Introduction
Stroke is the leading cause of morbidity and the second most common cause of mortality around the globe []. Patients who survived after a stroke or transient ischemic attack have increased risk of unfavorable outcome. Although, a number of individuals survive after stroke, they have to dependent on others for daily activities. The number of patients living with disabilities following stroke is expected to rise further in the future due to prolonged life expectancy with increasing number of aged people []. Therefore, it is important to be able to predict the unfavorable outcome after ischemic stroke with the formal predictions provided by statistical models instead of using the physician’s clinical experience.
Several prognostic scores [-], such as Acute Stroke Registry and Analysis of Lausanne score, Totaled Health Risks in Vascular Events score, have been developed to predict unfavorable functional outcome in patients with acute ischemic stroke. Age and stroke severity of patients have been established as the strongest predictors of unfavorable outcome in the acute phase of stroke [-]. However, these scores for individualized prediction of outcome are limited by the use of dichotomization/categorization of continuous variables such as age and National Institutes of Health Stroke Scale score (NIHSS), for the disadvantage of dichotomization is that it does not make use of within-category information and lead to the loss of information.
Using continuous variables including age and NIHSS, a nomogram is a better visual tool to predict clinical outcomes than aforementioned scores. The nomogram is a graphical statistical instrument that incorporates some variables to develop a continuous scoring system, which reflects the individual and precise risk probability. Nomograms are an important part of modern medical decision making, which has been established and validated in extensive medical applications including surgery, cancer, and myocardial infarction [-].
To the best of our knowledge, there have only been 3 scholarly works carried out on nomograms for individualized prediction of the probability of unfavorable outcome of ischemic stroke in Caucasians (Italian) [-]. The START nomogram was designed to predict outcome after intravenous thrombolysis for stroke [], and another nomogram model was developed in a study including 344 patients who started oral anticoagulants 1–7 days after atrial fibrillation-related stroke onset []. However, no nomograms were found to predict the probability of unfavorable outcome in Chinese patients with ischemic stroke.
The objectives of this study were to develop a nomogram based on the integration of parameters to predict the probability of 3-month unfavorable functional outcome in Chinese acute ischemic stroke patients.
Methods
Study Population
Patients who underwent acute ischemic stroke were identified retrospectively from the Stroke Center of the Nanjing First Hospital (China) Stroke Registry database. Patients were included if they underwent acute ischemic stroke from May 2013 to May 2018. All patients have given their informed consent, and the scientific use of the data obtained from the Nanjing First Hospital Stroke Registry (NFHSR) was approved by the Ethics Committees of Nanjing First Hospital in accord with the Helsinki declaration and internal protocol. All patients with complete clinical, demographic and laboratory data at the time of admission were included in the study. Patients treated with endovascular procedures were excluded from the study. All patients with signs of intracranial hemorrhage on baseline brain computed tomography scan, age < 18 years, lack of 3-month modified Rankin Scale (mRS) score, and NIHSS unknown were excluded from the analysis.
At the time of admission, detailed lists of all clinical parameters, demographic and laboratory characteristics were recorded for each patient. The following data were collected: age, sex, baseline NIHSS score, creatinine, fasting blood glucose, interval from onset to hospital within 4.5 h, medical history such as hypertension, diabetes mellitus, previous valvular heart disease, previous cerebral hemorrhage, and so on. All patients were clinically evaluated 3 months after the acute ischemic stroke by means of the mRS. The primary outcome was unfavorable functional result interpreted as mRS comprised between 3 and 6 (i.e., poor prognosis) at 3 months []. Baseline NIHSS and 3-month mRS were performed by assessors who were trained and certified in the use of these tools.
Statistical Analysis
One-sample Kolmogorov-Smirnov test was used for normality test. Continuous variables were described as median value and interquartile range. Proportions were achieved for categorical variables, dividing the number of events by the total number excluding unknown cases. The various groups were investigated for differences using the Mann-Whitney U-test for continuous variables. Differences between categorical variables were assessed by Fisher’s exact test or the χ2 test.
