denmark.
Funding Sources
This work was funded by the authors’ institutions.
Author Contributions
E.Z. wrote the first draft on prebiotics and S.T. on probiotics. The manuscript was completed in consensus and both authors approved the final version.
References
- 1. Abrahamsson TR, Jakobsson T, Böttcher MF, Fredrikson M, Jenmalm MC, Björkstén B, et al Probiotics in prevention of IgE-associated eczema: a double-blind, randomized, placebo-controlled trial. J Allergy Clin Immunol. 2007;119(5):1174–80.
- 2. Acevedo AM, Machado C, Rivera LE, Wolff M, Kleinberg I. The inhibitory effect of an arginine bicarbonate/calcium carbonate CaviStat-containing dentifrice on the development of dental caries in Venezuelan school children. J Clin Dent. 2005;16(3):63–70
- 3. Acevedo AM, Montero M, Rojas-Sanchez F, Machado C, Rivera LE, Wolff M, et al Clinical evaluation of the ability of CaviStat in a mint confection to inhibit the development of dental caries in children. J Clin Dent. 2008;19(1):1–8
- 4. Agnello M, Cen L, Tran NC, Shi W, McLean JS, He X. Arginine improves pH homeostasis via metabolism and microbiome modulation. J Dent Res. 2017;96(8):924–30.
- 5. Andrade MR, Salazar SL, de Sá LF, Portela M, Ferreira-Pereira A, Soares RM, et al Role of saliva in the caries experience and calculus formation of young patients undergoing hemodialysis. Clin Oral Investig. 2015;19(8):1973–80.
- 6. Ástvaldsdóttir Á, Naimi-Akbar A, Davidson T, Brolund A, Lintamo L, Attergren Granath A, et al Arginine and caries prevention: a systematic review. Caries Res. 2016;50(4):383–93.
- 7. Bijle MN, Yiu CK, Ekambaram M. Can oral ADS activity or arginine levels be a caries risk indicator? A systematic review and meta-analysis. Clin Oral Investig. 2018;22(2):583–96.
- 8. Blaabjerg S, Artzi DM, Aabenhus R. Probiotics for the prevention of antibiotic-associated diarrhea in outpatients— a systematic review and meta-analysis. Antibiotics (Basel). 2017;6(4):21.
- 9. Burne RA, Marquis RE. Alkali production by oral bacteria and protection against dental caries. FEMS Microbiol Lett. 2000;193(1):1–6.
- 10. Cagetti MG, Mastroberardino S, Milia E, Cocco F, Lingström P, Campus G. The use of probiotic strains in caries prevention: a systematic review. Nutrients. 2013;5(7):2530–50.
- 11. Chakraborty B, Burne RA. Effects of arginine on Streptococcus mutans growth, virulence gene expression, and stress tolerance. Appl Environ Microbiol. 2017;83(15):83.
- 12. Cummins D. Dentin hypersensitivity: from diagnosis to a breakthrough therapy for everyday sensitivity relief. J Clin Dent. 2009;20(1):1–9
- 13. Cummins D. Advances in the clinical management of dentin hypersensitivity: a review of recent evidence for the efficacy of dentifrices in providing instant and lasting relief. J Clin Dent. 2011;22(4):100–7
- 14. Cummins D. The development and validation of a new technology, based upon 1.5% arginine, an insoluble calcium compound and fluoride, for everyday use in the prevention and treatment of dental caries. J Dent. 2013;41Suppl 2:S1–11.
- 15. Dassi E, Ballarini A, Covello G, Quattrone A, Jousson O, De Sanctis V, et alHTM-CMB2013. Enhanced microbial diversity in the saliva microbiome induced by short-term probiotic intake revealed by 16S rRNA sequencing on the IonTorrent PGM platform. J Biotechnol. 2014;190:30–9.
- 16. Dibdin GH, Dawes C. A mathematical model of the influence of salivary urea on the pH of fasted dental plaque and on the changes occurring during a cariogenic challenge. Caries Res. 1998;32(1):70–4.
