Introduction
Capsular warning syndrome (CWS) was first described by Donnan et al. [] in 1993 as the recurrence, within 24 h, of ≥3 stereotyped episodes of subcortical TIAs, leading to the development of an early lacunar (L) stroke in 42% of cases. After Donnan’s study, CWS was mainly investigated in retrospective small series [-], except for a population-based study by Paul et al. [] which found a lower incidence (1.5%) and a very high early stroke risk (60%). Furthermore, the clinical definition of CWS became highly variable across studies, especially with respect to the timeframe and minimum TIAs number, ranging from ≥3 within 48 [] or 72 h [], to ≥2 within 7 days []. CWS pathophysiology is still unclear, and several mechanisms have been suggested, including perforating artery lipohyalinosis, microembolism, and, more recently, proximal stenosis of the middle cerebral artery (MCA) [, ]. Lastly, no conclusive data are available on the optimal management of CWS and on the effectiveness of intravenous thrombolysis (IVT) in CWS-related stroke. In our study, we aimed to compare clinical features, risk profile, and prognosis of CWS with L and nonlacunar (NL) TIAs from a large prospective cohort.
Methods
Study procedures are reported in detail elsewhere [, ]. In summary, consecutive patients aged ≥18 years with TIA symptoms presenting at the emergency department (ED) of S. Orsola-Malpighi Hospital of Bologna (Italy) between August 1, 2010, and December 31, 2017, were enrolled. From August 1, 2010, a fast-track TIA clinical pathway, to be performed <24 h from ED admission, was implemented. All patients underwent 3- and 12-month follow-up visits at our neurology unit after the index TIA. Outcome events and mRS were recorded during face-to-face interview. An experienced vascular neurologist (MG) confirmed the classification of the TIA subtype, as well as the occurrence of outcome events and the mRS score at each follow-up. For patients not traceable or missing the scheduled follow-up, information was retrieved from interviews with close relatives, hospital discharge records, specialty visits, and mortality registry. Study definitions and outcomes are reported in online supplementary file 1 (for all online suppl. material, see http://www.karger.com/doi/10.1159/000525954).
Statistical Analysis
Categorical variables were summarized as absolute and percentage frequencies, and continuous variables as mean ± standard deviation (SD) or median and interquartile range (IQR). The normality of variables was tested using Shapiro-Wilk test. Levene’s test was used to test variance homogeneity. Comparison between groups was performed using one-way ANOVA or Kruskal-Wallis tests for continuous variables and χ2 test for categorical variables, followed by post hoc pairwise comparisons with Benjamini-Hochberg-corrected significance level. Variables differing between CWS and other groups at p < 0.10 were included in a stepwise multivariable logistic regression to identify independent risk factors of CWS. Results were reported as odds ratios with 95% confidence interval. Overall survival from stroke was estimated using Kaplan-Meier curves. Differences between groups were tested using log-rank test. Competing risk analysis was carried out to estimate the cumulative incidence of stroke, with death as a competing event that prevents stroke occurrence. The significance level was set at p < 0.05. Statistical analysis was performed with SPSS statistical package, version 27.0 (SPSS Inc., Chicago, IL, USA).