A COACHS nomogram model was generated to predict the probability of 3-month unfavorable outcome. The COACHS nomogram, an important tool of modern medical decision making, was produced by inputing the predictors into a logistic regression model. The OR and its 95% CI were hence calculated for the variables found to be significantly associated with the primary endpoint in the multivariate analysis. The statistical analysis was carried out using SPSS version 22.0 (IBM Corporation, Armonk, NY, USA), Stata version 13.0 (Stata Corporation, College Station, TX, USA) statistical software, and the statistical software package R, version 3.3.3 (R Development Core Team, Auckland, New Zealand). In order to develop the nomogram, multivariate logistic regression analysis was performed for predicting the probability of unfavorable outcome using a forward stepwise method that included NIHSS score on admission, age, previous valvular heart disease, previous cerebral hemorrhage, fasting blood glucose and creatinine as preestablished variables and all variables with a probability value < 0.2 in the univariate analysis. The best model was selected based on Akaike’s information criterion. Collinearity of variables that entered the multivariate logistic regression analysis was assessed by the variation inflation factors (< 2 being considered nonsignificant) and condition index (< 30 being considered nonsignificant).
The COACHS nomogram enables discrimination of patients with favorable outcome from those with unfavorable outcome, the predictive accuracy of the nomogram model was assessed by calculation of the area under curve (AUC) of the receiver-operating characteristic. Calibration was carried out using a calibration plot, in which the predicted probabilities were plotted against the frequency of the observed unfavorable outcome. The prediction of a well-calibrated model should be mirrored by a 45° diagonal line. Given that all predictive equations tend to be over-fitted to the original sample, the model was internally validated using bootstrap resampling. All tests were two sided, and p < 0.05 was considered statistically significant.
Results
From the NFHSR database, we identified 3,419 patients who underwent acute ischemic stroke from May 2013 to May 2018. Among the 3,419 patients registered in the NFHSR cohort, 979 (28.6%) patients lack 3-month mRS score, 165 (4.8%) patients were excluded for being treated with endovascular procedure, and 257 (7.5%) patients lack of information of endovascular procedure. Additional patients were excluded for NIHSS unknown (n = 146; 4.3%), and a combination of the previous criteria (n = 847; 24.8%). Therefore, the final study population consisted of 1,025 patients (median age 68 years; IQR 60–78 years). Unfavorable outcome (mRS score 3–6) after 3 months from acute ischemic stroke was observed in 343 (33.5%) patients, and within the follow-up period 14 (1.4%) patients died (mRS score = 6).
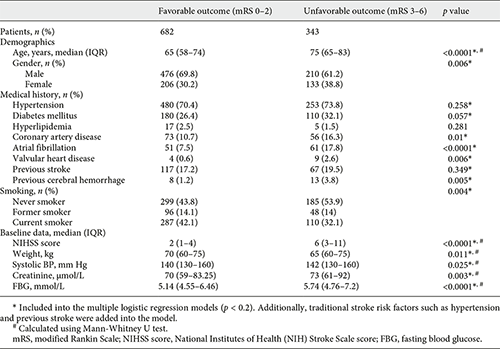
The clinical, demographic and laboratory characteristics of the patients in the favorable outcome cohorts (n = 682) and unfavorable outcome (n = 343) cohorts are shown in Table 1. In addition to age (65 vs. 75; p < 0.0001), NIHSS score on admission (2 vs. 6; p < 0.0001), fasting blood glucose (5.14 vs. 5.74; p < 0.0001), creatinine (70 vs. 73; p = 0.003), gender, coronary artery disease, atrial fibrillation, valvular heart disease, peripheral vascular disease, previous cerebral hemorrhage, smoking, drinking, weight, systolic BP, INR, TG, HbA1c, and post antiplatelet were the other factors associated with unfavorable outcome in the univariate analysis. Due to the family disagreed and patients have contraindication of thrombolysis, about 17% of patients arriving at the hospital from onset within 4.5 h didn’t carry out the thrombolytic therapy. The number of those patients is not significantly different between the outcome groups (p = 0.176).
