Tooth erosion is recognized as a multifactorial condition in which salivary flow rate and saliva components might impact the erosion initiation and progression and can be considered as predisposing factors [Järvinen et al., 1991; Meurman and ten Cate, 1996; Piangpach et al., 2009; Ramsay et al., 2015]. It seems to be biologically plausible that a reduced saliva flow rate might be associated with an increased risk for erosion [Jensdottir et al., 2013; Lieshout and Bots, 2014], since several protective functions of saliva, such as the neutralizing potential or the potential of pellicle formation might depend on salivary flow rate [Tenovuo, 1997; Hara and Zero, 2014]. However, the information in the literature on the relevance of the saliva flow rate in this context is contradictory. Whilst some studies found a relation to salivary flow and buffering capacity [O'Sullivan and Curzon, 2000], other studies reported no relation between erosion and any basic saliva parameter [Järvinen et al., 1991; Meurman and ten Cate, 1996; Johansson et al., 2002; Schlueter et al., 2012]. Even if the impact of changes in saliva flow rate on erosion initiation or progression is still under debate, it could be that changes in saliva flow impact the efficacy of oral health care products by changing interactions between tooth surface, protein coverage of tooth surface (pellicle) and active agents.
To maintain oral health, commonly, sodium fluoride (NaF)-containing toothpastes are recommended; however, their effects to prevent and treat tooth erosion are limited [Vieira et al., 2005; Huysmans et al., 2011; West et al., 2015; Hove et al., 2015]. Other fluorides turned out to be more effective. The best results can be achieved by the application of polyvalent metal cations, such as stannous ions containing fluoride preparations [Ganss et al., 2011, 2012; Hove et al., 2014; Algarni et al., 2015]. A further increase in efficacy can be achieved if novel technologies, such as the addition of biopolymers like chitosan to F/Sn preparations, are used [Lussi and Carvalho, 2015] both under in vitro [Ganss et al., 2012; Carvalho and Lussi, 2014; Pini et al., 2016] and under in situ conditions [Schlueter et al., 2013, 2014]. Nevertheless, even if a good effect of the mentioned preparations has been shown under in situ conditions with healthy volunteers showing a normal saliva flow rate, nothing is known about the impact of saliva flow rate reduction (hyposalivatory conditions) on the efficacy of the erosion-inhibiting agents stannous ions, fluoride and chitosan.
Therefore, the aim of this in situ pilot study was to assess the protective effect of different experimental toothpastes containing F/Sn in comparison to experimental NaF or placebo toothpastes against initial enamel erosion in participants with normal and low stimulated salivary flow rates. Moreover, the interaction between a salivary parameter (flow rate) and active ingredients of toothpastes were statistically evaluated.
Materials and Methods
Volunteers and Ethical Aspects
The study was conducted according to the Declaration of Helsinki and approved by the local ethic committee in research (process No. 010/2014). The report of the study follows the CONSORT statement [Schulz et al., 2010]. The study was conducted in the Dental Clinic and Oral Medicine Clinic (Orocentro) of Piracicaba Dental School, University of Campinas, Brazil.
All participants signed a written informed consent prior to screening. Twenty volunteers were selected: 10 with low and another 10 with normal salivary flow. The groups were matched according to gender: 6 males and 4 females in each group. The participants with reduced flow (45-65 years old) underwent head and neck radiotherapy during cancer treatment at least 2 years before the study. The inclusion criterion for those participants was reduced salivary flow rate (<0.8 mL/min in stimulated flow rate) [Dawes, 2008]. For the other participants with normal flow rate (25-35 years old), the inclusion criterion was stimulated flow rate >1.0 mL/min. All other inclusion criteria had to be fulfilled by all participants: no systemic disorders, healthy oral conditions (absence of caries, periodontal disease, or erosion), no use of orthodontic appliance, nonsmoking, not pregnant or breast-feeding, and no antibiotic use within a period of 2 months prior to the beginning of study. Flow rate was checked according to the description below for all participants before inclusion into the study. None of the participants took any medication with an impact on salivary flow rate, among them medications such as antidepressants, anxiolytics, antihypertensives or antiallergics.
Experimental Design
The study was planned and conducted as a single-center, crossover, randomized and double-blinded in situ study with 2 additional in vitro procedures (see also flow chart, Fig. 1). After preparation specimens underwent an in vitro erosive demineralization; next, the participants wore specimens in situ. Within this period, the specimens were treated intraorally with the test toothpastes and were exposed to saliva. After this phase a second in vitro phase was performed. Analyses of specimens were made at baseline (BL), after the first in vitro demineralization phase (D1), after the in situ phase and after the second in vitro demineralization phase (D2).
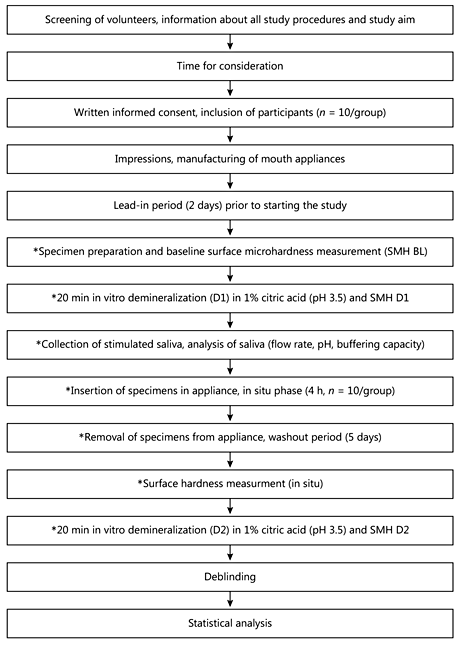
Fig. 1
Flow chart of study procedures. The procedures marked by an asterisk were performed for each toothpaste (crossover design); the order of toothpastes was randomly assigned. Prior to the use of each new preparation, a washout phase (5 days) was included.