Results
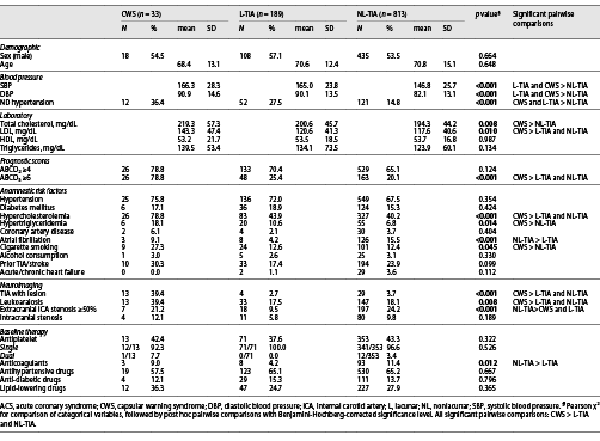
The study cohort consisted of 1,035 TIA patients (89.5% definite and 10.5% probable), including 33 CWS (3.3%), 189 L-TIAs (18.3%), and 811 NL-TIAs (78.5%) (Fig. 1). CWS accounted for 14.8% of all L-TIAs. Among TIA patients, 80.1% were admitted to the ED the same day of symptoms onset, and 14.5% 1 day after and the remaining 5.4% after 2–17 days. CWS patients presented a median of 3 (IQR 3–5) TIA episodes within 72 h with a significantly shorter median duration (15 min, IQR 5–30) compared to L-TIAs (60 min, IQR 5–160) and NL-TIAs (50, IQR 3–180; p = 0.001). Demographic and clinical characteristics are summarized in Table 1. Baseline mRS scores were similar between subgroups (median 0, IQR 0–0; p = 0.464). The most common clinical syndrome in CWS was pure motor hemiparesis (39.4%), followed by unilateral sensorimotor deficit (30.3%), unilateral pure hypoesthesia (21.2%), ataxic hemiparesis (6.1%), and dysarthria-clumsy hand syndrome (3.0%).
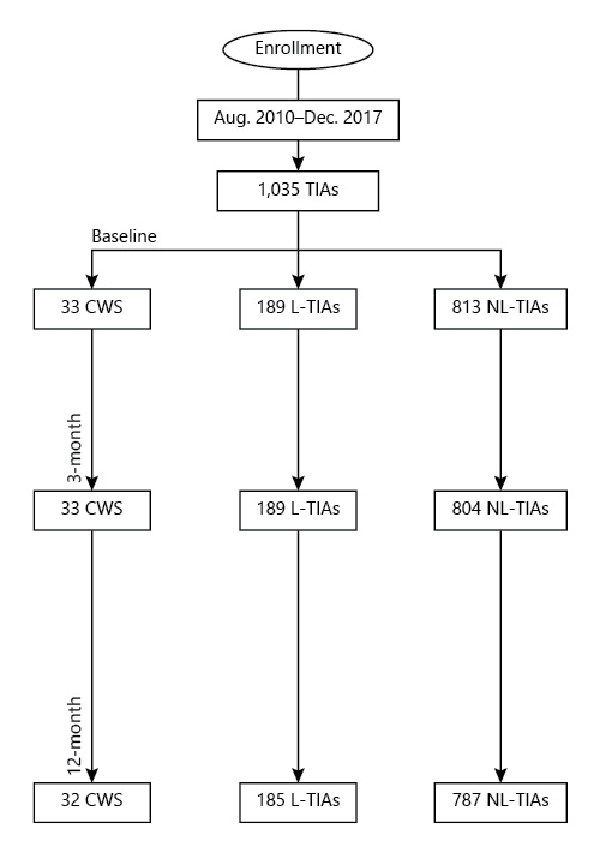
Fig. 1
Study flowchart.
As to anamnestic risk factors, CWS patients showed the highest prevalence of hypercholesterolemia, hypertriglyceridemia, and cigarette smoking (all p < 0.05). Newly diagnosed (ND) hypertension was significantly more frequent in CWS (36.4%) and L-TIAs (27.5%) as compared to NL-TIAs (14.8%; p < 0.001) (Table 1).
All patients underwent brain CT scan on ED admission. A second 24-h brain CT was conducted in 51.5% CWS, 15.9% L-TIAs, and 14.8% NL-TIAs. DWI MRI was performed in 33.3% CWS, 14.8% L-TIAs, and 17.1% NL-TIAs within a median of 1 (IQR 0–1) day after the admission. CDU extracranial vessel study was performed in all cases. Intracranial vessels were examined in all patients using transcranial CDU (100% CWS, 88.9% L-TIAs, and 76.7% NL-TIAs) and/or CTA/MRA (63.6% CWS, 7.9% L-TIAs, and 21.6% NL-TIAs). TIA with lesion and leukoaraiosis were significantly more frequent in CWS (p < 0.001 and p = 0.008, respectively). Intracranial stenosis frequency was similar between groups (p = 0.189). Specifically, we found proximal MCA stenosis in 3 CWS patients, while 1 patient exhibited an intracranial vertebral artery stenosis. Symptoms were congruous with mild MCA stenosis in one CWS. Conversely, extracranial internal carotid artery (ICA) stenosis was more frequent in NL-TIAs (p < 0.001). Baseline therapy did not significantly differ between subgroups except for a higher frequency of home anticoagulation in NL-TIA patients (p = 0.012), Table 1.
