Introduction
Hemorrhagic transformation (HT) is a frequent complication of acute ischemic stroke (AIS), which can occur in up to 40% patients []. Previous studies suggest HT is associated with worsened outcomes regardless of HT subtypes [, ]. Therefore, identifying patients at high risk of HT and choosing appropriate treatment accordingly may reduce the occurrence of HT and improve outcome.
As a routine part of standard complete blood count, red blood cell distribution width (RDW) reflects the variability in size of circulating red blood cells []. Recent studies have suggested that RDW may reflect the underlying inflammatory state and oxidative damage and is therefore associated with incidence and prognosis of several cardiovascular and cerebrovascular diseases [-]. One possible mechanism of HT is the increased oxidative damage to blood brain barrier, which may lead to vascular leakage and result in HT [, ]. Therefore, we speculate that RDW may associate with HT considering its association with inflammation response []. As far as we know, no study has focused on the relationship between RDW and HT. Under the light, we investigated whether RDW is associated with HT and HT subtypes in AIS patients.
Methods
Subjects
Consecutive AIS patients admitted to the Department of Neurology of West China Hospital, Sichuan University (Chengdu, China) between January 1, 2014, and December 31, 2018, were evaluated for eligibility. Patient data were obtained from the Chengdu Stroke Registry Database as previously described []. We included patients admitted within 24 h from stroke onset. Patients were excluded according to the following exclusion criteria: (1) younger than 18 years; (2) combined with malignant tumor; (3) with missing RDW data; (4) had hemorrhage on baseline computed tomography (CT); and (5) without follow-up brain images. All patients underwent CT within 24 h after admission. Follow-up CT or magnetic resonance imaging (MRI) was conducted within 14 days after admission or in any case of neurological deterioration. The diagnosis of ischemic stroke was based on clinical manifestation and confirmed by CT or MRI. Informed consent was obtained from patients or their next-on-kin. This project was approved by the Scientific Research Department of West China Hospital.
Data Collection
Blood samples were obtained within 24 h upon admission. Hemoglobin, platelet count, and RDW were determined using a Sysmex XE-2100 Hematology Automated Analyzer (Sysmex, Kobe, Japan). Patient demographics, admission stroke severity based on National Institute of Health Stroke Scale (NIHSS), vascular risk factors, and treatment during hospitalization were collected. Reperfusion therapy included thrombolysis and endovascular therapy. Stroke subtypes were determined according to the Trial of Org 10172 in Acute Stroke Treatment classification [].
The vascular risk factors include history of hypertension (defined as previous use of antihypertensive medication or blood pressure > 140/90 mm Hg on several different measurements during hospitalization), diabetes (defined as previous use of antidiabetic medication or fasting blood glucose >7 mmol/L), hyperlipidemia (defined as previous use of lipid-lowering medication or total cholesterol >6 mmol/L or low-density lipoprotein >4.14 mmol/L at admission), atrial fibrillation (based on history or newly diagnosed by 24-h electrocardiograms or electrocardiography during hospitalization), smoking (defined as a current smoker or experience of regular smoking habit), and alcohol consumption (defined as alcohol intake >60 g/day or >420 g/week).
Outcome Assessment
HT during hospitalization was diagnosed using follow-up CT or MRI and subcategorized as hemorrhagic infarct (HI) and parenchymal hematoma (PH) according to the recommendations of the European Cooperative Acute Stroke Study []. Two neurologists blinded to clinical data evaluated the neuroimaging and determined HT and its subtypes independently. Inconsistencies were resolved by consensus.