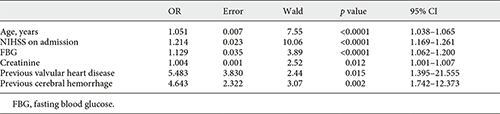
In multivariate analysis, NIHSS score on admission (OR 1.214, 95% CI 1.169–1.261), age (OR 1.051, 95% CI 1.038–1.065), previous valvular heart disease (OR 5.483, 95% CI 1.395–21.555), fasting blood glucose (OR 1.129, 95% CI 1.062–1.200), creatinine (OR 1.004, 95% CI 1.001–1.007), and previous cerebral hemorrhage (OR 4.643, 95% CI 1.742–12.373) were entered into a logistic regression model to construct the COACHS nomogram for prediction of the probability of 3-month negative outcome (Table 2, Fig. 1). No significant statistical collinearity was observed for any of the 6 independent risk factors that entered the multivariate logistic regression analysis. The logistic regression model resulted: Log (p[x]/1–p[x]) = – 6.213 + (0.194 × NIHSS score) + (0.05 × age) + (1.702 × previous valvular heart disease) + (0.121 × fasting blood glucose) + (0.004 × creatinine) + (1.535 × previous cerebral hemorrhage); where p(x) was the probability of 3-month unfavorable outcome.
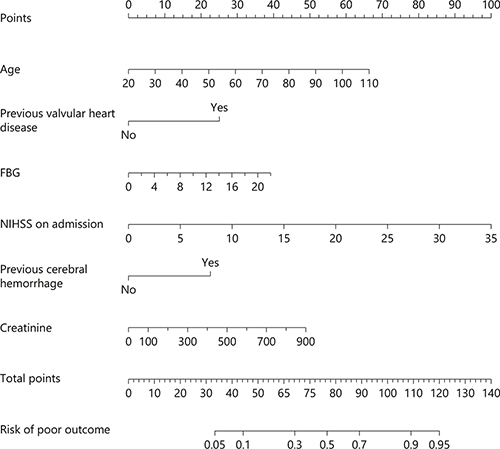
Fig. 1
The nomogram used for predicting 3-month unfavorable outcome after acute ischemic stroke in Chinese patients. The final score (i.e., total points) is calculated as the sum of the individual score of each of the 6 variables included in the nomogram. NIHSS, National Institutes of Health Stroke Scale; FBG, fasting blood glucose.
The nomogram was developed by assigning a graphic initial score to each of the 6 independent prognostic factors with a point range from 0 to 100, which was then summed to create a total score, finally converted into an individual risk of 3-month unfavorable outcome expressed in percentage, thus ranging from 0 to 100%. It was predicted that the higher total score of the nomogram was associated with the higher likelihood of unfavorable outcome, while the lower total score was associated with the lower likelihood of adverse outcome. The AUC-receiver-operating characteristic value of the COACHS nomogram was 0.799 (95% CI 0.770–0.828) in the NFHSR cohort (Fig. 2). The age values exhibited a modest diagnostic accuracy for identifying patients with unfavorable outcome after 3 months, displaying an AUC of 0.682 (95% CI 0.648–0.716; p < 0.0001). The NIHSS scores on admission exhibited a good diagnostic accuracy for identifying patients with adverse outcome after 3 months, displaying an AUC of 0.733 (95% CI 0.700–0.765; p < 0.0001). The total number of patients with a risk probability < 10% was 107/1,025 (10.4%), and only 10 of these had an unfavorable outcome (0.97 sensitivity, 0.14 specificity, 0.91 negative predictive value and 0.36 positive predictive value). The total number of patients with a risk probability < 40% was 728/1,025 (71.0%), 145 of whom had unfavorable outcome (0.58 sensitivity, 0.86 specificity, 0.80 negative predictive value and 0.67 positive predictive value). Finally, the total number of patients with a high-risk probability (i.e., > 80%) was 74/1,025 (7.2%), the vast majority of whom (62/74; 83.8%) had a poor prognosis (0.18 sensitivity, 0.98 specificity, 0.71 negative predictive value and 0.84 positive predictive value).
