Introduction
Advances in the prevention, diagnosis, and treatment of dental diseases combined with the aging of the world’s population [Department of Economic and Social Affairs, 2015] have favored the longer retention of natural teeth in the oral cavity. During the aging process, teeth become vulnerable to physiological, behavioral, and biological factors [Toto et al., 1971; Kinney et al., 2003, 2005; Arola and Reprogel, 2005; Nazari et al., 2009; Guiglia et al., 2010; Yahyazadehfar et al., 2014], which can alter the risk of developing oral diseases, including dental caries. Although the behavioral aspects of dental caries are of fundamental importance, in this study, we speculated that intrinsic and morphological changes of the dental structure due to aging can affect its susceptibility to dental caries.
Age is thought to significantly affect the tooth microstructure, with direct consequences on its mechanical and chemical properties. In general, the properties of dental elasticity, durability, fracture resistance, fatigue, and crack propagation deteriorate with age [Kinney et al., 2005; Zheng et al., 2005; Nazari et al., 2009], possibly affecting the susceptibility of aged enamel to demineralization. Early work in this field has focused on the impact of the posteruptive maturation process on susceptibility to caries, suggesting that older enamel is less prone to artificial caries development, with a marked effect shortly after eruption [Kotsanos and Darling, 1991]. While this posteruptive maturation has been recognized as an important protective factor for caries, it is not clear if this effect would extend into older ages, when advanced wear (either physiologic or pathologic) and the presence of wear facets could become factors. It is known that deeper enamel layers present with increasing solubility to acids [Theuns et al., 1986; Shellis, 1996]. Another interrelated aspect that is often neglected is the anatomical location of the tested enamel in the tooth crown, which may reflect a wide spectrum of important posteruptive changes related to the susceptibility of enamel to both demineralization and wear facets [Weatherell et al., 1972; Theuns et al., 1986; Bartlett and Dugmore, 2008]. Previous studies have indicated a gradual increase in fluoride content [Weatherell et al., 1972] and decrease in wear susceptibility following the occlusocervical direction of buccal enamel surfaces [Bartlett and Dugmore, 2008].
In this study, we aimed to explore this often-neglected side of dental caries research with regard to the effects of the estimated dental age, the anatomical location, and the presence of wear facets on the susceptibility of the enamel to this disease. A better understanding of these factors may impact both the establishment of standard protocols for specimen preparation and analysis as a part of in vitro/in situ dental caries simulation studies as well as the further development of clinical protocols for the management of dental caries. We performed a comprehensive analysis of the impact of tooth age, anatomical location, and the presence of wear facets on the susceptibility of enamel to demineralization. Our approach involved the use of extracted human premolars of a wide range of ages as estimated by established forensic methods. Our study hypothesis was that enamel structural changes and wear facets associated with aging would increase the enamel’s susceptibility to develop caries-like lesions.
Materials and Methods
Experimental Design
The dental age of 261 human extracted and deidentified premolars was estimated. Enamel specimens obtained from these teeth were prepared and submitted to an in vitro demineralization model to simulate the development of caries-like lesions on enamel natural surfaces at 3 different locations (the cervical, middle, and occlusal thirds). Natural enamel wear (facets), present in some of the specimens, was recorded and was also considered as an experimental factor in this study. The outcome measures were enamel lesion-integrated mineral concentration loss (ΔZ in µm/g/cm3) and lesion depth (LD in µm) measured at all 3 locations of the specimen by microtomography (micro-CT).
Estimation of Age
Age estimation was performed by a single previously calibrated examiner (intraclass correlation coefficient = 0.92) by forensic methods, according to root maturation. For teeth with an incomplete root apex, the Liversidge and Molleson method was used, whereby the distance between the tip of the buccal cusp and the edge of the developing root is measured. For fully formed teeth, the Bang and Ramm method was used, whereby the age of the tooth is estimated based on the measurement of root dentin translucency.
Sample Preparation
Two hundred and sixty-one human premolars were selected, cleaned, and stored in 0.1% thymol solution. A sample of the buccal enamel of each tooth (4 × 4 × 2.5 mm) was made (Isomet low-speed saw-cutting machine, Buehler, Lake Bluff, IL, USA) and had its pulp surface flattened. The central area of the specimen (4 × 1 mm, in the cervico-occlusal direction) was protected with nail polish and considered as an internal control. Each specimen was fixed on an acrylic block with the bottom and lateral sides protected by a thin layer of sticky wax.