Statistical Analyses
For continuous variables, data are presented as means with standard deviations or medians with interquartile range as appropriate. Categorical variables are shown as frequencies or percentages. Statistical differences between groups were evaluated by one-way ANOVA or Kruskal-Wallis test for continuous variables and chi-squared test or Fisher’s exact test for categorical variables. Subjects were divided into tertiles according to RDW level. Multivariate logistic regression was performed to calculate the ORs and the corresponding 95% CIs for HT, PH, and HI according to RDW levels. We used 2 models. Model 1 was adjusted for age and gender only. In model 2, we further adjusted variates within p < 0.1 in univariate analysis. A spline regression model was conducted to explore the pattern and magnitude of the association between RDW and HT with 4 knots (at the 25th, 50th, 75th, and 95th percentiles) [], and the reference point was the median value of RDW in the first tertile. Trends for the ORs of HT across increasing RDW categories (p for trend) were tested by using the median value of RDW in each tertile as a continuous variable. We further performed stratified logistic regression analyses to assess the potential effect modification by some significant variates (i.e., age, sex, hyperlipidemia, atrial fibrillation, stroke severity, antiplatelet, and reperfusion therapy). Interactions between RDW and variates on HT were tested by the likelihood ratio test, adjusting for variables in model 2 unless the variable was used as a subgroup variable. All statistical analyses were analyzed by the software packages R (http://www.R-project.org, The R Foundation), EmpowerStats (http://www.empowerstats.com, X&Y Solutions, Boston, MA, USA) and STATA 14.0 (Corporation, College Station, TX, USA). A 2-sided p value < 0.05 was considered statistically significant.
Results
Baseline Characteristics
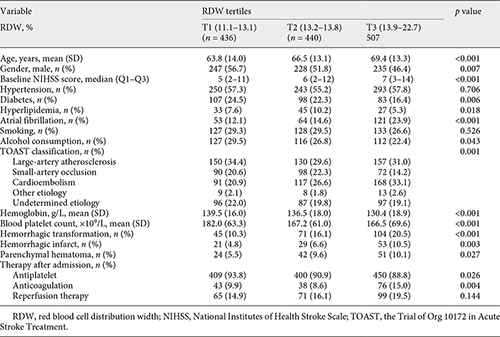
In the current study, 1,514 patients diagnosed with ischemic stroke were admitted within 24 h from stroke onset, and 131 patients were excluded due to malignant tumor (n = 91), hemorrhage on baseline CT (n = 11), younger than 18 years (n = 1), and without follow-up brain image (n = 25) or RDW value (n = 3). Therefore, 1,383 patients were eligible for the final analysis. The mean age was 66.7 ± 13.6 years and 710 (51.3%) were males. The average RDW level was 13.7 ± 1.1%. HT was observed in 220 (15.9%) patients, and the median time of follow-up imaging was 2 days (interquartile range 1–5 days). Among patients developed HT, 132 (60.0%) cases were determined by CT and 88 (40.0%) cases were determined by MRI; 103 (46.8%) cases were classified as HI and 117 (53.2%) cases were classified as PH. A total of 235 patients received reperfusion therapy (154 thrombolysis, 115 endovascular therapy, and 34 both). The demographic characteristics and clinical data of the included patients according to RDW tertiles are presented in Table 1. The incidence rates of HT in RDW tertiles (from low to high) were 10.3, 16.1, and 20.5%, respectively. Patients with higher RDW tended to be older, have higher prevalence of atrial fibrillation and higher baseline NIHSS score, and have higher incidence of HT than those with lower RDW.