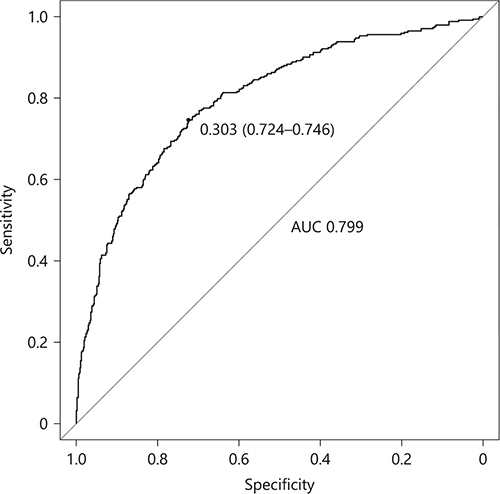
Fig. 2
ROC curve of the nomogram used for predicting 3-month unfavorable outcome after acute ischemic stroke in Chinese patients. AUC, area under curve; ROC, receiver operating characteristic.
The model was internally validated using 1,000 bootstrap samples to calculate the discrimination with accuracy, and the good predictive performance of the nomogram was confirmed, yielding a notable AUC of 0.799 (95% CI 0.769–0.829; p < 0.0001; Fig. 2). The bias-corrected calibration plot for the nomogram model showed the adequate agreement between predictors calculated with the COACHS nomogram and actual unfavorable outcomes at the end of the follow-up period (Fig. 3). Calibration graphic revealed adequate fit of the model predicting the risk of poor prognosis at 3 months. The Hosmer-Lemeshow goodness-of-fit test showed good calibration of the nomogram (p = 0.1376).
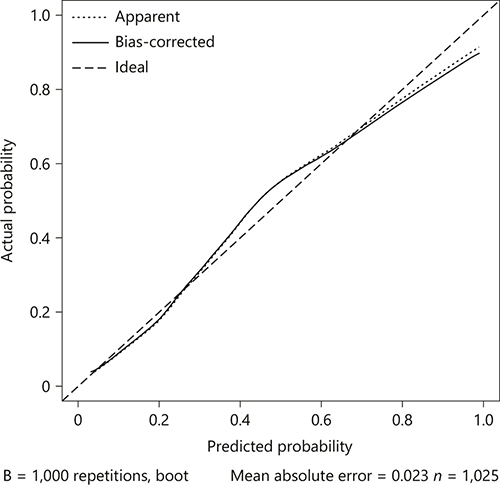
Fig. 3
The calibration plot for the nomogram used for predicting 3-month unfavorable outcome after acute ischemic stroke in Chinese patients. Dashed line is reference line where an ideal nomogram would lie. Dotted line is the performance of nomogram, while the solid line corrects for any bias in nomogram.
Discussion
Unfavorable outcome after ischemic stroke has become a public health problem; therefore, early identification of reliable predictors of unfavorable outcome should be a valuable perspective on precise clinical and care management. Several prognostic scores [-] and some models [, ] have identified some demographic and clinical characteristics that may be used to predict clinical outcome. However, all these scores and models for individualized prediction of outcome are limited by the use of dichotomization/categorization of predictors including NIHSS score and age.