Preparation of Specimens
A total of 320 enamel specimens (4 × 4 mm) from bovine incisors and with surface microhardness between 310 and 360 kg/mm2 were used for quantitative analysis. The bovine incisors were previously stored at least for 1 month in 0.1% thymol solution [Ingram et al., 1997; Amaechi et al., 1998], and specimens were obtained using a precision saw (Isomet 1000; Buehler, IL, USA) and a diamond disk (Buehler). The enamel surface was planed and flattened using silicon carbide papers (until #4000), felts, and diamond paste (1 µm) (all from Buehler) in a polishing machine, under water cooling (Arotec, São Paulo, SP, Brazil). Between each polishing step, the blocks were sonicated to remove debris in distilled water (15 min). The specimens were randomly assigned to the 2 experimental groups (normal and low salivary flow rate), and were further divided into 4 subgroups (toothpastes) after the first in vitro phase (D1). Additional specimens were included to be qualitatively analyzed by scanning electron microscopy (SEM). All specimens were stored in 100% humidity until use.
In vitro Erosive Challenge
The erosive agent was a citric acid solution (1%, pH 3.5, citric acid monohydrate >99.5% PA; Merck, Darmstadt, Germany) freshly prepared before each treatment. The specimens were fixed in a holder and immersed in 40 mL of the citric acid for 20 min at room temperature [Hara et al., 2009] and under agitation (100 rpm). After this treatment (demineralization 1, D1), the specimens were gently rinsed with tap water for 2 min [Hara et al., 2009]. This erosive treatment had been previously determined in a pilot study to cause a reduction of enamel surface microhardness by around 30%. After the in situ phase (in situ), a second demineralization (D2) was performed in the same way as D1.
In situ Phase
Before in situ procedures in each experimental phase, stimulated saliva was collected from each participant, resulting in a total of 4 collections per participants during participation in the study. They were instructed to chew unflavored parafilm (Parafilm M, Pechiney Plastic Packaging Company, Chicago, IL, USA) and dispense the saliva in a falcon tube (15 mL) until the collection of 5 mL of saliva; the time until collection was measured. As a longer exposure to the air could impair the analysis of the salivary parameters, the falcon tubes used for the patients were given to them inside a cup with ice and the patients were instructed to keep the tubes closed and to open them just at the moment to dispense saliva. Right after the collection, the tubes were put closed in a box with ice, and pH and buffering capacity of stimulated saliva were analyzed. The collection of saliva was performed always at the same time at least with 1 h of fasting and after oral hygiene.
At each in situ phase, the participants wore custom-made acrylic palatal appliances. Four specimens were mounted somewhat below the appliance surface in order to avoid any contact between the specimens' surface and the tongue, however, not too deep in order to allow sufficient brushing. After the collection of saliva, participants were instructed to wear their palatal appliance for 5 min to allow the specimens to equilibrate with oral fluids. Afterwards, the participants received one soft-bristle toothbrush (Curaprox-Curaden Swiss GmbH, Kriens, Switzerland) loaded with the assigned toothpaste (1.5 g per phase and participant). The participants were instructed to brush the buccal surface of their teeth for 25 s without contact to the specimens, to create a mixture of toothpaste/saliva (slurry). After this, they remained with the appliances in position for 2 min to enable the contact of the specimens with the slurry. Then, the appliance was removed, and 1 researcher (N.I.P.P.) brushed the specimen's surfaces with the same toothbrush used by the participant for 20 s (1 stroke/s) [Stenhagen et al., 2014]. The brushing procedure was always performed by the same researcher. The researcher was previously calibrated, in particular regarding load, brushing velocity, and duration. Velocity and duration were trained by using a stop watch, load was trained by using the respective load on an electronic scale. A new toothbrush was used per participant and experimental phase. After brushing, the participants reinserted the appliance, gently rinsed the mouth with 15 mL of water for 10 s and spit out. Appliances were worn intraorally for 4 h [Hara et al., 2009]. After this period, the specimens were removed and stored at 100% humidity in individual vials at 37°C until surface microhardness measurement. The same procedures were repeated in the subsequent phases, changing the toothpaste used according to the crossover design and randomization list.
During wearing appliances participants were instructed not to eat, they were only allowed to drink water. Before starting the study and between each phase, a lead-in period of 2 days and washout period of 5 days were planned. During the washout, the participants were instructed to use standard nonfluoridated toothpaste.
Toothpastes
Four experimental toothpastes were tested, with the same composition except for the active agent. All experimental toothpastes were formulated using the same basic composition (Drogal Pharmaceutics, Piracicaba, SP, Brazil): glycerin, silica, carboxymethyl cellulose, water, methyl paraben, saccharine, titanium dioxide, sodium lauryl sulfate, mint oil. Toothpastes were always freshly prepared immediately before each experimental phase by adding the respective amount of active agents to the basic formula: placebo, no fluoride, no stannous ions (pH 6.9); sodium fluoride (NaF: 1,450 ppm F-, pH 7.0); stannous fluoride and sodium fluoride (SnF/NaF; SnF2: 1,100 ppm F-, 1,090 ppm Sn2; NaF: 350 ppm F-, pH 4.3) and the combination of sodium fluoride, stannous chloride and chitosan (NaF/Sn/Ch, NaF: 1,450 ppm F-, SnCl2: 3,500 ppm Sn2+, 0.5% Ch, pH 5.0). All compounds were purchased from Sigma-Aldrich (Saint Louis, MO, USA).