Association between RDW and HT
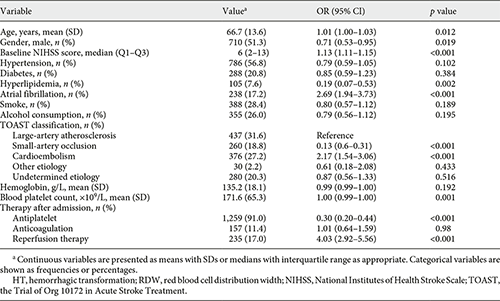
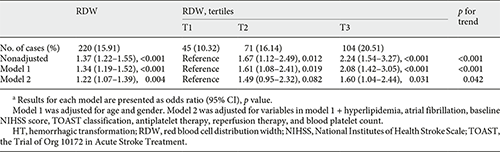
Univariate analysis showed that age, sex, baseline NIHSS score, hyperlipidemia, atrial fibrillation, Trial of Org 10172 in Acute Stroke Treatment classification, blood platelet count, antiplatelet therapy, and reperfusion therapy were regarded as confounding factors related to HT occurrence (p < 0.1, Table 2). In multivariate regression analysis, when RDW levels were divided into tertiles, patients in the upper (T3), and middle (T2) tertile had elevated risk of HT compared to patients in the lower (T1) tertile (nonadjusted: OR 2.24, 95% CI 1.54–3.27; OR 1.67, 95% CI 1.12–2.49, respectively, Table 3). After adjusting confounders, T3 still had a significant higher risk of HT than T1 (model 2: OR 1.60, 95% CI 1.04–2.44, p = 0.031), while the relationship between RDW and HT was no longer significant among patients in T2 (model 2: OR 1.49, 95% CI 0.95–2.32, p = 0.082). The risk of HT increased gradually across RDW tertiles (p for trend = 0.042). When RDW was tested as a continuous variable, it was positively associated with HT after adjusting confounders as well (model 2: OR 1.22, 95% CI 1.07–1.39, p = 0.003, Table 3). As shown in Figure 1, with an increase of RDW levels, the adjusted ORs of HT increased in a dose-response manner. Reperfusion therapy was associated with elevated risk of HT in model 2 no matter RDW was regarded as a continuous variable or divided into tertiles (OR 2.28, 95% CI 1.58–3.30, p < 0.001; OR 2.22, 95% CI 1.54–3.21, p < 0.001, respectively).
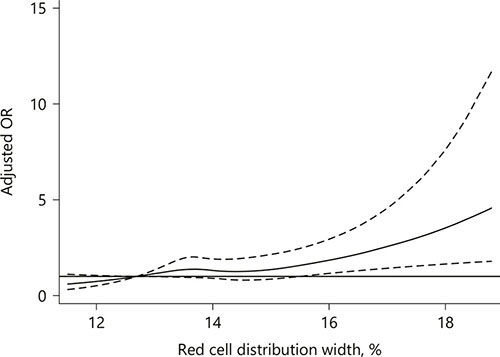
Fig. 1
The multiple spline regression analysis to investigate the relationship between RDW and HT. Solid lines represent the odds ratio of HT and dotted lines represent the corresponding 95% CI. ORs were adjusted for variates within p < 0.1 in univariate analysis. OR = 1 was set as the reference line.
Association between RDW and HT Subtypes
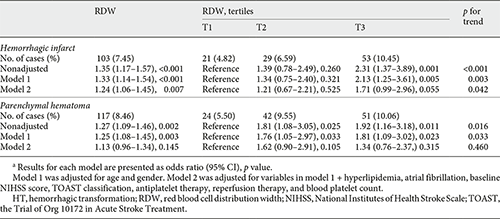
The relationship between RDW and HT subtypes was presented in Table 4. After controlling confounders, RDW significantly correlated with HI on continuous analysis (model 2: OR 1.24, 95% CI 1.06–1.45). The risk of HI increased across RDW tertiles (p for trend = 0.042), though T3 had a nonsignificant correlation with HI compared to T1 (OR 1.71, 95% CI 0.99–2.96, p = 0.055). RDW was positively associated with PH in nonadjusted model. However, their association became insignificant after multivariate adjustment.
Subgroup Analyses
As shown in Figure 2, in subgroup analyses stratified by sex, age, hyperlipidemia, atrial fibrillation, stroke severity and antiplatelet, the modest positive correlations between RDW and HT were observed in all subgroups and reached statistical significance in several subgroups. The association between RDW and HT could be modified by reperfusion therapy (p for interaction = 0.010). Among patients who had not received reperfusion therapy, RDW had a significant positive correlation with HT (OR 1.35, 95% CI 1.16–1.57, p < 0.001). However, this correlation attenuated and became insignificant when limiting the analysis to patients who had received reperfusion therapy (OR 1.00, 95% CI 0.74–1.34, p = 0.980).