By using continuous variables, such as age and NIHSS score, we are the first to present a visual COACHS nomogram, which is a better tool to predict the probability from 5 to 95% of 3-month unfavorable outcome after ischemic stroke in Chinese patients, as shown in Figure 1. That is, we observed that both NIHSS score on admission (OR 1.214; 95% CI 1.169–1.261) and age (OR 1.051; 95% CI 1.038–1.065) were significant and independent predictors of unfavorable outcome. Moreover, we found that previous valvular heart disease (OR 5.483, 95% CI 1.395–21.555), previous cerebral hemorrhage (OR 4.643, 95% CI 1.742–12.373), fasting blood glucose (OR 1.129, 95% CI 1.062–1.200), and creatinine (OR 1.004, 95% CI 1.001–1.007) were independently associated with adverse outcome. Therefore, the COACHS nomogram is defined as graphical computation instrument that incorporates 6 predictors to predict 3-month unfavorable outcome. In the present study, a > 80% risk limit was deduced from the nomogram. This risk limit was related to a positive predictive value of 0.84. This positive predictive value thereby allowed a more precise prognostication of undesirable functional consequences. Contrary to the nomogram-derived > 80% risk limit, probability risk cutoff of < 10% significantly demonstrated a much more negative prognostic value of 0.91. This negative prognostic value enabled the precise exclusion of the likelihood of developing unfavorable functional consequences. For example, in the present study, the COACHS nomogram designated a chance of approximately 95% probability of having an unfavorable outcome in an 80-year-old (45 points) stroke patient, with a history of valvular heart disease (25 points), NHISS score of 10 (28 points), no previous cerebral hemorrhage (0 point), fasting blood glucose of 6 (10 points) and creatinine level of 300 (16 points), with a total score of 124 points. On the other hand, < 10% probability of adverse consequence was assign to a 40-year-old (15 points), with no history of valvular heart disease (0 points), NHISS score of 5 (10 points), no previous cerebral hemorrhage (0 point), fasting blood glucose of 6 (10 points), and creatinine level of 100 (5 point), with a total score of 40 points.
The main findings of COACHS nomogram were that fasting blood glucose, creatinine, previous valvular heart disease, and previous cerebral hemorrhage were observed to be independently associated with poor outcome at 3 months after ischemic stroke in Chinese patients, and the effect remained significant even after adjusting other clinical, laboratory, and demographic variables. First, some studies also found that hyperglycemia in acute stroke could predict worse outcome independently [-]. Hyperglycemia is associated with an increased recruitment of ischemic penumbra, which is likely to be found in the early hours after stroke onset but may also be seen up to 24 h due to increase brain lactate production [, ]. Overtime, high blood glucose from diabetes can damage the blood vessels and the nerves that control the heart which increases the chance of developing heart disease. The aforementioned mechanisms may account for the unfavorable outcome experienced by the patients following the onset of ischemic stroke. Second, we confirmed previous studies which observed that increased serum creatinine level was associated with higher mortality rates [, ]. Serum creatinine concentration is widely used as an index of renal function, impaired renal function is also associated with increased long-term mortality and poor outcome after stroke [], but this concentration is affected by factors other than glomerular filtration rate. A further investigation of the mechanisms of creatinine in the pathogenesis of stroke in future is important. Third, the results of our study showed a relationship between previous valvular heart disease and poor outcome in patients with ischemic stroke although data on the association between previous valvular heart disease and outcome after acute stroke are scarce. Patients with previous valvular heart disease who experience ischemic stroke or transient ischemic attack have increased rates of death, but not recurrent stroke, compared with expected rates. Finally, in previous experimental and clinical studies, history of previous cerebral hemorrhage was associated with poor prognosis both in terms of mortality and functional recovery []. Despite improvements in surgical and medical treatments, it is still a serious disease with high case fatality and morbidity rates [, ]. These findings are consistent with the results of our study.
Moreover, our study also demonstrated that NHISS on admission and age are stronger, significant and independent predictors of 3-month unfavorable outcome in ischemic stroke patients. As previously reported [, -, -], NIHSS score constitutes a strong independent predictor of poor outcome in patients with ischemic stroke, and age contribute to a long-term mortality and unfavorable outcome, this could be due to the fact that old age is associated with one or more cardiovascular disease such as coronary artery disease, high blood pressure, high cholesterol, atrial fibrillation, heart failure, peripheral artery disease, and cerebral amyloid angiopathy.
Our study has some limitations. First, our data are retrospectively collected in a single center, causing much data missing. Second, data on the association between previous valvular heart disease/previous cerebral hemorrhage and unfavorable outcome after acute ischemic stroke are scarce. Third, known neurobiological predictors such as infarct size [] are not available in the cohort. Therefore, the lack of imaging data may influence accuracy of our model to predict the probability of 3-month unfavorable outcome. However, our nomogram still has the good performance of prediction. In future research, the neuroimaging predictors should be integrated into our model, which may help to increase its discriminative performance and improve the accuracy of the nomogram prediction in stroke patients. Despite these limitations, as far as we know, the present study is the first attempt to develop a nomogram to predict the probability of unfavorable outcome in a Chinese cohort of stroke patients. Finally, an external validation in a completely different cohort of patients is necessary.