Caries-Like Lesion Formation
Caries-like lesions were induced using the chemical model proposed by Moi et al. [2008]. The specimens were individually immersed (3 mL/mm2) in demineralizing solution (0.1 M acetic acid, 1.28 mM Ca, 0.74 mM Pi, and 0.03 µg F/mL, pH 5.0) for 8 days at 37°C. The solution was kept static and replaced on the fourth day. After lesion formation, the specimens were rinsed with deionized water and stored in a humid environment at 4°C.
3-Dimensional X-Ray Micro-CT
Samples were scanned by 3-dimensional X-ray micro-CT (Skyscan 1,172, Bruker MicroCT, Kontich, Belgium) along with a reference standard (1-mm thick aluminum wire of high purity >99.999%; Sigma-Aldrich, St. Louis, USA) and linear attenuation coefficient of 0.767326/cm. For image acquisition, a 4.88-μm pixel, 100-kV voltage (a peak emission energy of 59 keV; according to the manufacturer), a 100-μA amperage, 180°-rotation, 3-frame average, 0.5°-rotation pitch, and AlCu filter were used. The images were reconstructed and converted to bitmap by nRecon software v1.5.23 with 10-smoothing, 18-ring artifact reduction and 25% beam-hardening. The images were aligned with the DataViewer morphometric visualization software (Bruker MicroCT). One 2-dimensional (2D) image was extracted from each of the 3 studied locations of the specimen, i.e., the occlusal (500 μm from the occlusal side), cervical (500 μm from the cervical side), and middle thirds. From each 2D image, 2 areas (left and right to the internal control) were analyzed, totaling 6 areas analyzed per sample (Fig. 1, sagittal view). The left and right outcomes were averaged and considered for the statistical analysis. The presence of wear facets was determined using 2D images in DataViewer software (Bruker MicroCT). The facets had a flat appearance (Fig. 1c). The anatomical location and the side where the wear facet was observed were tabulated and used in the data analysis.
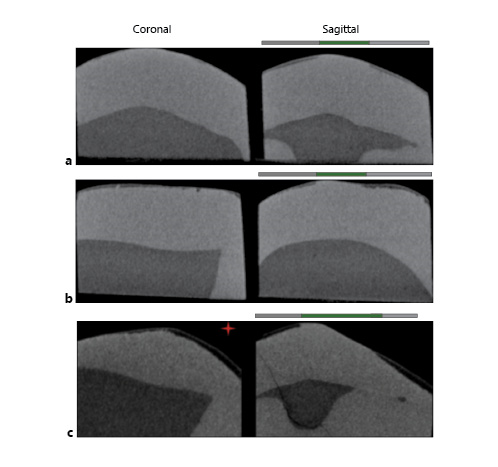
Fig. 1
Micro-CT images. a Sample with young dental age. b Sample with advanced dental age. c Sample with wear facet (red star). Coronal sections show only the decayed area of the samples. Sagittal sections: gray lines signal areas exposed to demineralization. The green line signals the central area of the block that has been protected (internal control). Left side shows the wear facet.
ΔZ and LD analyses were performed using ImageJ software v1.8.0_66 by a previously trained examiner, who was blinded to the estimated dental age. ΔZ consisted of the difference in the mineral concentration profiles of the internal control and experimental enamel, up to a depth of 200 µm from the surface. The internal control and experimental profiles were 150 µm apart from each other. The quantification of the mineral concentration was performed from an equation described previously [Wong et al., 2004], using an enamel mineral empirical formula with a density of 2.99 g/cm3 [Elliott, 1997]. Based on the X-ray emission peak energy of 59 keV, the mass attenuation coefficient for enamel mineral was 0.406434 cm2/g, and the linear attenuation coefficient for aluminum was 0.767326/cm. Areas with mineral concentration <2.63 g/cm3 (corresponding to a mineral volume of 88%) in the control area and enamel blocks with <3 areas for analysis were excluded.