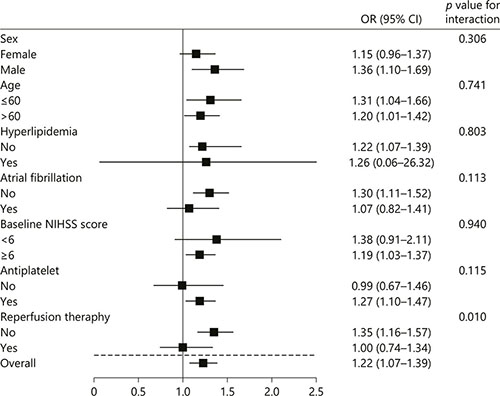
Fig. 2
Stratified logistic regression analysis to identify variables that modify the correlation between RDW and HT. For subcategories, black squares represent point estimates, and horizontal lines represent 95% CIs. Each subcategory was adjusted for age, sex, hyperlipidemia, atrial fibrillation, baseline NIHSS score, the Trial of Org 10172 in Acute Stroke Treatment classification, antiplatelet therapy, reperfusion therapy, and blood platelet count, except for the stratification variable. For baseline NIHSS score, subgroups were dichotomized by median value.
Discussion
In the current study, we found that elevated baseline RDW level was independently associated with increased risk of HT in a dose-response manner after adjustment for potential confounders. This relationship was significant in patients without reperfusion therapy, but not in those underwent reperfusion therapy. Moreover, RDW was related to HI rather than PH.
The mechanism underlying the association between RDW and HT remains unclear. Oxidative stress and inflammation have been thought as important determinants of RDW []. Oxidative stress can decrease erythrocytes lifespan [], while inflammation is strongly associated with inhibited erythropoiesis [], both of which may increase RDW level. Ischemia and reperfusion injury can lead to oxidative stress via production of reactive oxygen species. Oxidative damage toward blood brain barrier may result in vascular rupture []. Inflammation can overproduce free radicals []. Free radical damage toward cerebral vascular endothelium may lead to vascular rupture and extravasation of blood, which causes HT [].
There is a possible explanation for why in patients underwent reperfusion therapy the association between RDW and HT attenuated and became insignificant. As previously reported, recanalization of occluded arteries is related to the risk of HT and reperfusion injury plays an important role in the development of HT [, ]. In our multivariate analysis, reperfusion therapy was shown to be associated with HT no matter RDW was tested as a continuous or categorical variable (OR 2.28, 95% CI 1.58–3.30, p < 0.001; OR 2.22, 95% CI 1.54–3.21, p < 0.001, respectively). Therefore, we speculate that in patients who had not received reperfusion therapy, higher RDW reflects a greater degree of inflammation and oxidative stress and relates to higher likelihood of HT occurrence; while in patients who had received reperfusion therapy, the relative strong effect of reperfusion therapy upon HT attenuates the association between RDW and HT makes the role of RDW become inconspicuous.
We further explored the association between RDW and HT subtypes. RDW was significantly associated with HI and the risk of HT increased step-wise across RDW tertiles. However, RDW was not related to PH. It seems that mild HT, instead of severe HT, was related to RDW in our study. This correlation needs further confirmation in future studies.
Our study had several strengths. First, as far as we know, this is the first study to demonstrate the relationship between RDW and HT. Second, we performed subgroup analysis to identify potential effect modification by covariates and detected an interaction between reperfusion therapy and RDW. Finally, RDW significantly and positively related to HT no matter it was tested as a continuous or categorical variable in multiple logistic regression analysis, which improves the reliability of our results.
Some limitations need to be noticed. First, this is a single-center based retrospective analysis. Though the relative large sample size may minimize the selection bias, future larger multicenter studies are warranted to verify our results and improve generalizability. Second, MRI is more sensitive than CT for detection of HT, so it is possible that some mild HT could be overlooked by CT []. Third, the relationship between RDW and HT subtypes needs further confirmation. Finally, we did not evaluate the association between RDW and patient functional outcome. Future studies are needed to explore their relationship.