In medical practice, the prediction of prognosis is challenging. The difficulty is even enhanced in the prognostication of functional progress of stroke. This is due to the complexity of the interaction between the factors associated stroke (which include the severity and subtype of the stroke developed) and other pathophysiologic factors (such as renal failure, valvular heart disease, uncontrolled diabetes mellitus, atrial fibrillation, and congestive heart failure). However, the visual COACHS nomogram may provide a number of significant applications, such as discussing prognosis with patients and their families on which realistic expectations can achieve and modifying rehabilitation programs. Furthermore, the COACHS nomogram can also be used to identify patients who need early intensive rehabilitation, or to aid in establishing of reasonable decisions for institutionalization of patients with immense likelihood of negative consequences of ischemic stroke. In summary, our study demonstrates that clinical, laboratory and demographic parameters (such as age and NIHSS score on admission) may be better predictors of clinical outcome in patients with acute ischemic stroke and thus can be easily used in clinical settings to aid clinicians make better decisions during management and rehabilitation strategies for patients with stroke.
Conclusion
The COACHS nomogram may be used to predict unfavorable outcome at 3 months after acute ischemic stroke in Chinese population. It may be also a useful tool that is effective in its clinical utilization to risk-stratify acute stroke patients.
Disclosure Statement
The authors have indicated that they have no conflicts of interest regarding the content of this article. B.S. and Y.L.: contributed equally to this work. J.Z. and JianJun Zou: concepted, designed, and supervized the study. Y.L., X.C., T.J., and W.W.: acquired the data. B.S., D.T., and JianJun Zou: analyzed and interpreted the data, provided statistical analysis, had full access to all of the data in the study, and are responsible for the integrity of the data and the accuracy of the data analysis. L.N., M.I., and C.S.: drafted the manuscript, JianJun Zou, J.Y., and C.C.: critically revised the manuscript for important intellectual content.
Funding Source
This study was supported by National Natural Science Foundation of China grant 81673511, Jiangsu Key Research and Development Plan grant BE2017613, Jiangsu Six Talent Peaks Project grant WSN-151, and Nanjing Medical Science and Technique Development Foundation grants QRX17020 and ZKX15027.
References
- 1. Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, et al.; American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2014 update: a report from the American Heart Association. <X00_Journal>Circulation</X00_Journal>. 2014 Jan; 129(3):e28–292.
- 2. Weimar C, König IR, Kraywinkel K, Ziegler A, Diener HC; German Stroke Study Collaboration. Age and National Institutes of Health Stroke Scale Score within 6 hours after onset are accurate predictors of outcome after cerebral ischemia: development and external validation of prognostic models. <X00_Journal>Stroke</X00_Journal>. 2004 Jan; 35(1): 158–62.
- 3. Ntaios G, Faouzi M, Ferrari J, Lang W, Vemmos K, Michel P. An integer-based score to predict functional outcome in acute ischemic stroke: the ASTRAL score. <X00_Journal>Neurology</X00_Journal>. 2012 Jun; 78(24): 1916–22.
- 4. Flint AC, Faigeles BS, Cullen SP, Kamel H, Rao VA, Gupta R, et al.; VISTA Collaboration. THRIVE score predicts ischemic stroke outcomes and thrombolytic hemorrhage risk in VISTA. <X00_Journal>Stroke</X00_Journal>. 2013 Dec; 44(12): 3365–9.
- 5. Saposnik G, Fang J, Kapral MK, Tu JV, Mamdani M, Austin P, et al.; Investigators of the Registry of the Canadian Stroke Network (RCSN); Stroke Outcomes Research Canada (SORCan) Working Group. The iScore predicts effectiveness of thrombolytic therapy for acute ischemic stroke. <X00_Journal>Stroke</X00_Journal>. 2012 May; 43(5): 1315–22.