Measurement Methods
Microhardness Analysis
The enamel surface microhardness (SMH) was measured at BL, after D1, after salivary exposure (in situ) and after D2. A Knoop indenter, loaded with 50 g with an indentation time of 5 s in a microhardness tester (HMV-2000 Shimadzu, Tokyo, Japan) was used. Five indentations, 100 µm apart, were made in the center of the enamel surface, and the mean value of the measured points was calculated. From the SMH means obtained, 4 variables were calculated [Carvalho and Lussi, 2014; Stenhagen et al., 2014; Creeth et al., 2015]:
- Initial SMH loss after D1 expressed as percent of initial hardness
%SMHinitial = 100 × [(SMH_D1 - SMH_BL)/SMH_BL]
- Total or after in situ SMH loss considers the exposure to saliva and the effect of antierosive agents
%SMHtotal = 100 × [(SMH_in situ - SMH_BL)/SMH_BL])
- Residual SMH loss represents the effect of brushing, saliva and active agents on changes of enamel SMH
%RHL = (-100 × [(SMH_D1 - SMH_in situ)/(SMH_D1 - SMH_BL)]) - 100
- Relative erosion resistance assesses the behavior of treated enamel facing new erosive challenges, indicating its acquired acid resistance
%RER = 100 × [(SMH_D1 - SMH_D2)/(SMH_D1 - SMH_BL)]
The value per specimen was calculated as the mean of the 5 values measured at 1 time point. From the specimens per participant the mean was calculated per time point. Therefore, each participant was considered as an experimental unit (the entire sample was n = 20). The calculations of %SMHinitial, %SMHtotal, %RHL and %RER were performed using the values for each participant and treatment.
Analysis of Salivary Parameters
From the stimulated saliva collected, the volume was measured and the flow rate (mL/min) was calculated [Moritzuka et al., 2006]. Besides, saliva pH was measured directly using a pH meter (Orion 290A+, São Paulo, Brazil) previously calibrated. The buffering capacity was assessed in accordance with Ericsson [1959]. For this analysis, 0.5 mL of stimulated saliva was added to 1.5 mL of 0.005 M hydrochloric acid, the mixture was agitated for 30 s, and after letting it rest for 5 min for liberation of carbonic acid, the pH was measured.
Scanning Electron Microscopy
The additionally analyzed specimens were treated just until the in situ phase, and afterwards, they were submitted to SEM analysis. Four specimens for each group concerning toothpaste and salivary flow were randomly selected and sputtered with gold (90 s, Baltec, MED/JEOL Ltd., Tokyo, Japan) to be evaluated under microscopy (JSM 6510 - JEOL Ltd., Tokyo, Japan). Representative areas were investigated using an original magnification of ×3,000.
Sample Size Calculation
Sample size was based on feasibility of the study. Recruiting of persons with extremely low saliva flow is limited; therefore it was decided to include 10 participants per group. This number was based on different studies in the literature [Hara et al., 2009; Wiegand et al., 2010; Hove et al., 2014].
Responsibilities, Randomization, and Blinding
Two study directors were responsible for study design, randomization and blinding procedures, and due realization of the study (D.A.N.L.L. and F.H.B.A.). All investigators were involved in the recruitment selection of the participants. One investigator (N.I.P.P.) performed all clinical procedures and specimen analyses, while all procedures for study preparation were performed by a “technical” investigator (D.S.). The order of using the toothpastes in the crossover design was defined by a randomization list. According to the list the manufacturer of the toothpastes and 1 study director (D.A.N.L.L.) coded all toothpaste containers with numbers, which were allocated to the participants. The study was blinded for the participants and the clinical investigator (N.I.P.P.). All individuals' specimens were also coded with the respective numbers by the technical investigator (D.S.) and were randomly distributed between the 4 experimental phases/toothpastes. The clinical investigator (N.I.P.P.), who performed the in situ procedures, the brushing of the samples, and the microhardness measurements, was blinded in relation to which toothpaste was used for each participant (during the in situ procedures) and in relation to from whom the specimen stemmed that was analyzed during the microhardness test. Deblinding was performed after analysis of the last specimen. No interim analysis was planned or performed.
Statistical Analysis
The statistical analysis was performed using SPSS 23 for Windows (Armonk, NY, USA). Statistics have been performed for measured surface microhardness values only for BL. All other statistics have been performed for the calculated values %SMHinitial, %SMHtotal, %RHL, and %RER.
The Kolmogorov-Smirnov test was used to check for normal distribution (no significant deviation). Comparison of toothpastes within 1 group (normal and low salivary flow rate) was performed with an ANOVA and the Tamhane post hoc test (significant deviation from the homogeneity of variances, Levene test). Comparison of groups (normal and low salivary flow rate) was performed with t tests for independent data including a Bonferroni adjustment (level of significance 0.0125).
To analyze whether interactions between factors “salivary flow rate” and “toothpaste” exist, a factorial ANOVA (GLM 3, test of between-subject effects) was performed. The level of significance was, if not otherwise stated, 0.05.
Results
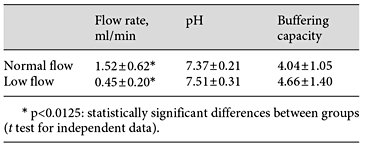
All volunteers completed the study, and none of them reported adverse effects due to the toothpastes or procedures applied. Salivary flow rate of each participant as well as pH and buffering capacity values are given in Table 1. There was a significant difference between both groups for flow rate but not for the other parameters.