Statistical Analysis
Means of the 2 values obtained in each third of the sample were calculated and considered in the analysis. The effects of aging, specimen position (cervical, middle, or occlusal third), and the presence or absence of wear facets on ΔZ and LD were evaluated using a linear mixed-effects models. This model performs a regression analysis of ΔZ and LD versus tooth age (treated as a continuous variable) while including a random effect for specimen to account for correlation of the 3 locations on the same specimen. The models for ΔZ and LD included an inflection point in the regression line at the age of 30 years based on visual observation of the data. A simulation-based analysis was performed to account for measurement error of the estimated tooth age assessments. The measurement error for tooth age was assumed to have an SD of 10 years. The simulated analysis used 10,000 replications, where a normally distributed random error with mean 0 and SD 10 was added to each tooth age measurement. A 5% significance level was used for all tests. Statistical analyses were performed using SAS v9.4 (SAS Institute Inc., Cary, NC, USA).
Results
Effect of Estimated Tooth Age on ΔZ and LD
The relationship between estimated tooth age and ΔZ was evaluated using the slope of the regression line for tooth age. The slope indicates the increase in ΔZ for each 1-year increase in tooth age. For a 10-year difference in tooth age, the difference in ΔZ is 10 × slope. For example, the slope for ΔZ for the cervical third after the age of 30 years was 0.52, so a 10-year difference in age would lead to a difference of 5.2 in ΔZ, while a 30-year difference in age would lead to a 15.6 difference in ΔZ (Table 1; Fig. 2).
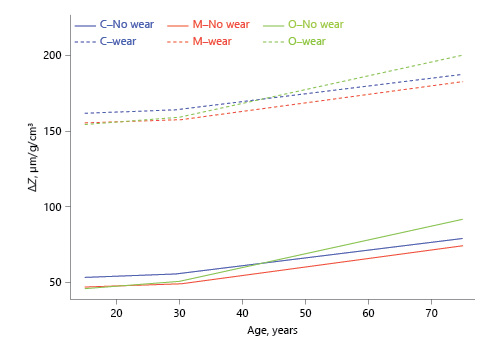
Fig. 2
Association between estimated dental age and lesion integrated mineral concentration loss (ΔZ; inflection point in the regression line at age 30 years, based on visual observation).
For ΔZ, slopes for all 3 locations were significantly >0 before and after the age of 30 years, indicating that ΔZ increased with age for all 3. The slope change at the age of 30 years was also significant at all 3 locations, i.e., ΔZ increased faster with age after 30 years than before 30 years at all 3 locations. Before the age of 30 years, slopes did not differ significantly etween locations, but after the age of 30 years, the slope was higher for the occlusal third than for the other 2.
For LD, before the age of 30 years, only the slope for the occlusal third was significantly >0, indicating that under the age of 30 years, age had no effect on LD for the cervical or middle thirds. The slope before the age of 30 years was significantly higher for the occlusal than for the cervical third. After the age of 30 years, all 3 slopes were significantly >0 and not significantly different from each other (Table 1; Fig. 3).
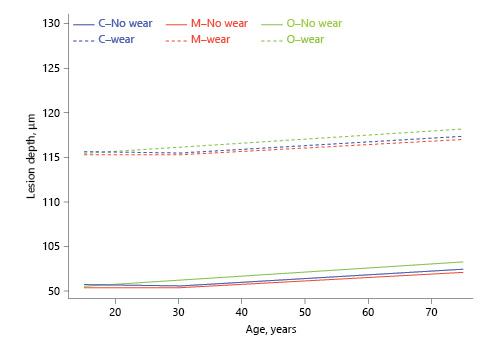
Fig. 3
Association between estimated dental age and lesion depth (inflection point in the regression line at age 30 years, based on visual observation).
Enamel Location Effect
The evaluated locations (cervical, middle, and occlusal) were compared at empirical estimated ages of 15, 30, and 75 years to evaluate the differences throughout the age range considered in this study (Table 2).
The ΔZ in the cervical third was significantly higher than the middle and occlusal thirds for younger teeth (15 and 30 years). Inverse results were observed starting at the age of 40 years and, at the age of 75 years, the ΔZ in the cervical third was still higher than the middle third but lower than the occlusal third. The occlusal and middle thirds were not significantly different at younger ages, but the difference increased with age and became significantly higher in the occlusal third at the age of 75 years.
For LD, the cervical third was significantly larger than the middle third at the age of 15 and 75 years but not at 30 years. The occlusal third was significantly larger than middle and cervical thirds at the age of 30 and 75 years.
Analysis of enamel thickness showed that there was an inverse linear relationship between thickness and age in the middle third (a slope of –0.85, p = 0.015).