- 17. Dodds MW. The oral health benefits of chewing gum. J Ir Dent Assoc. 2012;58(5):253–61
- 18. EFSA Panel on Dietetic Products. Nutrition and Allergies: scientific opinion on the substantiation of health claims related to sugar-free chewing gum with carbamide and plaque acid neutralisation (ID 1153) pursuant to Article 13(1) of Regulation (EC) No 1924/2006. EFSA J. 2011;9(4):2071–85.
- 19. Fontana M. Enhancing fluoride: clinical human studies of alternatives or boosters for caries management. Caries Res. 2016;50Suppl 1:22–37.
- 20. Fure S, Lingström P, Birkhed D. Effect of three months’ frequent use of sugar-free chewing gum with and without urea on calculus formation. J Dent Res. 1998;77(8):1630–7.
- 21. Gibson GR, Hutkins R, Sanders ME, Prescott SL, Reimer RA, Salminen SJ, et al Expert consensus document: the International Scientific Association for Probiotics and Prebiotics (ISAPP) consensus statement on the definition and scope of prebiotics. Nat Rev Gastroenterol Hepatol. 2017;14(8):491–502.
- 22. Gibson GR, Roberfroid MB. Dietary modulation of the human colonic microbiota: introducing the concept of prebiotics. J Nutr. 1995;125(6):1401–12.
- 23. Goldenberg JZ, Yap C, Lytvyn L, Lo CK, Beardsley J, Mertz D, et al Probiotics for the prevention of Clostridium difficile-associated diarrhea in adults and children. Cochrane Database Syst Rev. 2017;12:CD006095.
- 24. Gruner D, Paris S, Schwendicke F. Probiotics for managing caries and periodontitis: systematic review and meta-analysis. J Dent. 2016;48:16–25.
- 25. Guyatt GH, Oxman AD, Kunz R, Falck-Ytter Y, Vist GE, Liberati A, et alGRADE Working Group. Going from evidence to recommendations. BMJ. 2008;336(7652):1049–51.
- 26. Hassan H, Lingström P, Carlén A. Plaque pH in caries-free and caries-active young individuals before and after frequent rinses with sucrose and urea solution. Caries Res. 2015;49(1):18–25.
- 27. Hasslöf P, West CE, Videhult FK, Brandelius C, Stecksén-Blicks C. Early intervention with probiotic Lactobacillus paracasei F19 has no long-term effect on caries experience. Caries Res. 2013;47(6):559–65.
- 28. Hatakka K, Savilahti E, Pönkä A, Meurman JH, Poussa T, Näse L, et al Effect of long term consumption of probiotic milk on infections in children attending day care centres: double blind, randomised trial. BMJ. 2001;322(7298):1327.
- 29. He J, Hwang G, Liu Y, Gao L, Kilpatrick-Liverman L, Santarpia P, et al L-arginine modifies the exopolysaccharide matrix and thwarts Streptococcus mutans outgrowth within mixed-species oral biofilms. J Bacteriol. 2016;198(19):2651–61.
- 30. Hedayati-Hajikand T, Lundberg U, Eldh C, Twetman S. Effect of probiotic chewing tablets on early childhood caries—a randomized controlled trial. BMC Oral Health. 2015;15(1):112.
- 31. Hu DY, Yin W, Li X, Feng Y, Zhang YP, Cummins D, et al A clinical investigation of the efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride, as sodium monofluorophosphate in a calcium base, on primary root caries. J Clin Dent. 2013;24 Spec no A:A23–31
- 32. Huang X, Palmer SR, Ahn SJ, Richards VP, Williams ML, Nascimento MM, et al A highly arginolytic Streptococcus species that potently antagonizes Streptococcus mutans. Appl Environ Microbiol. 2016;82(7):2187–201.
- 33. Huang X, Zhang K, Deng M, Exterkate RA, Liu C, Zhou X, et al Effect of arginine on the growth and biofilm formation of oral bacteria. Arch Oral Biol. 2017;82:256–62.
- 34.
- 35. Jørgensen MR, Castiblanco G, Twetman S, Keller MK. Prevention of caries with probiotic bacteria during early childhood. Promising but inconsistent findings. Am J Dent. 2016;29(3):127–31
- 36. Kanapka JA, Kleinberg I. Catabolism of arginine by the mixed bacteria in human salivary sediment under conditions of low and high glucose concentration. Arch Oral Biol. 1983;28(11):1007–15.