Figure 2 shows the SMH values for the different groups and time points tested. No significant differences were found between groups regarding initial SMH (BL). These values were used as the basis for the calculation of the parameters under evaluation: %SMH, %RHL, and %RER.
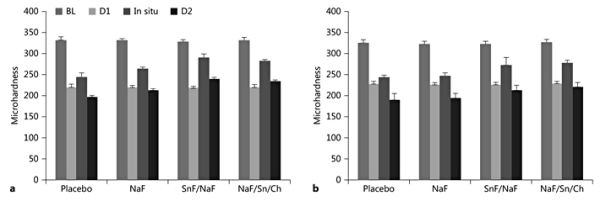
Fig. 2
SMH results for the different toothpastes and groups of study. a Normal flow. b Low flow.
Table 2 presents the calculated values after D1 (%SMHinitial), after in situ phase (%SMHtotal) as well as %RHL and %RER.
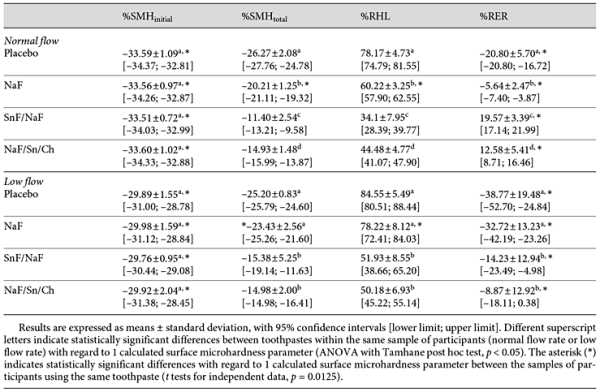
Regarding normal flow rate, best performance was found for SnF/NaF for %SMHtotal, %RHL, and %RER, followed by NaF/Sn/Ch, NaF and placebo, with statistical difference between each of them (p < 0.05). In case of low flow, SnF/NaF and NaF/Sn/Ch presented the best result for all parameters, without significant differences between both Sn preparations. NaF and placebo were comparable with no statistically significant difference between them. Comparing the flow rate conditions (normal and low), differences for %SMHtotal and for %RHL after application of NaF and for %RER in case of all preparations were found (p = 0.0125), with a better performance under normal salivary flow conditions. The resistance was distinctly lower in case of low flow rate; however, the Sn-containing preparations were able to compensate this saliva effect.
Factorial ANOVA showed an impact of saliva flow on study outcome for all calculated values (%SMHinitial, %RHL, %RER, all p < 0.001; %SMHtotal, p < 0.01); an impact of toothpaste type was found for %SMHtotal, %RHL, %RER (all p < 0.001). An effect of interaction between salivary flow rate and toothpaste on study outcome was shown for %SMHtotal (p < 0.01) and for %RHL (p < 0.05), but not for %SMHinitial and %RER.
Representative SEM images are shown in Figure 3. Very similar patterns were observed for placebo and NaF, irrespective of salivary flow. The etching pattern is more evident for these groups in case of low salivary flow. In general, it is possible to recognize some etching pattern with visibility of the orientation and location of the prisms in all groups. However, for SnF/NaF and NaF/Sn/Ch these characteristics were clearly less evident, mainly under normal salivary flow.
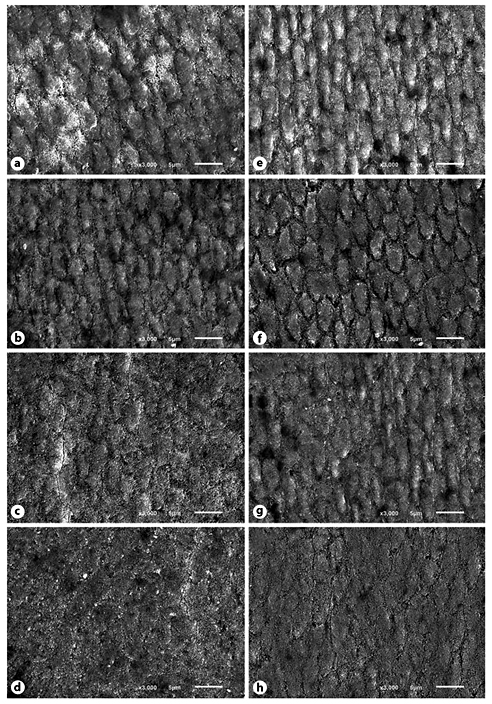
Fig. 3
Representative SEM images of the enamel surfaces after the in situ phase from each toothpaste used under normal (a-d) and hyposalivatory (e-h) conditions. a, e Placebo. b, f NaF. c, g SnF/NaF. d, h NaF/Sn/Ch.
Discussion
The present study aimed to assess the effect of toothpastes containing different active agents on SMH in enamel after a single erosive challenge in a short-term in situ application and ex vivo abrasion and erosion model. Most studies investigated the in situ effect in persons with a normal saliva flow rate [Magalhães et al., 2008; Hara et al., 2009; Wiegand et al., 2010; Schlueter et al., 2013; Hove et al., 2014; Stenhagen et al., 2014], showing the effect under more or less healthy conditions. The novelty of the present study is, however, to investigate the toothpastes' effect in persons with an extremely low flow rate (xerostomia). In persons, who were treated with ionizing radiation in the head-neck area, the salivary glands, which are in the field of radiation, are often injured and therefore the saliva flow rate is severely impaired [Jendsottir et al., 2013; Lieshout and Bots, 2014]. As resting saliva is often in this group virtually not present anymore [Andrews and Griffiths, 2001; Jhan et al., 2008], it was decided to classify according only to the stimulated saliva in order to homogenize this group. As the flow rate was so low in the present study, it was not possible to collect saliva in the appropriate time. Therefore, the saliva was collected according to volume, and the needed time was measured. This increased the blinding of the analysis since an allocation to one group by volume was excluded.