Caries-Like Lesion versus Wear Facets
Enamel locations with wear facets showed significantly higher ΔZ and LD than the locations without facets (Fig. 1c). Of the 261 samples, estimated tooth age ranged from 10.9 to 93.4 years. Of the 6 areas analyzed in each specimen, 12.7% of them had wear facets, which were more frequent in the occlusal third of the specimens. Based on linear regression, the ΔZ value for the youngest (10.9 years old) was 10.57 µm/g/cm3 and for the oldest (93.5 years old) it was 197.92 µm/g/cm3.
Discussion
This study contributes to the limited literature on the effect of enamel age on caries susceptibility. Although conceptually ideal, designing a clinical study for this purpose would be challenging, due to the difficulty in obtaining enough dental samples of different ages for the specific laboratory analytical testing, within a reasonable amount of time. To overcome this limitation, we used already extracted and deidentified human premolars. The ages of the teeth were estimated by validated forensic methods, similar to the approach used in our previous study [Algarni et al., 2018]. The controlled conditions of this in vitro study allowed us to observe a significant decrease in the integrated mineral concentration loss and increase in the depth of the caries-like lesions, as functions of a tooth aging (Fig. 1a, b). This contrasts with the previous findings that indicated reduction in the artificial caries susceptibility in older enamel [Kotsanos and Darling, 1991]. Structural, chemical, and physical factors related to enamel can help clarify such differences.
The different study designs and methodologies should also be considered when trying to understand the differences between our study and that of Kotsanos and Darling [1991]. Due to our experimental approach, we were able to examine a larger number of samples, with an even distribution of ages. In the case of older teeth (>30 years old), Kotsanos and Darling [1991] used impacted teeth that had neither been exposed to the oral environment nor subjected to tooth wear. We were also able to quantify the ΔZ and LD. In the previously mentioned study, the depth of carious lesions was evaluated by birefringence of enamel immersed in water. We used micro-CT in this study, so the volume of organic material due to X-ray absorption was negligible, allowing for more accurate measurements. Although transversal microradiography has been considered the gold standard for analysis of the mineral content of carious lesions, the use of micro-CT for mineral quantification, depth measurement, and visualization of carious lesions allows for a precise, high-resolution, and nondestructive approach [He et al., 2011; Shahmoradi and Swain, 2016, 2017]. Quantifying enamel mineral content is one of the most important aspects of studying dental enamel [Angmar et al., 1963]. This study considered a minimum 88% mineral volume value (corresponding to a mineral concentration of 2.63 g/cm3) for sound enamel [Angmar et al., 1963] corrected by a mineral density of 2.99 g/cm3 [Elliott, 1997; Medeiros et al., 2012], so specimens with internal control areas (sound enamel) presenting with lower mineral volume values were excluded. This avoided the use of specimens with intrinsic enamel defects in this study.
The structure of the enamel is subjected to changes with aging. The crystallinity of the apatite atomic unit cell is reduced due to an increased carbonate content over time [Leventouri et al., 2009]. The natural wear process continually removes the surface layer of the enamel and exposes inner layers; this leads to an increasing dissolution gradient towards the enamel-dentin junction [Theuns et al., 1986]. The most significant chemical changes that occur in enamel are related to the mineral exchange between the oral environment and the enamel surface [Carvalho and Lussi, 2017]. Studies show an increased concentration of magnesium [Lappalainen et al., 1981] and carbonate [Leventouri et al., 2009] and a decrease of fluorine [Kidd et al., 1984; Kotsanos and Darling, 1991] in older enamel. The dissolution rate of apatite is proportional to its carbonate content and inversely proportional to the fluorine content [LeGeros and Tung, 1983], which may partially explain a reduction in the acid resistance with tooth aging [Kidd et al., 1984]. Moreover, reduced enamel protein content along with aging [Miake et al., 2016] may also be related to the higher susceptibility of older enamel to demineralization (dental erosion-like lesions) [Lubarsky et al., 2014]. This is possibly explained by the lower permeability caused by decreased organic content in carious enamel [Barbosa de Sousa et al., 2013].