- 37. Keller MK, Brandsborg E, Holmstrøm K, Twetman S. Effect of tablets containing probiotic candidate strains on gingival inflammation and composition of the salivary microbiome: a randomised controlled trial. Benef Microbes. 2018;9(3):487–94.
- 38. Keller MK, Nøhr Larsen I, Karlsson I, Twetman S. Effect of tablets containing probiotic bacteria (Lactobacillus reuteri) on early caries lesions in adolescents: a pilot study. Benef Microbes. 2014;5(4):403–7.
- 39. Kesel RG. The effectiveness of dentifrices, mouthwashes, and ammonia-urea compounds in the control of dental caries. J Dent Res. 1948;27(2):244–58.
- 40. Kilian M, Chapple IL, Hannig M, Marsh PD, Meuric V, Pedersen AM, et al The oral microbiome - an update for oral healthcare professionals. Br Dent J. 2016;221(10):657–66.
- 41. Kleinberg I. Effect of urea concentration on human plaque pH levels in situ. Arch Oral Biol. 1967;12(12):1475–84.
- 42. Kleinberg I. A new saliva-based anticaries composition. Dent Today. 1999;18(2):98–103
- 43. Kleinberg I. SensiStat. A new saliva-based composition for simple and effective treatment of dentinal sensitivity pain. Dent Today. 2002;21(12):42–7
- 44. Koopman JE, Hoogenkamp MA, Buijs MJ, Brandt BW, Keijser BJ, Crielaard W, et al Changes in the oral ecosystem induced by the use of 8% arginine toothpaste. Arch Oral Biol. 2017;73:79–87.
- 45. Koopman JE, Röling WF, Buijs MJ, Sissons CH, ten Cate JM, Keijser BJ, et al Stability and resilience of oral microcosms toward acidification and Candida outgrowth by arginine supplementation. Microb Ecol. 2015;69(2):422–33.
- 46. Kraivaphan P, Amornchat C, Triratana T, Mateo LR, Ellwood R, Cummins D, et al Two-year caries clinical study of the efficacy of novel dentifrices containing 1.5% arginine, an insoluble calcium compound and 1,450 ppm fluoride. Caries Res. 2013;47(6):582–90.
- 47. Laleman I, Detailleur V, Slot DE, Slomka V, Quirynen M, Teughels W. Probiotics reduce mutans streptococci counts in humans: a systematic review and meta-analysis. Clin Oral Investig. 2014;18(6):1539–52.
- 48. Laursen RP, Hojsak I. Probiotics for respiratory tract infections in children attending day care centers-a systematic review. Eur J Pediatr. 2018;177(7):979–94.
- 49. Ledder RG, Mistry H, Sreenivasan PK, Humphreys G, McBain AJ. Arginine exposure decreases acidogenesis in long-term oral biofilm microcosms. MSphere. 2017;2(4):2.
- 50. Li J, Huang Z, Mei L, Li G, Li H. Anti-caries effect of arginine-containing formulations in vivo: a systematic review and meta-analysis. Caries Res. 2015a;49(6):606–17.
- 51. Li X, Zhong Y, Jiang X, Hu Deyu, Mateo LR, Morrison BM Jr, et al Randomized clinical trial of the efficacy of dentifrices containing 1.5% arginine, an insoluble calcium compound and 1450 ppm fluoride over two years. J Clin Dent. 2015b;26(1):7–12
- 52. Liu Y, Ren Z, Hwang G, Koo H. Therapeutic strategies targeting cariogenic biofilm microenvironment. Adv Dent Res. 2018;29(1):86–92.
- 53. Liu YL, Nascimento M, Burne RA. Progress toward understanding the contribution of alkali generation in dental biofilms to inhibition of dental caries. Int J Oral Sci. 2012;4(3):135–40.
- 54. López-López A, Camelo-Castillo A, Ferrer MD, Simon-Soro Á, Mira A. Health-Associated Niche Inhabitants as Oral Probiotics: The Case of Streptococcus dentisani. Front Microbiol. 2017;8:379.
- 55. Machiulskiene V, Nyvad B, Baelum V. Caries preventive effect of sugar-substituted chewing gum. Community Dent Oral Epidemiol. 2001;29(4):278–88.