In conclusion, the present study suggests that RDW is associated with HT among AIS patients without reperfusion therapy, and that the risk of HT increased in a dose-response manner with increasing RDW levels. Therefore, patients with elevated RDW levels should be carefully treated. Future studies are required to explicit the mechanism underlying the association between RDW and HT.
Statement of Ethics
This study protocol has been approved by the Ethics Committee of West China Hospital of Sichuan University.
Disclosure Statement
The authors have no conflicts of interest to declare.
Funding Sources
B.W. and M.L. obtained public funding. This study was supported by National Natural Science Foundation of China (81671146, 81870937), Major International (Regional) Joint Research Project, National Natural Science Foundation of China (Grant No. 81620108009), the National Key Development Plan for Precision Medicine Research (2017YFC09s10004), and Key Research and Development Program, Science and Technology Department of Sichuan Province (Grant No. 2017SZ0007).
Author Contributions
B.W. and M.L. conceived and designed the study. C.W., L.W., D.Z., L.D., S.Q., and Y.L. acquired the data, which L.D., S.Q., Y.L., and C.W. analyzed. C.W., L.W., and D.Z. aided in data interpretation and wrote the manuscript. All authors were involved in revising the article and approved the final version.
References
- 1. Jickling GC, Liu D, Stamova B, Ander BP, Zhan X, Lu A, et al. Hemorrhagic transformation after ischemic stroke in animals and humans. <X00_Journal>J Cereb Blood Flow Metab</X00_Journal>. 2014 Feb;34(2):185–99.
- 2. Lei C, Wu B, Liu M, Chen Y. Asymptomatic hemorrhagic transformation after acute ischemic stroke: is it clinically innocuous? <X00_Journal>J Stroke Cerebrovasc Dis</X00_Journal>. 2014 Nov-Dec;23(10):2767–72.
- 3. Berger C, Fiorelli M, Steiner T, Schäbitz WR, Bozzao L, Bluhmki E, et al. Hemorrhagic transformation of ischemic brain tissue: asymptomatic or symptomatic? <X00_Journal>Stroke</X00_Journal>. 2001 Jun;32(6):1330–5.
- 4. Salvagno GL, Sanchis-Gomar F, Picanza A, Lippi G. Red blood cell distribution width: A simple parameter with multiple clinical applications. <X00_Journal>Crit Rev Clin Lab Sci</X00_Journal>. 2015;52(2):86–105.
- 5. Borné Y, Smith JG, Melander O, Engström G. Red cell distribution width in relation to incidence of coronary events and case fatality rates: a population-based cohort study. <X00_Journal>Heart</X00_Journal>. 2014 Jul;100(14):1119–24.
- 6. Chen PC, Sung FC, Chien KL, Hsu HC, Su TC, Lee YT. Red blood cell distribution width and risk of cardiovascular events and mortality in a community cohort in Taiwan. <X00_Journal>Am J Epidemiol</X00_Journal>. 2010 Jan;171(2):214–20.
- 7. Lappegård J, Ellingsen TS, Skjelbakken T, Mathiesen EB, Njølstad I, Wilsgaard T, et al. Red cell distribution width is associated with future risk of incident stroke. The Tromsø Study. <X00_Journal>Thromb Haemost</X00_Journal>. 2016 Jan;115(1):126–34.
- 8. Kim J, Kim YD, Song TJ, Park JH, Lee HS, Nam CM, et al. Red blood cell distribution width is associated with poor clinical outcome in acute cerebral infarction. <X00_Journal>Thromb Haemost</X00_Journal>. 2012 Aug;108(2):349–56.
- 9. Wang X, Lo EH. Triggers and mediators of hemorrhagic transformation in cerebral ischemia. <X00_Journal>Mol Neurobiol</X00_Journal>. 2003 Dec;28(3):229–44.