The recruitment of persons with low flow rate is difficult, and motivating them for attendance in a study is often complicated. Therefore, it was planned to use a very short-term in situ model (4 h) [Hara et al., 2009]. The specimens were pre-eroded in order to shorten the in situ period, since it is well known that antierosive agents react much more quickly with erosively altered surfaces than with sound or polished ones [Attin et al., 2000]. In addition, with this model, the very first interaction between active agent, abrasives and acid could be investigated. Even if an intraoral brushing model was to be preferred, the brushing procedure was performed extraorally in order to increase standardization regarding duration, force, and velocity, which is according to current recommendations [Wiegand and Attin, 2011]. For further standardization, only 1 calibrated examiner performed the abrasive procedures, who was extensively trained. Laboratory-prepared toothpastes were chosen to exclude the influence of other ingredients, which can be different between commercially available products.
In both groups results for placebo were as expected for D1, with a reduction of hardness by approximately 30% [Attin et al., 1997; Carvalho and Lussi, 2014]. Treatment with placebo inclusive brushing led to a slight increase in microhardness (%RHL ranged between 78 and 85% in the placebo groups), which is plausible since the brushing procedure removed parts of the outer, partially demineralized enamel. As no remineralization occurs during a 4-h in situ period [Ganss et al., 2007; Lussi et al., 2014], the recovery of hardness in the placebo group was mainly to be attributed to the partial removal of the outer demineralized structures. However, this outer layer was not completely removed by brushing [Kuroiwa et al., 1994] indicated by the still reduced surface hardness confirming results of the literature [Carvalho and Lussi, 2014].
The findings of the erosion resistance calculations are of particular interest, turning the relevance of saliva during the development of erosion out. Interestingly, no differences in pH and buffering capacity were found between both groups, which is in concordance to the literature. Buffering capacity can be reduced during radiotherapy; however, this parameter seems to normalize after a period of 3 months after therapy [Marangoni-Lopes et al., 2016]. Other parameters than pH and buffering capacity might be of more relevance in the present study. The saliva forms the acquired pellicle on the surface, which has substantial thickness after the 4-h in situ phase under normal flow conditions [Hannig et al., 2003], showing a considerable erosion protecting effect [Hara et al., 2006], relevant for the second demineralization (D2). In the low flow rate group, the protective effect seems to be impaired. Qualitative and quantitative effects could play a role. Either the pellicle is thinner due to the minor amount of saliva as well as the minor presence of pellicle-forming proteins in the oral cavity, or the pellicle is differently structured, since the saliva composition changes under certain pathological conditions [Paszynska et al., 2015; Marangoni-Lopes et al., 2016]. Both options could lead to a less protective pellicle. The SEM pictures show indeed differences between the groups, perhaps due to different pellicle effects in each group (Fig. 3).
The results of the active agents' effect in the normal flow rate group are in good concordance to the literature. The sodium fluoride preparation showed a relative increase in efficacy in comparison to placebo by approximately 25% (%SMHtotal), an effect dimension which was previously reported [Schlueter et al., 2009a, b; Huysmans et al., 2011; West et al., 2015]. In contrast, this effect could not be verified under hyposalivatory conditions, under which the effect of NaF was reduced to a dimension similar to placebo for all evaluated parameters. The effect of NaF is apparently modulated by the amount of saliva [Scaramucci et al., 2013]. The efficacy of sodium fluoride-containing preparations is based on the formation of CaF2-like particles on the surface. These precipitates are rapidly dissolved under erosive in vitro conditions; however, they seem to be more stable under in situ conditions due to the impact of saliva and pellicle [Ganss et al., 2007]. Maybe, the effect of the preparation tends more towards in vitro than in situ conditions if the saliva flow is reduced.
The stannous ion-containing preparations showed better efficacy regarding postprocedure in situ relative %SMHtotal, %RHL and %RER under both low and normal flow conditions, confirming protective effects found in previous in situ studies [Hove et al., 2008; Huysmans et al., 2011; Hove et al., 2014; Schlueter et al., 2014; Hove et al., 2015] and in vivo studies [Young et al., 2006] including participants with a normal saliva flow rate. Stannous ions strongly react with hydroxyapatite, forming stable coatings composed of different salts already after a single application, or being incorporated into the enamel after multiple applications creating a more resistant substrate [Babcock et al., 1978; Schlueter et al., 2009a]. In the presence of saliva, the stannous ions interact not only with mineralized tissue, but also with proteins of the acquired pellicle [Huysmans et al., 2011; Rakhmatullina et al., 2013; Bellamy et al., 2014], like mucins and albumins [Algarni et al., 2015]. Such an interaction can lead to an increase in pellicle thickness [Algarni et al., 2015] resulting in a stronger barrier against acids [Buzalaf et al., 2008; Hara et al., 2014]. These findings support the above hypothesized relevance of pellicle thickness. Interestingly, the stannous ions seem to compensate, at least in part, the reduced protective effect of saliva under hyposalivatory conditions. Whether it is due to an increased acid resistance of the dental hard tissue by the interaction of stannous ions with the dental hard tissue, the increase in pellicle thickness or a combination of both cannot be answered with the present study. However, the SEM pictures (Fig. 3) showed an enamel surface with less evidence of an etching pattern for the Sn-based toothpastes, strengthening the effect of this ion on acid solubility of enamel and, when comparing salivary conditions, the suspected pellicle effect.
