Introduction
Intravenous thrombolysis (IVT) is an effective treatment for acute ischemic stroke (AIS) when started within 3–4.5 h of onset [, ]. Although IVT is relatively successful, the overall vascular recanalization rate is less than 50%, and the probability of recanalized vessels being reoccluded is 14–34% [, ]. The incidence of early neurological deterioration 24 h after IVT was 13.8% []. Early reocclusion is the most common mechanism of early clinical fluctuation and deterioration after IVT for stroke. Its cause remains unclear, but it may be related to residual fibrinogen and platelet aggregation in microcirculation after IVT []. The Antiplatelet therapy in combination with Rt-PA Thrombolysis in Ischemic Stroke (ARTIS) trial showed that intravenous administration of 300 mg aspirin early after IVT did not improve 3-month functional outcomes but increased the risk of symptomatic intracranial hemorrhage (sICH) []. Therefore, current guidelines do not recommend antiplatelet therapy immediately after IVT in AIS. A Korean study concluded that early antiplatelet administration does not increase the risk of intracranial hemorrhage (ICH) after recanalization and may benefit some stroke patients []. The safety of antiplatelet therapy within 24 h of thrombolytic therapy remains controversial. As a novel antiplatelet drug and inhibitor of the glycoprotein (GP) IIb/IIIa receptor, tirofiban has been shown to reduce myocardial infarction and mortality in patients with acute coronary syndromes [, ]. Tirofiban is also beginning to be used for AIS. Therefore, we performed a meta-analysis to evaluate the safety and efficacy of tirofiban combined with IVT in AIS compared with patients not receiving tirofiban.
Materials and Methods
This meta-analysis was conducted according to the recommendations of the preferred reporting items for systematic reviews and meta-analyses guidelines []. The checklist is available in the online supplementary material (for all online suppl. material, see http://www.karger.com/doi/10.1159/000527861).
Search Strategy
A systematic search was conducted using the PubMed and Embase databases for all English literature published up to and including August 31, 2021. The following keywords were used to retrieve relevant studies: “Tirofiban,” “GP IIb/IIIa receptor inhibitor,” “intravenous thrombolysis,” “recombinant tissue plasminogen activator,” “rt-PA,” and “Alteplase.” Further information on the literature search is available in the online supplementary material.
At the same time, references of the relevant literature were manually searched, and authors of all potentially included studies were contacted. After obtaining all possible related literature, Endnote (Thomson ResearchSoft, Stanford, Connecticut, USA) was used to detect and delete duplicate studies. The two authors independently reviewed the titles, abstracts, and keywords of all included studies and resolved any differences. The full texts of all eligible studies were examined in detail.
Study Selection
The inclusion criteria of this meta-analysis were as follows: (1) studies comparing tirofiban with non-tirofiban in patients with AIS after IVT treatment; (2) randomized controlled trials (RCTs) or cohort studies; (3) at least one of the following outcomes were reported: sICH, any ICH, mortality and modified Rankin Scale (mRS) score at the 3-month follow-up; and (4) data of studies can be obtained through articles or by contacting authors. The exclusion criteria of this meta-analysis were as follows: (1) studies on acute myocardial infarction; (2) patients with AIS who did not receive IVT; (3) duplicate studies of data; (4) single-arm tests, conference abstracts, review articles, case reports, meta-analyses, and animal studies; and (5) articles from which we could not extract specific data.
Outcomes
Safety outcome endpoints included sICH, any ICH, and mortality at 3 months. sICH was defined according to the European Cooperative Acute Stroke Study III []. Any ICH was defined according to the definition of Heidelberg hemorrhage classification []. The efficacy outcome endpoint was the mRS score at the 3-month follow-up. A favorable functional outcome was defined as a mRS score of 0–2.
Data Extraction and Quality Assessment
Data from studies were extracted as follows: first author name, year of publication, country, study design, study center, occlusion location, sample size, therapeutic strategy, and tirofiban administration. For the disputed data, the third author extracted the data and evaluated it if it could not be determined after discussion. The quality of cohort studies was assessed by using the Newcastle-Ottawa Scale (NOS) []. Cohort studies with NOS scores of 7–9 were considered high quality. RCTs were assessed by using the Cochrane Collaboration risk assessment tool []. Study quality was assessed with low risk, unclear risk, or high risk for seven items (random sequence generation, allocation concealment, blinding of participants and personnel, blinding of outcome assessment, incomplete outcome data, selective reporting, and other biases).