- 6. Strbian D, Meretoja A, Ahlhelm FJ, Pitkäniemi J, Lyrer P, Kaste M, et al. Predicting outcome of IV thrombolysis-treated ischemic stroke patients: the DRAGON score. <X00_Journal>Neurology</X00_Journal>. 2012 Feb; 78(6): 427–32.
- 7. Cooray C, Mazya M, Bottai M, Dorado L, Skoda O, Toni D, et al. External Validation of the ASTRAL and DRAGON Scores for Prediction of Functional Outcome in Stroke. <X00_Journal>Stroke</X00_Journal>. 2016 Jun; 47(6): 1493–9.
- 8. Emberson J, Lees KR, Lyden P, Blackwell L, Albers G, Bluhmki E, et al.; Stroke Thrombolysis Trialists’ Collaborative Group. Effect of treatment delay, age, and stroke severity on the effects of intravenous thrombolysis with alteplase for acute ischaemic stroke: a meta-analysis of individual patient data from randomised trials. <X00_Journal>Lancet</X00_Journal>. 2014 Nov; 384(9958): 1929–35.
- 9. Moulla Y, Lyros O, Adolf D, Kaiser T, Dietrich A. A Nomogram Based on Clinical Factors to Predict the Serum Myoglobin Levels Following Bariatric Surgery. <X00_Journal>Obes Surg</X00_Journal>. 2018 Jun; 28(6): 1697–703.
- 10. Battersby NJ, Bouliotis G, Emmertsen KJ, Juul T, Glynne-Jones R, Branagan G, et al.; UK and Danish LARS Study Groups. Development and external validation of a nomogram and online tool to predict bowel dysfunction following restorative rectal cancer resection: the POLARS score. <X00_Journal>Gut</X00_Journal>. 2018 Apr; 67(4): 688–96.
- 11. Song W, Zhu ZG, Wu Q, Lv CG, Wang YG, Chen L, et al. A nomogram to predict overall survival for biliary tract cancer. <X00_Journal>Cancer Manag Res</X00_Journal>. 2018 Jun; 10: 1535–41.
- 12. Zhou X, Sun Z, Zhuang Y, Jiang J, Liu N, Zang X, et al. Development and Validation of Nomogram to Predict Acute Kidney Injury in Patients with Acute Myocardial Infarction Treated Invasively. <X00_Journal>Sci Rep</X00_Journal>. 2018 Jun; 8(1): 9769.
- 13. Cappellari M, Turcato G, Forlivesi S, Micheletti N, Tomelleri G, Bonetti B, et al. Introduction of direct oral anticoagulant within 7 days of stroke onset: a nomogram to predict the probability of 3-month modified Rankin Scale score >2. <X00_Journal>J Thromb Thrombolysis</X00_Journal>. 2018 Oct; 46(3): 292–8.
- 14. Cappellari M, Turcato G, Forlivesi S, Bagante F, Cervellin G, Lippi G, et al. The START nomogram for individualized prediction of the probability of unfavorable outcome after intravenous thrombolysis for stroke. <X00_Journal>Int J Stroke</X00_Journal>. 2018 Oct; 13(7): 700–6.
- 15. Turcato G, Cervellin G, Cappellari M, Bonora A, Zannoni M, Bovi P, et al. Early function decline after ischemic stroke can be predicted by a nomogram based on age, use of thrombolysis, RDW and NIHSS score at admission. <X00_Journal>J Thromb Thrombolysis</X00_Journal>. 2017 Apr; 43(3): 394–400.
- 16. Swarowska M, Polczak A, Pera J, Klimkowicz-Mrowiec A, Slowik A, Dziedzic T. Hyperfibrinogenemia predicts long-term risk of death after ischemic stroke. <X00_Journal>J Thromb Thrombolysis</X00_Journal>. 2014 Nov; 38(4): 517–21.