In multivariable logistic regression, including ND hypertension, hypercholesterolemia, hypertriglyceridemia, ABCD3 ≥6, atrial fibrillation, cigarette smoking, extracranial ICA stenosis ≥50%, TIA with lesion and leukoaraiosis, only ND hypertension, hypercholesterolemia, cigarette smoking, and leukoaraiosis resulted significant independent risk factors of CWS (online suppl. Table 1). All patients received timely secondary prevention (≤24 h from the admission), including single or dual antiplatelet therapy (DAPT), oral anticoagulation, blood pressure-lowering with target <140/90 mm Hg, and statin treatment with LDL target <100 mg/dL. 48.4% of CWS underwent DAPT (aspirin + clopidogrel), while the remaining (42.4%) were treated with single antiplatelet or continued anticoagulation for prior atrial fibrillation (9.1%). A shift toward DAPT was documented over the study period: in the absence of contraindications (e.g., lack of tolerance, high bleeding risk), all high-risk TIAs enrolled after 2014 were treated with DAPT for 21 days, followed by single antiplatelet therapy (aspirin when naïve, clopidogrel when already on home aspirin), basing on the evidence available at the time []. None of CWS or L-TIA patients underwent carotid surgery, since the extracranial ICA stenosis was in all cases asymptomatic. On the other hand, 87/197 (44.2%) NL-TIAs with symptomatic extracranial ICA stenosis ≥50% were treated with endarterectomy (n = 81) or stenting (n = 6).
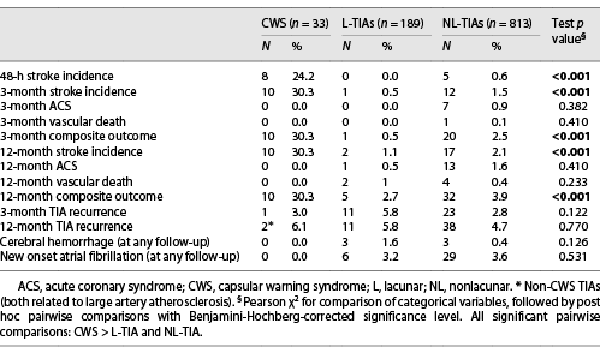
CWS patients had a significantly higher cumulative incidence of stroke (Table 2) and composite outcome at each follow-up versus other groups. In line with this finding, Kaplan-Meier survival analysis showed that most stroke after CWS patients occurred in the first 2 days after admission (CWS vs. L-TIAs and NL-TIAs, log-rank test; p < 0.001) (Fig. 2a). Competing risk analysis confirmed the highest cumulative incidence of stroke (Fig. 2b). Ischemic stroke after CWS was recorded in 10 patients (30.3%) at 3 months, mostly (80.0%) <48 h (median 1 day, IQR 0–1 day), while stroke after non-CWS TIAs occurred after a median of 77 days (IQR 3–200 days). A small vessel occlusion etiology was detected in all strokes after CWS, with more frequent involvement of the internal capsule (60.0%), followed by the corona radiate (20.0%), pons (10.0%), and putamen (10.0%). Strokes after CWS showed a median National Institute of Health Stroke Scale (NIHSS) score of 4 (IQR 4–8) at the presentation. None of the CWS patients with extracranial ICA stenosis ≥50% (n = 7) nor with intracranial stenosis (n = 4) progressed to stroke. Patients with stroke after CWS showed better 3-month functional outcome (90.0% mRS 0–2) compared to those with stroke after other TIAs (36.8% mRS 0–2; p = 0.002). Nor cases of vascular death, acute coronary syndrome (ACS), or CWS recurrence were recorded. As regards secondary outcomes, we found no significant differences between TIA subgroups (Table 2). Concerning secondary prevention, we found a lower 3-month cumulative stroke incidence among CWS patients treated with DAPT (2/16, 12.5%) compared with those treated with single antiplatelet therapy (8/14, 57.1%; p = 0.010). IVT was administered in 60.0% (6/10) of strokes after CWS and 21.1% (4/19) of strokes after other TIAs, with comparable 3-month median mRS scores (0, IQR 0–1 in both groups; p = 0.323) and without hemorrhagic complications.