Chitosan shows notable antierosive as well as antierosive/antiabrasive effects under normal flow conditions if used in combination with stannous and fluoride ions in a toothpaste [Ganss et al., 2012; Schlueter et al., 2014; Carvalho and Lussi, 2014] or mouthrinse [Pini et al., 2016]. Chitosan is a cationic biopolymer able to bind with proteins [Van der Mei et al., 2007], fluoride [Keegan et al., 2012], and other ions from saliva and enamel surface [Lussi & Carvalho, 2015], and abrasives from toothpastes [Lundin et al., 2008]. Furthermore, chitosan is able to increase tin retention on tooth surfaces [Hove et al., 2008] and to reduce friction effects between the dental hard tissue and the abrasive agent by forming layers on the surface [Raviv et al., 2003], explaining the better efficacy of preparations with a chitosan additive. However, in contrast to the literature, the NaF/Sn/Ch formulation was less effective than the SnF/NaF formulation in the present study. Most likely the biopolymer needs several applications to form the protective layer or to increase tin retention for developing the antierosive effect in case of a normal flow pellicle, which was not the case in the single application model of the present study. Interestingly, under low flow conditions, both stannous ion-containing toothpastes showed the same efficacy (%RER), with an in tendency better effect of the chitosan-containing preparation. The chitosan seems to compensate in parts the reduced protective effect of saliva in low flow persons.
Besides all advantages of the study, one has to consider also the shortcomings of the study. The design only investigated the very initial stages of erosion, which is only a small part of the disease. Therefore, further studies with a longer observation period and a multiple application of the preparations should be performed in order to confirm the protective effect of the stannous or even stannous and chitosan-containing preparations in case of hyposalivation. Another shortcoming is the method for quantification. Surface hardness is only appropriate in case of very initial erosion, which should be simulated in the present study. However, it cannot be fully excluded that a surface loss was provoked. Furthermore, it is well stated that the dental surface hardness loss by an erosive attack cannot be recovered. Maybe, a small rehardening by antierosive agents in the presence of saliva can be achieved. However, it could also be that the measurement was performed in precipitates formed on the surface by pellicle, chitosan or minerals. To compensate at least in part this shortcoming, the interpretation of results was mainly focused on the %RER values, since these values seem to be more independent of potentially formed precipitates. A third shortcoming is the sample size, which is quite small, not allowing drawing final conclusions from the results, which have to be confirmed with a larger study.
From the findings of this pilot study, it can be concluded that salivary flow is important to modulate the efficacy of antierosive preparations probably influencing their action mechanism, which deserves more focus in future research. The NaF toothpaste showed a limited effect against enamel erosion in case of normal and even no efficacy in case of low salivary flow. Sn-based toothpastes provided greater antierosive protection, regardless of the salivary flow, being considered the most effective agent to be used against enamel erosion in patients with xerostomia, even if the efficacy is worse than under a normal flow rate. Although final conclusions cannot be drawn from the results of the present pilot study, they indicate that stannous ions are quite effective, and it seems to be worth paying attention to further research in this area to develop strategies for the care of patients suffering from hyposalivation due to different reasons. Chitosan may also be a promising additive, since it showed a slight increase in antierosive action of stannous ion-containing preparations even after a single application. As it was found for stannous ions, showing a better effect after multiple application, it could be that after long-term use the biopolymer is able to compensate the reduced effect of a low flow rate to a greater dimension, which should be elucidated in further studies.
Acknowledgments
The authors would like to thank the Department of Oral Diagnosis (Semiology and Oral Pathology Sections) for their support with the patients who received head and neck radiotherapy. Besides, the authors are grateful to Drogal Pharmaceutics (Piracicaba, SP, Brazil) for their support in formulating the toothpastes used, to Mr. Waldomiro Vieira Filho (Laboratory of Biochemistry of Piracicaba Dental School) and Mrs. Birgit Meier (University of Giessen) for their technical assistance. Financial support was provided by Coordination of Training of Higher Education Graduate/Capes, Brazil.
Ethics Statement
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments, or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Disclosure Statement
The authors declare that they have no conflict of interest.
References
- 1. Algarni AA, Mussi MC, Moffa EB, Lippert F, Zero DT, Siqueira WL: The impact of stannous, fluoride ions and its combination on enamel pellicle proteome and dental erosion prevention. PLoS One 2015;10:e0128196.
- 2. Amaechi BT, Higham SM, Edgar WM. Efficacy of sterilisation methods and their effect on enamel demineralisation. Caries Res 1998;32:441-446.
- 3. Andrews N, Griffiths C: Dental complications of head and neck radiotherapy. Part 1. Aust Dent J 2001;46:88-94.
- 4. Attin T, Dumont B, Buchalla W. Fluoride uptake in carious, eroded and sound enamel after application of a 2,000 ppm fluoride solution. Dtsch Zahnärztl Z 2000;55:455-460.
- 5. Attin T, Koidl U, Buchalla W, Schaller HG, Kielbassa AM, Hellwig E: Correlation of microhardness and wear in differently eroded bovine dental enamel. Arch Oral Biol 1997;42:243-250.
- 6. Babcock FD, King JC, Jordan TH: The reaction of stannous fluoride and hydroxyapatite: J Dent Res 1978;57:933-938.
- 7. Bellamy PG, Harris R, Date RG, Mussett AJS, Manly A, Barker ML, et al: In situ clinical evaluation of a stabilized, stannous fluoride dentifrice. Int Dent J 2014;64(suppl 1):43-50.