- 56. Marquis RE, Burne RA, Parsons DT, Casiano-Colon AE. Arginine deiminase and alkali generation in plaque. In: Bowen WH, Tabak LA. Cariology for the nineties. UK: University of Rochester Press; 1993. pp. 309–17.
- 57. Marsh PD. Ecological Events in Oral Health and Disease: New Opportunities for Prevention and Disease Control?J Calif Dent Assoc. 2017;45:525–37
- 58. Marsh PD, Moter A, Devine DA. Dental plaque biofilms: communities, conflict and control. Periodontol 2000. 2011;55(1):16–35.
- 59. Martin-Cabezas R, Davideau JL, Tenenbaum H, Huck O. Clinical efficacy of probiotics as an adjunctive therapy to non-surgical periodontal treatment of chronic periodontitis: a systematic review and meta-analysis. J Clin Periodontol. 2016;43(6):520–30.
- 60. Morou-Bermudez E, Elias-Boneta A, Billings RJ, Burne RA, Garcia-Rivas V, Brignoni-Nazario V, et al Urease activity in dental plaque and saliva of children during a three-year study period and its relationship with other caries risk factors. Arch Oral Biol. 2011;56(11):1282–9.
- 61. Morou-Bermudez E, Loza-Herrero MA, Garcia-Rivas V, Suarez-Perez E, Billings RJ. Oral bacterial acid-base metabolism in caries screening: a proof-of-concept study. JDR Clin Trans Res. 2017;2(2):132–41.
- 62. Nascimento MM. Potential uses of arginine in dentistry. Adv Dent Res. 2018;29(1):98–103.
- 63. Nascimento MM, Browngardt C, Xiaohui X, Klepac-Ceraj V, Paster BJ, Burne RA. The effect of arginine on oral biofilm communities. Mol Oral Microbiol. 2014;29(1):45–54.
- 64. Nascimento MM, Gordan VV, Garvan CW, Browngardt CM, Burne RA. Correlations of oral bacterial arginine and urea catabolism with caries experience. Oral Microbiol Immunol. 2009;24(2):89–95.
- 65. Nascimento MM, Liu Y, Kalra R, Perry S, Adewumi A, Xu X, et al Oral arginine metabolism may decrease the risk for dental caries in children. J Dent Res. 2013;92(7):604–8.
- 66. Näse L, Hatakka K, Savilahti E, Saxelin M, Pönkä A, Poussa T, et al Effect of long-term consumption of a probiotic bacterium, Lactobacillus rhamnosus GG, in milk on dental caries and caries risk in children. Caries Res. 2001;35(6):412–20.
- 67. Ohshima T, Kojima Y, Seneviratne CJ, Maeda N. Therapeutic application of synbiotics, a fusion of probiotics and prebiotics, and biogenics as a new concept for oral candida infections: a mini review. Front Microbiol. 2016;7:10.
- 68. Panagakos F, Schiff T, Guignon A: Dentin hypersensitivity: effective treatment with an in-office desensitizing paste containing 8% arginine and calcium carbonate. Am J Dent 2009;22 Spec No A:3a-7a.
- 69. Parwani SR, Parwani RN. Nitric oxide and inflammatory periodontal disease. Gen Dent. 2015;63(2):34–40
- 70. Petersen PE, Hunsrisakhun J, Thearmontree A, Pithpornchaiyakul S, Hintao J, Jürgensen N, et al School-based intervention for improving the oral health of children in southern Thailand. Community Dent Health. 2015;32(1):44–50
- 71. Petersen PE, Razanamihaja N. Carbamide-containing polyol chewing gum and prevention of dental caries in schoolchildren in Madagascar. Int Dent J. 1999;49(4):226–30.
- 72. Petersson LG, Magnusson K, Hakestam U, Baigi A, Twetman S. Reversal of primary root caries lesions after daily intake of milk supplemented with fluoride and probiotic lactobacilli in older adults. Acta Odontol Scand. 2011;69(6):321–7.
- 73. Reid G. Probiotics: definition, scope and mechanisms of action. Best Pract Res Clin Gastroenterol. 2016;30(1):17–25.