Another important aspect is the loss of the more superficial and acid-resistant layer of enamel by physiological or pathological tooth wear. It is estimated that there is a reduction of approximately one-third of the enamel thickness over a lifetime [Kidd et al., 1984]. In addition to the structural changes mentioned above, the inner enamel layers have different mechanical properties, with the modulus of elasticity and hardness reduced by approximately 16 and 12%, respectively, near the enamel-dentin junction [He et al., 2011; Carvalho and Lussi, 2015]. These factors also explain the increase in the susceptibility of enamel to undergo demineralization that we observed in this study, since older enamel tended to be thinner, as shown by our results. Similarly, the increased susceptibility of enamel located on the occlusal third compared to the cervical and middle thirds may have been due to both the decrease in fluoride content at this location and the surface wear that occurs with advancing age [Weatherell et al., 1972; Bartlett and Dugmore, 2008].
Despite the advantages of our study approach, some methodological limitations should be acknowledged. The extracted teeth used were deidentified; there was thus no demographic or oral health information about the donors which could have potentially impacted the results. The estimation of dental age by the forensic methods used had an error of approximately ±10 years. The results should therefore be interpreted with caution with a focus on the observed trends. Despite being aligned to the clinical anatomical location of the crown, the different locations studied (occlusal, middle, and cervical thirds) refer to the site determined in the specimen and may not represent them exactly. In fact, the differences in susceptibility observed in this study due to the enamel location may be even more pronounced in the teeth, since the enamel specimens were prepared from the middle area of the crown. Finally, our findings have a very important application in future in vitro/in situ studies involving enamel demineralization (i.e., caries and dental erosion simulation models), which must clearly account for the tooth age, either by collecting data on the donors or by using tooth age estimation models as done in this study. Another important aspect is the standardization and identification of the coronal location of the tooth used for the preparation and analyses of enamel specimens.
Considering the inherent limitations to this study, we conclude that the susceptibility of enamel to develop caries-like lesions increased with the increase in estimated dental age, and this was modulated by the anatomical location of the enamel and increased in the presence of enamel wear facets.
Acknowledgement
This research was part of Dr. Barreto de Olivera’s dissertation to be submitted in partial fulfilment of her MSD degree in Dental Sciences at the Federal University of Paraiba, Brazil.
Statement of Ethics
This study was reviewed and approved by the local Institutional Review Board (Indiana University Purdue University of Indianapolis, process NSO 911-07).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
Dr. Barreto de Oliveira received a scholarship from the Brazilian Government, through the Coordination for the Improvement of Higher Education Personnel (CAPES) (Process 1757972). This project was supported by the Oral Health Research Institute, Indiana University School of Dentistry.
Author Contributions
R.D.B.O., A.T.H., and F.B.S. contributed to the conception and design of the study. R.D.B.O. and A.A.A. conducted the laboratory phase. G.J.E. conducted statistical analysis. All authors interpreted the results, drafted the manuscript, and approved its final version.
References
- 1. Algarni AA, Ungar PS, Lippert F, Martínez-Mier EA, Eckert GJ, González-Cabezas C, et al Trend-analysis of dental hard-tissue conditions as function of tooth age. J Dent. 2018;74:107–12.
- 2. Angmar B, Carlström D, Glas JE. Studies on the ultrastructure of dental enamel. IV. The mineralization of normal human enamel. J Ultrastruct Res. 1963;8(1-2):12–23.
- 3. Arola D, Reprogel RK. Effects of aging on the mechanical behavior of human dentin. Biomaterials. 2005;26(18):4051–61.
- 4. Barbosa de Sousa F, Dias Soares J, Sampaio Vianna S. Natural enamel caries: a comparative histological study on biochemical volumes. Caries Res. 2013;47(3):183–92.
- 5. Bartlett D, Dugmore C. Pathological or physiological erosion—is there a relationship to age?Clin Oral Investig. 2008;12(S1Suppl 1):S27–31.
- 6. Carvalho TS, Lussi A. Age-related morphological, histological and functional changes in teeth. J Oral Rehabil. 2017;44(4):291–8.
- 7. Carvalho TS, Lussi A. Susceptibility of enamel to initial erosion in relation to tooth type, tooth surface and enamel depth. Caries Res. 2015;49(2):109–15.
- 8. De Medeiros RC, Soares JD, De Sousa FB. Natural enamel caries in polarized light microscopy: differences in histopathological features derived from a qualitative versus a quantitative approach to interpret enamel birefringence. J Microsc. 2012;246(2):177–89.
- 9. Department of Economic and Social Affairs: World Population Ageing United Nations, 2015.
- 10. Elliott JC. Structure, crystal chemistry and density of enamel apatites. Ciba Found Symp. 1997;205:54–67.