- 17. Prefasi D, Martínez-Sánchez P, Fuentes B, Díez-Tejedor E. Severity and outcomes according to stroke etiology in patients under 50 years of age with ischemic stroke. <X00_Journal>J Thromb Thrombolysis</X00_Journal>. 2016 Aug; 42(2): 272–82.
- 18. Vora NA, Shook SJ, Schumacher HC, Tievsky AL, Albers GW, Wechsler LR, et al. A 5-item scale to predict stroke outcome after cortical middle cerebral artery territory infarction: validation from results of the Diffusion and Perfusion Imaging Evaluation for Understanding Stroke Evolution (DEFUSE) Study. <X00_Journal>Stroke</X00_Journal>. 2011 Mar; 42(3): 645–9.
- 19. Yoo DS, Chang J, Kim JT, Choi MJ, Choi J, Choi KH, et al. Various blood glucose parameters that indicate hyperglycemia after intravenous thrombolysis in acute ischemic stroke could predict worse outcome. <X00_Journal>PLoS One</X00_Journal>. 2014 Apr; 9(4):e94364.
- 20. Parsons MW, Barber PA, Desmond PM, Baird TA, Darby DG, Byrnes G, et al. Acute hyperglycemia adversely affects stroke outcome: a magnetic resonance imaging and spectroscopy study. <X00_Journal>Ann Neurol</X00_Journal>. 2002 Jul; 52(1): 20–8.
- 21. Toni D, De Michele M, Fiorelli M, Bastianello S, Camerlingo M, Sacchetti ML, et al. Influence of hyperglycaemia on infarct size and clinical outcome of acute ischemic stroke patients with intracranial arterial occlusion. <X00_Journal>J Neurol Sci</X00_Journal>. 1994 May; 123(1-2): 129–33.
- 22. MacWalter RS, Wong SY, Wong KY, Stewart G, Fraser CG, Fraser HW, et al. Does renal dysfunction predict mortality after acute stroke? A 7-year follow-up study. <X00_Journal>Stroke</X00_Journal>. 2002 Jun; 33(6): 1630–5.
- 23. Yahalom G, Schwartz R, Schwammenthal Y, Merzeliak O, Toashi M, Orion D, et al. Chronic kidney disease and clinical outcome in patients with acute stroke. <X00_Journal>Stroke</X00_Journal>. 2009 Apr; 40(4): 1296–303.
- 24. Lavine SD, Masri LS, Levy ML, Giannotta SL. Temporary occlusion of the middle cerebral artery in intracranial aneurysm surgery: time limitation and advantage of brain protection. <X00_Journal>J Neurosurg</X00_Journal>. 1997 Dec; 87(6): 817–24.
- 25. Hop JW, Rinkel GJ, Algra A, van Gijn J. Case-fatality rates and functional outcome after subarachnoid hemorrhage: a systematic review. <X00_Journal>Stroke</X00_Journal>. 1997 Mar; 28(3): 660–4.
- 26. Inagawa T. Trends in incidence and case fatality rates of aneurysmal subarachnoid hemorrhage in Izumo City, Japan, between 1980-1989 and 1990-1998. <X00_Journal>Stroke</X00_Journal>. 2001 Jul; 32(7): 1499–507.
- 27. Weimar C, Ziegler A, König IR, Diener HC. Predicting functional outcome and survival after acute ischemic stroke. <X00_Journal>J Neurol</X00_Journal>. 2002 Jul; 249(7): 888–95.
- 28. Welsh P, Barber M, Langhorne P, Rumley A, Lowe GD, Stott DJ. Associations of inflammatory and haemostatic biomarkers with poor outcome in acute ischaemic stroke. <X00_Journal>Cerebrovasc Dis</X00_Journal>. 2009; 27(3): 247–53.
- 29. Cappellari M, Turcato G, Forlivesi S, Zivelonghi C, Bovi P, Bonetti B, et al. STARTING-SICH Nomogram to Predict Symptomatic Intracerebral Hemorrhage After Intravenous Thrombolysis for Stroke. <X00_Journal>Stroke</X00_Journal>. 2018 Feb; 49(2): 397–404.
Mr. BaiLi Song and Mr. YuKai Liu contributed equally to this work.