Statistical Analysis
Statistical analysis was performed by Review Manager (Version 5.4, Cochrane Collaboration, 2020) and STATA (Version 16.0, Stata Corp LLC, USA). The χ2 test was used to analyze whether there was heterogeneity among studies of the same outcome index, and p < 0.1 was considered statistically significant. The value of I2 was used to quantify heterogeneity. If I2 < 50%, indicating homogeneity, a fixed-effect model was used for statistical analysis of effect indicators. If I2 > 50%, indicating the existence of heterogeneity, a random-effect model was used for statistical analysis of effect indicators, and the sources and possible causes of heterogeneity were analyzed by using subgroup analysis. The outcomes were dichotomous variables, and effect indices were reported by odds ratios (OR) and 95% confidence intervals (CI). p < 0.05 was considered statistically significant. Sensitivity analysis was performed by leave-one-out cross-validation to assess the stability of the meta-analysis results. The risk of bias was evaluated by funnel plot.
Results
Search Results and Study Characteristics
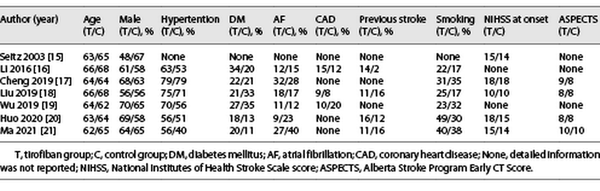
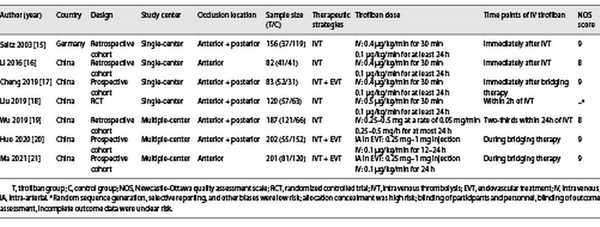
After preliminary retrieval, 1,849 relevant English studies were collected, 149 studies were culled by Endnote software, 1,680 irrelevant studies were removed after reading the title and abstract, and 13 studies that did not meet the inclusion criteria were removed after reading the full text. Seven studies [-] met all inclusion criteria, including one RCT [], three retrospective cohort studies [, , ], and three prospective cohort studies [, , ]. The baseline of the included studies {age, male, hypertension, diabetes mellitus, atrial fibrillation, coronary heart disease, previous stroke, smoking, NIHSS (National Institute of Health stroke scale) at onset, and ASPECTS (Alberta Stroke Program Early CT Score)} is summarized in Table 1. The literature screening process of this meta-analysis is shown in Figure 1, and the study characteristics and quality assessment results are shown in Table 2. In the only RCT study, although it was concluded that IVT combined with tirofiban was safe and effective, there were limitations due to its small sample size and not including patients receiving endovascular therapy (EVT). Therefore, we conducted this meta-analysis and divided into two subgroups according to whether EVT was performed: (1) the bridging therapy group and (2) the non-EVT group.
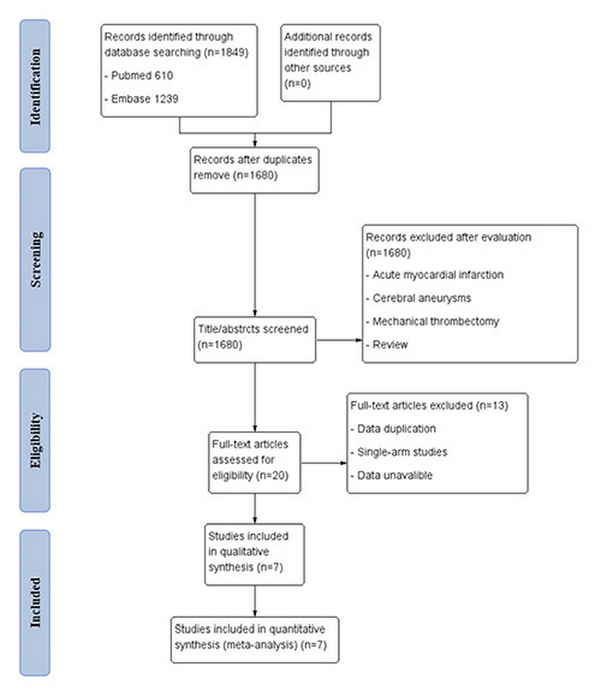
Fig. 1
Flowchart of the screening process.
Safety Outcomes
sICH
The overall incidence of sICH was 41 in 953 patients enrolled in six studies (4.3%). sICH occurred in 14 of 392 (3.6%) in the tirofiban group and 27 of 561 (4.8%) in the control group. Heterogeneity tests between studies showed no heterogeneity (p = 0.81, I2 = 0%), and meta-analysis showed that tirofiban use after IVT did not increase the risk of sICH (OR 0.98; 95% CI 0.50–1.93; p = 0.96) (Fig. 2a).