- 8. Buzalaf MAR, Hannas AR, Kato MT: Saliva and dental erosion. J Appl Oral Sci 2012;20:493-502.
- 9. Carvalho TS, Lussi A: Combined effect of a fluoride-, stannous- and chitosan-containing toothpaste and stannous-containing rinse on the prevention of initial enamel erosion-abrasion. J Dent 2014;42:450-459.
- 10. Creeth JE, Kelly SA, Martinez-Mier EA, Hara AT, Bosma ML, Butler A, et al: Dose-response effect of fluoride dentifrice on remineralisation and further demineralisation of erosive lesions: a randomized in situ clinical study. J Dent 2015;43:823-831.
- 11. Dawes C. Salivary flow patterns and the health of hard and soft oral tissues. J Am Dent Assoc 2008;139:18S-24S.
- 12. Ericsson Y: Clinical investigation on the salivary buffering action. Acta Odontol Scand 1959;17:131-165.
- 13. Ganss C, Lussi A, Grunau O, Klimek J, Schlueter N: Conventional and anti-erosion fluoride toothpastes: effect on enamel erosion and erosion-abrasion. Caries Res 2011;45:581-589.
- 14. Ganss C, Schlueter N, Friedrich D, Klimek J: Efficacy of waiting periods and topical fluoride treatment on toothbrush abrasion of eroded enamel in situ. Caries Res 2007;41:146-151.
- 15. Ganss C, von Hinckeldey J, Tolle A, Schulze K, Klimek J, Schlueter N: Efficacy of the stannous ion and a biopolymer in toothpastes on enamel erosion/abrasion. J Dent 2012;40:1036-1043.
- 16. Hannig M, Hess NJ, Hoth-Hannig W, De Vrese M: Influence of salivary pellicle formation time on enamel demineralization - an in situ pilot study. Clin Oral Investig 2003;7:158-161.
- 17. Hara AT, Ando M, González-Cabezas C, Cury JA, Serra MC, Zero DT: Protective effect of the dental pellicle against erosive challenges in situ. J Dent Res 2006;85:612-616.
- 18. Hara AT, Kelly SA, González-Cabezas C, Eckert GJ, Barlow AP, Mason SC, et al: Influence of fluoride availability of dentifrices on eroded enamel remineralization in situ. Caries Res 2009;43:57-63.
- 19. Hara AT, Zero BT: The potential of saliva in protecting against dental erosion. Monograph Oral Sci 2014;25:197-205.
- 20. Hove LH, Holme B, Young Z, Tveit AB: The protective effect of TiF4, SnF2 and NaF against erosion-like lesions in situ. Caries Res 2008;42:68-72.
- 21. Hove LH, Stenhagen KR, Holme B, Tveit AB: The protective effect of SnF2 containing toothpaste and solution on enamel surfaces subjected to erosion and abrasion in situ. Eur Arch Paediatr Dent 2014;15:237-243.
- 22. Hove LH, Stenhagen KR, Mulic A, Holme B, Tveit AB: May caries-preventive fluoride have an effect on dental erosive wear? An in situ study. Acta Odontol Scand 2015;73:114-120.
- 23. Huysmans MC, Jager DH, Ruben JL, Unk DE, Klijn CP, Vieira AM: Reduction of erosive wear in situ by stannous fluoride containing toothpaste. Caries Res 2011;45:518-523.
- 24. Ingram GS, Higham SM, Wilkinson SC, Edgar WM: Methods for the sterilisation of dentine (abstract). J Dent Res 1997;76:1070.
- 25. Järvinen VK, Rytömaa JI, Leinonen OP: Risk factor in dental erosion. J Dent Res 1991;70:942-947.
- 26. Jensdottir T, Buchwald C, Nauntofte B, Hansen HS, Bardow A: Saliva in relation to dental erosion before and after radiotherapy. Acta Odontol Scand 2013;71:1008-1013.
- 27. Jhan BC, Reis PM, Miranda EI, Lopes RC, Carvalho AL, Scheper MA, et al: Oral health status of 207 head neck cancer patients before, during and after radiotherapy. Clin Oral Investig 2008;12:19-24.
- 28. Johansson AK, Lingstrom P, Birkhed D: Comparison of factors potentially related to the occurrence of dental erosion in high- and low-erosion groups. Eur J Oral Sci 2002;110:204-211.
- 29. Keegan GM, Smart JD, Ingram MJ, Barnes LM, Burnett GR, Rees GD: Chitosan microparticles for the controlled delivery of fluoride. J Dent 2012;40:229-240.
- 30. Kuroiwa M, Kodaka T, Kuroiwa M, Abe M: Brushing-induced effects with and without a non-fluoride abrasive dentifrice on remineralization of enamel surfaces etched with phosphoric acid. Caries Res 1994;28:309-314.
- 31. Lieshout HFJ, Bots CP: The effect of radiotherapy on dental hard tissue - a systematic review. Clin Oral Investig 2014;18:17-24.
- 32. Lundin M, Macakova L, Dedinaite A, Claesson P: Interactions between chitosan and SDS at a low-charged silica substrate compared to interactions in the bulk - the effect of ionic strength. Langmuir 2008;24:3814-3827.
- 33. Lussi A, Carvalho TS: The future of fluorides and other protective agents in erosion prevention. Caries Res 2015;49(suppl 1):18-29.
- 34. Lussi A, Lussi J, Carvalho TS, Cvikl B: Toothbrushing after an erosive attack: will waiting avoid tooth wear? Eur J Oral Sci 2014;122:353-359.