- 74. Reyes E, Martin J, Moncada G, Neira M, Palma P, Gordan V, et al Caries-free subjects have high levels of urease and arginine deiminase activity. J Appl Oral Sci. 2014;22(3):235–40.
- 75. Rodríguez G, Ruiz B, Faleiros S, Vistoso A, Marró ML, Sánchez J, et al Probiotic compared with standard milk for high-caries children: a cluster randomized trial. J Dent Res. 2016;95(4):402–7.
- 76. Romani Vestman N, Chen T, Lif Holgerson P, Öhman C, Johansson I. Oral microbiota shift after 12-week supplementation with Lactobacillus reuteri DSM 17938 and PTA 5289; a randomized control trial. PLoS One. 2015;10(5):e0125812.
- 77.
- 78. Sanders ME. Probiotics: definition, sources, selection, and uses. Clin Infect Dis. 2008;46(s2Suppl 2):S58–61.
- 79. Sharma S, Lavender S, Woo J, Guo L, Shi W, Kilpatrick-Liverman L, et al Nanoscale characterization of effect of L-arginine on Streptococcus mutans biofilm adhesion by atomic force microscopy. Microbiology. 2014;160(Pt 7):1466–73.
- 80. Shu M, Morou-Bermudez E, Suárez-Pérez E, Rivera-Miranda C, Browngardt CM, Chen YY, et al The relationship between dental caries status and dental plaque urease activity. Oral Microbiol Immunol. 2007;22(1):61–6.
- 81. Sissons CH, Cutress TW, Hoffman MP, Wakefield JS. A multi-station dental plaque microcosm (artificial mouth) for the study of plaque growth, metabolism, pH, and mineralization. J Dent Res. 1991;70(11):1409–16.
- 82. Sjögren K, Ruben J, Lingström P, Lundberg AB, Birkhed D. Fluoride and urea chewing gums in an intra-oral experimental caries model. Caries Res. 2002;36(1):64–9.
- 83. Slomka V, Hernandez-Sanabria E, Herrero ER, Zaidel L, Bernaerts K, Boon N, et al Nutritional stimulation of commensal oral bacteria suppresses pathogens: the prebiotic concept. J Clin Periodontol. 2017;44(4):344–52.
- 84. Slomka V, Herrero ER, Boon N, Bernaerts K, Trivedi HM, Daep C, et al Oral prebiotics and the influence of environmental conditions in vitro. J Periodontol. 2018;89(6):708–17.
- 85. Smith CA, Higham SM, Smith PW, Verran J. The effect of chewing urea-containing gum on plaque acidogenic and alkaligenic parameters. Caries Res. 2004;38(2):124–9.
- 86. Son S, Mühlemann HR. The effect of human supragingival calculus formation of acetohydroxamic acid. Helv Odontol Acta. 1971;15(2Suppl 7):7
- 87. Souza ML, Cury JA, Tenuta LM, Zhang YP, Mateo LR, Cummins D, et al Comparing the efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride to a dentifrice containing 1450 ppm fluoride alone in the management of primary root caries. J Dent. 2013;41Suppl 2:S35–41.
- 88. Srisilapanan P, Korwanich N, Yin W, Chuensuwonkul C, Mateo LR, Zhang YP, et al Comparison of the efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride to a dentifrice containing 1450 ppm fluoride alone in the management of early coronal caries as assessed using Quantitative Light-induced Fluorescence. J Dent. 2013;41Suppl 2:S29–34.
- 89. Stecksén-Blicks C, Sjöström I, Twetman S. Effect of long-term consumption of milk supplemented with probiotic lactobacilli and fluoride on dental caries and general health in preschool children: a cluster-randomized study. Caries Res. 2009;43(5):374–81.
- 90. Stensson M, Koch G, Coric S, Abrahamsson TR, Jenmalm MC, Birkhed D, et al Oral administration of Lactobacillus reuteri during the first year of life reduces caries prevalence in the primary dentition at 9 years of age. Caries Res. 2014;48(2):111–7.
- 91. Stephan RM. The effect of urea in counteracting the influence of carbohydrates on the pH of dental plaques. J Dent Res. 1943;22(1):63–71.