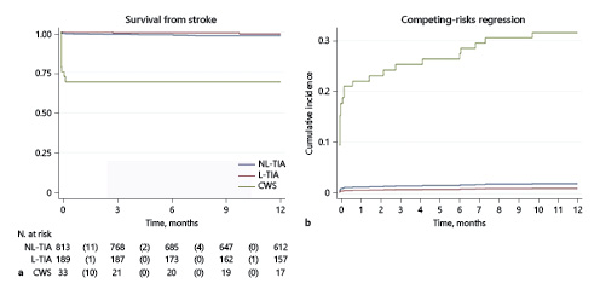
Fig. 2
Kaplan-Meier estimates of stroke-free survival (a) and cumulative incidence of stroke in competing risk analysis (b). CWS, capsular warning syndrome; L, lacunar; NL, nonlacunar.
Discussion
In this study, we investigated frequency, clinical characteristics, risk profile, and prognosis of CWS in a large prospective TIA cohort treated with a fast-track care path. To the best of our knowledge, our study was the first to compare CWS features and outcomes with those of L and NL-TIAs from the same prospective cohort. After Donnan’s study [], data on CWS came mostly from small retrospective studies and poor information is available on long-term prognosis [, ]. The only population-based study [] classified as CWS patients with ≥2 transient stereotyped pure motor symptoms <7 days, thus questioning the adherence to the original CWS definition that requires closer transient episodes [].
Our findings confirmed that CWS is a rare condition, although we found a higher frequency (3.3%) compared to the study by Paul et al. [] (1.5%), which included only patients with stereotyped motor symptoms. As concerns risk profile, we found that ND hypertension was a significant independent risk factor of CWS. ND hypertension was confirmed during the hospital stay through repeated BP measurements ≥24 h after symptom resolution, in order to exclude cases with transient BP elevation possibly related to compensatory mechanisms during the acute phase. We suggest that the lack of stabilizing effects of antihypertensive treatment could elicit blood pressure fluctuations [, ], potentially enhancing hypoperfusion of the penetrating artery. Terai et al. [] performed serial MRI during L symptoms disclosing penetrating artery hypoperfusion and a correspondence between thrombus development into the hypoperfused vessel and ultimate core infarction. Furthermore, early neurologic deterioration in L stroke was recently associated with hypoperfusion lesion on brain imaging []. The shorter duration of CWS episodes versus other TIAs in our population may suggest a quickly reversible interruption of the blood flow in the distal territory of penetrating artery promoted by intermittent hypoperfusion. Symptom claudication in CWS could be explained by the presence of structural changes in the wall of penetrating artery affected by lipohyalinosis, which could favor hypoperfusion and ultimately lead to clot formation []. CWS patients exhibited also a high frequency of other independent risk factors usually associated with small vessel disease (SVD), including cigarette smoking, hypertriglyceridemia, hypercholesterolemia with increased LDL levels, and leukoaraiosis. This finding suggests a more severe small vessel pathology compared even to L (non-CWS) TIAs. Chronic hypoperfusion associated with leukoaraiosis is known to induce pathologic changes at both glial and endothelial levels, which in turn may promote the failure of penetrating artery to address metabolic stresses in the supplied territory []. The early and irreversible failure could also explain our frequent finding of an acute ischemic lesion at neuroimaging close to CWS onset (TIA with lesion, 39.4%). In light of these observations, we might hypothesize that CWS results from the interplay of several risk factors related to SVD, which contribute to determining severe in situ alterations of small penetrating vessels. Hence, early TIA recurrence in CWS may be the marker of a higher burden of cerebrovascular impairment compared to other TIAs, placing this condition at the boundary of L syndrome spectrum.