- 11. Guiglia R, Musciotto A, Compilato D, Procaccini M, Lo Russo L, Ciavarella D, et al Aging and oral health: effects in hard and soft tissues. Curr Pharm Des. 2010;16(6):619–30.
- 12. He B, Huang S, Zhang C, Jing J, Hao Y, Xiao L, et al Mineral densities and elemental content in different layers of healthy human enamel with varying teeth age. Arch Oral Biol. 2011;56(10):997–1004.
- 13. Kidd EA, Richards A, Thylstrup A, Fejerskov O. The susceptibility of ‘young’ and ‘old’ human enamel to artificial caries in vitro. Caries Res. 1984;18(3):226–30.
- 14. Kinney JH, Marshall SJ, Marshall GW. The mechanical properties of human dentin: a critical review and re-evaluation of the dental literature. Crit Rev Oral Biol Med. 2003;14(1):13–29.
- 15. Kinney JH, Nalla RK, Pople JA, Breunig TM, Ritchie RO. Age-related transparent root dentin: mineral concentration, crystallite size, and mechanical properties. Biomaterials. 2005;26(16):3363–76.
- 16. Kotsanos N, Darling AI. Influence of posteruptive age of enamel on its susceptibility to artificial caries. Caries Res. 1991;25(4):241–50.
- 17. Lappalainen R, Knuuttila M, Salminen R. The concentrations of Zn and Mg in human enamel and dentine related to age and their concentrations in the soil. Arch Oral Biol. 1981;26(1):1–6.
- 18. LeGeros RZ, Tung MS. Chemical stability of carbonate- and fluoride-containing apatites. Caries Res. 1983;17(5):419–29.
- 19. Leventouri T, Antonakos A, Kyriacou A, Venturelli R, Liarokapis E, Perdikatsis V. Crystal structure studies of human dental apatite as a function of age. Int J Biomater. 2009;2009:698547.
- 20. Lubarsky GV, Lemoine P, Meenan BJ, Deb S, Mutreja I, Carolan P, et al Enamel proteins mitigate mechanical and structural degradations in mature human enamel during acid attack. Mater Res Express. 2014;2(2):1–20.
- 21. Miake Y, Tsutsui S, Eshita Y. Variation in the color of Japanese teeth and structural changes in enamel rod sheath associated with age. J Hard Tissue Biol. 2016;25(2):131–6.
- 22. Moi GP, Tenuta LM, Cury JA. Anticaries potential of a fluoride mouthrinse evaluated in vitro by validated protocols. Braz Dent J. 2008;19(2):91–6.
- 23. Nazari A, Bajaj D, Zhang D, Romberg E, Arola D. Aging and the reduction in fracture toughness of human dentin. J Mech Behav Biomed Mater. 2009;2(5):550–9.
- 24. Shahmoradi M, Swain MV. Micro-CT analysis of naturally arrested brown spot enamel lesions. J Dent. 2017;56:105–11.
- 25. Shahmoradi M, Swain MV. Quantitative characterization and micro-CT mineral mapping of natural fissural enamel lesions. J Dent. 2016;46:23–9.
- 26. Shellis RP. A scanning electron-microscopic study of solubility variations in human enamel and dentine. Arch Oral Biol. 1996;41(5):473–84.
- 27. Theuns HM, Driessens FC, van Dijk JW, Groeneveld A. Experimental evidence for a gradient in the solubility and in the rate of dissolution of human enamel. Caries Res. 1986;20(1):24–31.
- 28. Toto PD, Kastelic EF, Duyvejonck KJ, Rapp GW. Effect of age on water content in human teeth. J Dent Res. 1971;50(5):1284–5.
- 29. Weatherell JA, Robinson C, Hallsworth AS. Changes in the fluoride concentration of the labial enamel surface with age. Caries Res. 1972;6(4):312–24.
- 30. Wong FS, Anderson P, Fan H, Davis GR. X-ray microtomographic study of mineral concentration distribution in deciduous enamel. Arch Oral Biol. 2004;49(11):937–44.
- 31. Yahyazadehfar M, Ivancik J, Majd H, An B, Zhang D, Arola D. On the Mechanics of Fatigue and Fracture in Teeth. Appl Mech Rev. 2014;66(3):0308031–3080319.
- 32. Zheng L, Nakajima M, Higashi T, Foxton RM, Tagami J. Hardness and Young’s modulus of transparent dentin associated with aging and carious disease. Dent Mater J. 2005;24(4):648–53.