- 35. Magalhães AC, Rios D, Marthinho CCR, Delbem ACB, Buzalaf MAR, Machado MAAM: The influence of residual salivary fluoride from dentifrice on enamel erosion: an in situ study. Braz Oral Res 2008;22:67-71.
- 36. Marangoni-Lopes L, Rodrigues LP, Mendonça RH, Nobre-Dos Santos M: Radiotherapy changes salivary properties and impacts quality of life of children with Hodgkin disease. Arch Oral Biol 2016;21:99-105.
- 37. Meurman JH, ten Cate HM: Pathogenesis and modifying factors of dental erosion. Eur J Oral Sci 1996;104:199-206.
- 38. Moritzuka M, Kitasaki Y, Burrow MF, Ikeda M, Tagami J, Nomura S: Quantitative assessment for stimulated saliva flow rate and buffering capacity in relation to different ages. J Dent 2006;34:716-720.
- 39. O'Sullivan EA, Curzon ME: Salivary factors affecting dental erosion in children. Caries Res 2000;34:82-87.
- 40. Paszynska E, Schlueter N, Slopien A, Dmitrzak-Weglarz M, Dyszkiewicz-Konwinska M, Hannig C: Salivary enzyme activity in anorexic persons - a controlled clinical trial. Clin Oral Investig 2015;19:1981-1989.
- 41. Piangpach T, Hengtrakool C, Kukiattrakoon B, Kedjarune-Leggat U: The effect of salivary factors on dental erosion in various age groups and tooth surfaces. J Am Dent Assoc 2009;140:1137-1143.
- 42. Pini NI, Lima DA, Lovadino JR, Ganss C, Schlueter N: In vitro efficacy of experimental chitosan-containing solutions as anti-erosive agents in enamel. Caries Res 2016;50:337-345.
- 43. Rakhmatullina E, Beyeler B, Lussi A: Inhibition of enamel erosion by stannous and fluoride containing rinsing solutions. Schweiz Monatsschr Zahnmed 2013;123:296-302.
- 44. Ramsay DS, Rothen M, Scott JM, Cunha-Cruz J: Tooth wear and the role of salivary measures in general practice patients. Clin Oral Investig 2015;19:85-95.
- 45. Raviv U, Giasson S, Kampf N, Gohy JF, Jerome R, Klein R: Lubrication by charged polymers. Nature 2003;425:163-165.
- 46. Scaramucci T, Borges AB, Lippert F, Frank NE, Hara AT: Sodium fluoride effect on erosion-abrasion under hyposalivatory simulating conditions. Arch Oral Biol 2013;58:1457-1463.
- 47. Schlueter N, Ganss C, Pötschke S, Klimek J, Hannig C: Enzyme activities in the oral fluids of patients suffering from bulimia - a controlled clinical trial. Caries Res 2012;46:130-139.
- 48. Schlueter N, Hardt M, Lussi A, Engelmann F, Klimek J, Ganss C: Tin-containing fluoride solutions as anti-erosive agents in enamel: an in vitro tin-uptake, tissue-loss, and scanning electron micrograph study. Eur J Oral Sci 2009a;117:427-434.
- 49. Schlueter N, Klimek J, Ganss C: In vitro efficacy of experimental tin- and fluoride-containing mouth rinses as anti-erosive agents in enamel. J Dent 2009b;37:944-948.
- 50. Schlueter N, Klimek J, Ganss C: Randomized in situ study on the efficacy of a tin/chitosan toothpaste on erosive-abrasive enamel loss. Caries Res 2013;47:574-581.
- 51. Schlueter N, Klimek J, Ganss C: Effect of a chitosan additive to a Sn2+-containing toothpaste on its anti-erosive/anti-abrasive efficacy - a controlled randomized in situ trial. Clin Oral Investig 2014;18:107-115.
- 52. Schulz KF, Altman DG, Moher D; CONSORT Group: CONSORT 2010 statement: updated guidelines for reporting parallel group randomised trials. BMC Med 2010;24:8-18.
- 53. Stenhagen KR, Hove LH, Holme B, Tveit AB: The effect of daily fluoride mouth rinsing on enamel erosive/abrasive wear in situ. Caries Res 2014;47:2-8.
- 54. Tenovuo J: Salivary parameters of relevance for assessing caries activity in individuals and populations. Community Dent Oral Epidemiol 1997;25:82-86.
- 55. Van der Mei HC, Engels E, de Vries J, Dijkstra RJB, Busscher HJ: Chitosan adsorption to salivary pellicles. Eur J Oral Sci 2007;115:303-307.
- 56. Vieira A, Ruben JL, Huysmans MC: Effect of titanium tetrafluoride, amine fluoride and fluoride varnish on enamel erosion in vitro. Caries Res 2005;39:371-379.
- 57. West NX, Seong J, Hellin N, Eynon H, Barker ML, He T: A clinical study to measure anti-erosion properties of a stabilized stannous fluoride dentifrice relative to a sodium fluoride/triclosan dentifrice. Int J Dent Hyg 2017;15:113-119.
- 58. Wiegand A, Attin T: Design of erosion/abrasion studies - insights and rational concepts. Caries Res 2011;45(suppl 1):53-59.
- 59. Wiegand A, Hiestand B, Sener B, Magalhães AC, Ross M, Attin T: Effect of TiF4, ZrF4, HfF4 and AmF on erosion and erosion/abrasion of enamel and dentin in situ. Arch Oral Biol 2010;55:223-228.
- 60. Young A, Thrane PS, Saxegaard E, Jonsk G, Rölla G: Effect of stannous fluoride toothpaste on erosion-like lesions: an in vivo study. Eur J Oral Sci 2006;114:180-183.