- 92. Taipale T, Pienihäkkinen K, Alanen P, Jokela J, Söderling E. Administration of Bifidobacterium animalis subsp. lactis BB-12 in early childhood: a post-trial effect on caries occurrence at four years of age. Caries Res. 2013;47(5):364–72.
- 93. Taipale TJ, Pienihäkkinen K, Isolauri E, Jokela JT, Söderling EM. Bifidobacterium animalis subsp. lactis BB-12 in reducing the risk of infections in early childhood. Pediatr Res. 2016;79(1-1):65–9.
- 94. Teanpaisan R, Piwat S, Tianviwat S, Sophatha B, Kampoo T. Effect of long-term consumption of Lactobacillus paracasei SD1 on reducing mutans streptococci and caries risk: a randomized placebo-controlled trial. Dent J (Basel). 2015;3(2):43–54.
- 95. Toiviainen A, Jalasvuori H, Lahti E, Gursoy U, Salminen S, Fontana M, et al Impact of orally administered lozenges with Lactobacillus rhamnosus GG and Bifidobacterium animalis subsp. lactis BB-12 on the number of salivary mutans streptococci, amount of plaque, gingival inflammation and the oral microbiome in healthy adults. Clin Oral Investig. 2015;19(1):77–83.
- 96. Twetman S. Prevention of dental caries as a non-communicable disease. Eur J Oral Sci. 2018;126Suppl 1:19–25.
- 97. Twetman S, Jørgensen M, Keller M. Fifteen years of probiotic therapy in the dental context: what has been achieved?J Calif Dent Assoc. 2017;45:539–45
- 98. Villavicencio J, Villegas LM, Arango MC, Arias S, Triana F. Effects of a food enriched with probiotics on Streptococcus mutans and Lactobacillus spp. salivary counts in preschool children: a cluster randomized trial. J Appl Oral Sci. 2018;26(0):e20170318.
- 99. Wattanarat O, Makeudom A, Sastraruji T, Piwat S, Tianviwat S, Teanpaisan R, et al Enhancement of salivary human neutrophil peptide 1-3 levels by probiotic supplementation. BMC Oral Health. 2015;15(1):19.
- 100. Wijeyeweera RL, Kleinberg I. Acid-base pH curves in vitro with mixtures of pure cultures of human oral microorganisms. Arch Oral Biol. 1989;34(1):55–64.
- 101. Wong L, Sissons CH, Pearce EI, Cutress TW. Calcium phosphate deposition in human dental plaque microcosm biofilms induced by a ureolytic pH-rise procedure. Arch Oral Biol. 2002;47(11):779–90.
- 102. Yin W, Hu DY, Fan X, Feng Y, Zhang YP, Cummins D, et al A clinical investigation using quantitative light-induced fluorescence (QLF) of the anticaries efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride as sodium monofluorophosphate. J Clin Dent. 2013a;24 Spec no A:A15–22
- 103. Yin W, Hu DY, Li X, Fan X, Zhang YP, Pretty IA, et al The anti-caries efficacy of a dentifrice containing 1.5% arginine and 1450 ppm fluoride as sodium monofluorophosphate assessed using Quantitative Light-induced Fluorescence (QLF). J Dent. 2013b ;41Suppl 2:S22–8.
- 104. Yu Y, Wang X, Ge C, Wang B, Cheng C, Gan YH. Effects of rinsing with arginine bicarbonate and urea solutions on initial enamel lesions in situ. Oral Dis. 2017;23(3):353–9.
- 105. Zaura E, Brandt BW, Prodan A, Teixeira de Mattos MJ, Imangaliyev S, Kool J, et al On the ecosystemic network of saliva in healthy young adults. ISME J. 2017;11(5):1218–31.
- 106. Zheng X, He J, Wang L, Zhou S, Peng X, Huang S, et al Ecological effect of arginine on oral microbiota. Sci Rep. 2017;7(1):7206.
- 107. Zuccotti G, Meneghin F, Aceti A, Barone G, Callegari ML, Di Mauro A, et alItalian Society of Neonatology. Probiotics for prevention of atopic diseases in infants: systematic review and meta-analysis. Allergy. 2015;70(11):1356–71.