Another suggested pathophysiology relies on the presence of an atherosclerotic stenosis of proximal MCA. This hypothesis was supported by findings from few reports [, ], sometimes disregarding the original CWS definition, especially for symptom duration (up to 1 month in the report by Lee et al. []). We disclosed a similar rate of intracranial stenosis in TIA subgroups. Specifically, we observed low-grade proximal stenosis of congruous MCA in a single CWS patient who did not progress to stroke, thus suggesting a sporadic finding. Systematic investigation of large CWS samples with MRI vessel wall study could add important information.
As regards prognosis, ischemic stroke was significantly more frequent after CWS versus other TIAs and occurred <48 h in most cases, thus underpinning a strict association with the CWS phenotype. We found a consistently lower cumulative stroke incidence (30.3%) in comparison with prior CWS studies (66–71% [, ]). This observation suggests a positive impact of our fast-track path on prognosis, likely by enhancing timely prevention strategy and close monitoring over time. Regardless of the high cumulative incidence of early stroke, CWS did not recur and showed better post-stroke functional prognosis compared to other TIAs. Concerning CWS treatment, various interventions have been sparsely evaluated, including IVT, single or DAPT, anticoagulation, and vasopressors, without conclusive evidence [, , , ]. In a few reports, DAPT was effective in preventing subsequent stroke [], decreased clinical fluctuations, and improved outcome in progressive L stroke [], capsular or stuttering L syndrome []. In our cohort, we found a lower 3-month cumulative stroke incidence in CWS patients treated with DAPT (12.5%) compared to those treated with single antiplatelet therapy (57.1%; p = 0.010). This finding strengthens the importance of a strict adherence to the most recent guidelines on TIA treatment [], recommending DAPT in all high-risk TIAs. Indeed, the rapid initiation of DAPT might be beneficial, perhaps by hindering the ultimate formation of a thrombus within the hypoperfused vessel. However, patients were not randomized to receive single or dual antiplatelet treatment; thus, clinical trials are requested to provide evidence on this point. Although the small number of treated patients does not allow to draw a firm conclusion, IVT seems reasonable in CWS-related strokes when administrated within the appropriate time window or in the presence of a favorable DWI-fluid attenuated inversion recovery (FLAIR) mismatch.
Our study has some limitations. First, our estimated stroke risk cannot be generalized because of the unknown proportion of TIA patients not seeking medical attention acutely. However, the stereotyped clustering of transient symptoms within a short timeframe should dramatically reduce this potential bias in CWS. Furthermore, given its rarity, CWS accounted for only 3.5% of the whole TIA cohort. Therefore, the small number of outcome events in this subgroup (n = 10) did not allow a reliable evaluation of risk factors associated with stroke occurrence. Another limitation was the inability to perform early brain MRI in all cases, potentially hampering the detection of TIA with lesions. The strengths of our study include its prospective design, the large cohort dimension, the outcome comparison between different TIAs, and the timely comprehensive assessment and management, as provided by our fast-track path.
In conclusion, we found a peculiar risk profile of CWS consistent with a more severe degree of SVD, even in comparison with L (non-CWS) TIAs. In particular, we identified unknown hypertension as a novel independent risk factor of CWS. Regardless of the high cumulative incidence of early stroke, CWS did not recur and showed better post-stroke functional prognosis compared to other TIAs. Nevertheless, the main challenge remains the identification of preventing strategies that may stop the progression to clinical stroke. In this regard, timely initiation of DAPT might be beneficial, while IVT seems effective and safe.
Statement of Ethics
All participants gave written informed consent. The Ethics Committee of S. Orsola-Malpighi University Hospital approved the study on 07/18/2018 (approval code: 398/2018).
Conflict of Interest Statement
The authors have no conflicts of interest to declare.
Funding Sources
The authors declare no funding was received for this study.