- 10. Álvarez-Sabín J, Maisterra O, Santamarina E, Kase CS. Factors influencing haemorrhagic transformation in ischaemic stroke. <X00_Journal>Lancet Neurol</X00_Journal>. 2013 Jul;12(7):689–705.
- 11. Fan L, Gui L, Chai EQ, Wei CJ. Routine hematological parameters are associated with short- and long-term prognosis of patients with ischemic stroke. <X00_Journal>J Clin Lab Anal</X00_Journal>. 2018 Feb;32(2):e22244.
- 12. Wu B, Lin S, Hao Z, Yang J, Xu Y, Wu L, et al. Proportion, risk factors and outcome of lacunar infarction: a hospital-based study in a Chinese population. <X00_Journal>Cerebrovasc Dis</X00_Journal>. 2010 Jan;29(2):181–7.
- 13. Adams HP Jr, Bendixen BH, Kappelle LJ, Biller J, Love BB, Gordon DL, et al. Classification of subtype of acute ischemic stroke. Definitions for use in a multicenter clinical trial. TOAST. Trial of Org 10172 in Acute Stroke Treatment. <X00_Journal>Stroke</X00_Journal>. 1993 Jan;24(1):35–41.
- 14. Hacke W, Kaste M, Fieschi C, von Kummer R, Davalos A, Meier D, et al.; Second European-Australasian Acute Stroke Study Investigators. Randomised double-blind placebo-controlled trial of thrombolytic therapy with intravenous alteplase in acute ischaemic stroke (ECASS II). <X00_Journal>Lancet</X00_Journal>. 1998 Oct;352(9136):1245–51.
- 15. Durrleman S, Simon R. Flexible regression models with cubic splines. <X00_Journal>Stat Med</X00_Journal>. 1989 May;8(5):551–61.
- 16. Montagnana M, Cervellin G, Meschi T, Lippi G. The role of red blood cell distribution width in cardiovascular and thrombotic disorders. <X00_Journal>Clin Chem Lab Med</X00_Journal>. 2011 Dec;50(4):635–41.
- 17. Marinkovic D, Zhang X, Yalcin S, Luciano JP, Brugnara C, Huber T, et al. Foxo3 is required for the regulation of oxidative stress in erythropoiesis. <X00_Journal>J Clin Invest</X00_Journal>. 2007 Aug;117(8):2133–44.
- 18. Li N, Zhou H, Tang Q. Red Blood Cell Distribution Width: A Novel Predictive Indicator for Cardiovascular and Cerebrovascular Diseases. <X00_Journal>Dis Markers</X00_Journal>. 2017;2017:7089493.
- 19. Turcato G, Cappellari M, Follador L, Dilda A, Bonora A, Zannoni M, et al. Red Blood Cell Distribution Width Is an Independent Predictor of Outcome in Patients Undergoing Thrombolysis for Ischemic Stroke. <X00_Journal>Semin Thromb Hemost</X00_Journal>. 2017 Feb;43(1):30–5.
- 20. Sumii T, Lo EH. Involvement of matrix metalloproteinase in thrombolysis-associated hemorrhagic transformation after embolic focal ischemia in rats. <X00_Journal>Stroke</X00_Journal>. 2002 Mar;33(3):831–6.
- 21. Pan J, Konstas AA, Bateman B, Ortolano GA, Pile-Spellman J; J P. Reperfusion injury following cerebral ischemia: pathophysiology, MR imaging, and potential therapies. <X00_Journal>Neuroradiology</X00_Journal>. 2007 Feb;49(2):93–102.
- 22. Arnould MC, Grandin CB, Peeters A, Cosnard G, Duprez TP. Comparison of CT and three MR sequences for detecting and categorizing early (48 hours) hemorrhagic transformation in hyperacute ischemic stroke. <X00_Journal>AJNR Am J Neuroradiol</X00_Journal>. 2004 Jun-Jul;25(6):939–44.
C.W., L.W., and D.Z. contributed equally to this work.