Author Contributions
Matteo Foschi: manuscript writing; Paola Rucci: statistical analysis and manuscript revision for intellectual content; Maria Guarino: study design and coordination, data revision, and manuscript writing and revision for intellectual content; Lucia Pavolucci, Francesca Rondelli, Valentina Barone, Rita Rinaldi, Luca Spinardi, Elisabetta Favaretto, Fabrizio Giostra, Benilde Cosmi, Claudio Borghi, and Pietro Cortelli: major role in data acquisition.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material. Further inquiries can be directed to the corresponding author.
Appendix
Bologna TIA Study Group
Andrea Stracciari MD, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy. Susanna Mondini, MD, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy. Roberto D’Angelo, MD, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy. Marianna Nicodemo, MD, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy. Luca Faccioli, MD, Neuroradiology Unit, IRCCS Istituto delle Scienze Neurologiche di Bologna, Bologna, Italy. Alfio Amato, MD, Angiology and Blood Coagulation Unit, IRCCS S. Orsola-Malpighi, Bologna, Italy. Carlotta Brusi, MD, Angiology and Blood Coagulation Unit, IRCCS S. Orsola-Malpighi, Bologna, Italy. Giovanni Maria Puddu, MD, Internal Medicine Unit, IRCCS S. Orsola-Malpighi, Bologna, Italy. Enrico Strocchi, MD, Internal Medicine Unit, IRCCS S. Orsola-Malpighi, Bologna, Italy. Daniela Degli Esposti, MD, Internal Medicine Unit, IRCCS S. Orsola-Malpighi, Bologna, Italy. Gianluca Faggioli, MD, Vascular Surgery Unit, IRCCS S. Orsola-Malpighi, Bologna, Italy. Mauro Gargiulo, MD, Vascular Surgery Unit, IRCCS S. Orsola-Malpighi, Bologna, Italy. Chiara Lanzarini, MD, Emergency Department, Medicina d’Urgenza ePronto Soccorso, IRCCS S. Orsola-Malpighi, Bologna, Italy. Daniela Paola Pomata, MD, Emergency Department, Medicina d’Urgenza ePronto Soccorso, IRCCS S. Orsola-Malpighi, Bologna, Italy.
References
- 1. Donnan GA, O’Malley HM, Quang L, Hurley S, Bladin PF. The capsular warning syndrome: pathogenesis and clinical features. Neurology. 1993;43(5):957–62. https://doi.org/10.1212/wnl.43.5.957.
- 2. Lee J, Albers GW, Marks MP, Lansberg MG. Capsular warning syndrome caused by middle cerebral artery stenosis. J Neurol Sci. 2010;296(1–2):115–20. https://doi.org/10.1016/j.jns.2010.06.003.
- 3. Tassi R, Cerase A, Acampa M, D’Andrea P, Guideri F, Lo Giudice G, et al. Stroke warning syndrome: 18 new cases. J Neurol Sci. 2013;331(1–2):168–71. https://doi.org/10.1016/j.jns.2013.05.027.
- 4. Camps-Renom P, Delgado-Mederos R, Martínez-Domeño A, Prats-Sánchez L, Cortés-Vicente E, Simón-Talero M, et al. Clinical characteristics and outcome of the capsular warning syndrome: a multicenter study. Int J Stroke. 2015;10(4):571–5. https://doi.org/10.1111/ijs.12432.
- 5. Ladeira F, Barbosa R, Calado S, Viana-Baptista M. Capsular warning syndrome: the role of blood pressure. J Neurol Sci. 2017;381:20–1. https://doi.org/10.1016/j.jns.2017.08.008.
- 6. He L, Xu R, Wang J, Zhang L, Zhang L, Zhou F, et al. Capsular warning syndrome: clinical analysis and treatment. BMC Neurol. 2019;19(1):285. https://doi.org/10.1186/s12883-019-1522-0.
- 7. Paul NLM, Simoni M, Chandratheva A, Rothwell PM. Population-based study of capsular warning syndrome and prognosis after early recurrent TIA. Neurology. 2012;79(13):1356–62. https://doi.org/10.1212/WNL.0b013e31826c1af8.
- 8. Xu X, Wei Y, Zhang X, Yang L, Cui Z, Yan J. Value of higher-resolution MRI in assessing middle cerebral atherosclerosis and predicting capsular warning syndrome. J Magn Reson Imag. 2016;44(5):1277–83. https://doi.org/10.1002/jmri.25265.
- 9. Guarino M, Rondelli F, Favaretto E, Stracciari A, Filippini M, Rinaldi R, et al. Short- and long-term stroke risk after urgent management of transient ischaemic attack: the bologna TIA clinical pathway. Eur Neurol. 2015;74(1–2):1–7. https://doi.org/10.1159/000430810.
- 10. Foschi M, Pavolucci L, Rondelli F, Spinardi L, Favaretto E, Filippini M, et al. Prospective observational cohort study of early recurrent TIA: features, frequency, and outcome. Neurology. 2020;95(12):e1733–44. https://doi.org/10.1212/WNL.0000000000010317.
- 11. Wang Y, Wang Y, Zhao X, Liu L, Wang D, Wang C, et al. Clopidogrel with aspirin in acute minor stroke or transient ischemic attack. N Engl J Med. 2013;369(1):11–9. https://doi.org/10.1056/nejmoa1215340.
- 12. Webb AJS, Fischer U, Mehta Z, Rothwell PM. Effects of antihypertensive-drug class on interindividual variation in blood pressure and risk of stroke: a systematic review and meta-analysis. Lancet. 2010 Mar 13;375(9718):906–15. https://doi.org/10.1016/S0140-6736(10)60235-8.
- 13. Terai S, Hori T, Miake S, Tamaki K, Saishoji A. Mechanism in progressive lacunar infarction: a case report with magnetic resonance imaging. Arch Neurol. 2000;57(2):255–8. https://doi.org/10.1001/archneur.57.2.255.
- 14. Vynckier J, Maamari B, Grunder L, Goeldlin MB, Meinel TR, Kaesmacher J, et al. Early neurologic deterioration in lacunar stroke: clinical and imaging predictors and association with long-term outcome. Neurology. 2021 Aug 16;97(14):e1437–46. https://doi.org/10.1212/wnl.0000000000012661.
- 15. Hervé D, Gautier-Bertrand M, Labreuche J, Amarenco PGENIC Investigators. Predictive values of lacunar transient ischemic attacks. Stroke. 2004;35(6):1430–5. https://doi.org/10.1161/01.STR.0000127365.49448.0f.
- 16. Grueter BE, Schulz UG. Age-related cerebral white matter disease (leukoaraiosis): a review. Postgrad Med J. 2012;88(1036):79–87. https://doi.org/10.1136/postgradmedj-2011-130307.
- 17. Kawano H, Nakajima M, Inatomi Y, Yonehara T, Ando Y. Loading dose of clopidogrel in combination with other antithrombotic therapy for capsular warning syndrome. J Stroke Cerebrovasc Dis. 2014;23(5):1265–6. https://doi.org/10.1016/j.jstrokecerebrovasdis.2013.09.020.
- 18. Berberich A, Schneider C, Reiff T, Gumbinger C, Ringleb PA. Dual antiplatelet therapy improves functional outcome in patients with progressive lacunar strokes. Stroke. 2019;50(4):1007–9. https://doi.org/10.1161/STROKEAHA.118.023789.
- 19. Hawkes MA, Braksick SA, Zhang W, Wijdicks EFM, Rabinstein AA. Can we stop the stuttering in stroke? Interventions in 40 patients with acute lacunes. J Neurol Sci. 2019;401:1–4. https://doi.org/10.1016/j.jns.2019.04.009.
- 20. Fonseca AC, Merwick Á, Dennis M, Ferrari J, Ferro JM, Kelly P, et al. European Stroke Organisation (ESO) guidelines on management of transient ischaemic attack. Eur Stroke J. 2021;6(2):V. https://doi.org/10.1177/23969873211027003.