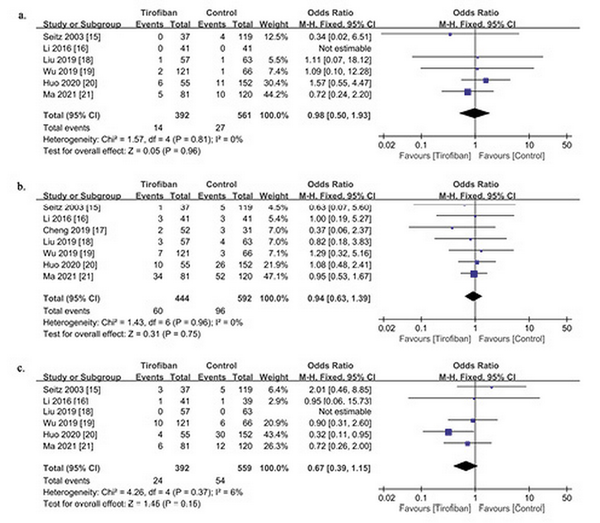
Fig. 2
Forest plot of efficacy outcome in patients with and without tirofiban. Safety outcomes include sICH (a), any ICH (b), and mortality (c).
Any ICH
The overall incidence of any ICH was 156 of 1,036 (15.1%) in the seven studies, including 60 of 444 (13.5%) in the tirofiban group and 96 of 592 (16.2%) in the control group. In evaluating the association between tirofiban and any ICH, pooled analysis showed that tirofiban did not increase the risk of any ICH in patients with IVT (OR 0.94; 95% CI, 0.63–1.39; p = 0.75). There was no significant heterogeneity among these studies (p = 0.96; I2 = 0%) (Fig. 2b).
Mortality
Six studies examined mortality 3 months after IVT. The overall mortality was 78 of 951 (8.2%), of which 24 of 392 (6.1%) were associated with tirofiban, and 54 of 559 (9.7%) were in the control group. In a study [], 2 patients were lost to follow-up at 3 months. Pooled analysis showed that there was no significant association between tirofiban and the reduction of mortality (OR 0.67; 95% CI 0.39–1.15; p = 0.15). Heterogeneity among these studies was low (p = 0.37; I2 = 6%) (Fig. 2c).
Efficacy Outcome
Favorable Functional Outcome
A favorable functional outcome was defined as mRS 0–2 at 3 months in five studies and mRS 0–1 in one study []. The incidence of mRS 0–2 was obtained by contacting the authors. The incidence of overall favorable functional outcomes (mRS 0–2) after IVT was 477 of 838 (57.0%), with 229 of 378 (60.6%) in the tirofiban group and 248 of 460 (53.9%) in the control group. Although there was no statistically significant difference in functional outcomes between the tirofiban group and the control group, it can be seen that the tirofiban group tended to obtain better functional outcomes (OR 1.33; 95% CI 0.99–1.78; p = 0.06) (Fig. 3).
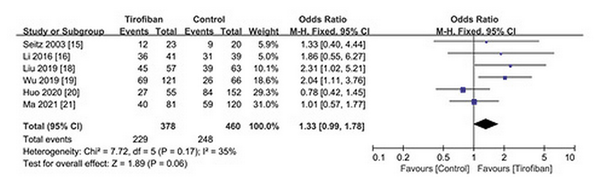
Fig. 3
Forest plot of efficacy outcome (favorable functional outcome) in patients with and without tirofiban.
Subgroup Analysis of Mortality and Favorable Functional Outcome
We performed subgroup analysis on mortality and favorable functional outcomes. Six studies were divided into two groups according to whether EVT was performed: (1) bridging therapy group: two studies [, ] performed EVT (including intraoperative intra-arterial tirofiban injection) before intravenous tirofiban administration; and (2) non-EVT group: four studies [, , , ] only performed IVT before tirofiban intravenous administration. Pooled analysis showed that (1) tirofiban administration after bridging therapy could reduce 3-month mortality (OR 0.47; 95% CI 0.23–0.98; p = 0.04; I2 = 13%) (Fig. 4); (2) tirofiban administration significantly increased the rate of favorable functional outcomes for non-EVT (IVT only) patients (OR 1.98; 95% CI 1.30–3.02; p = 0.002; I2 = 0%) (Fig. 5).
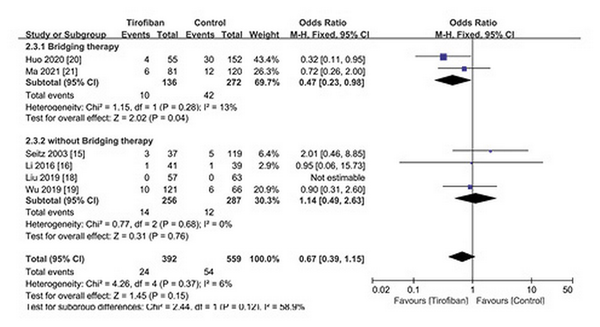
Fig. 4
Subgroup analysis of mortality for tirofiban.
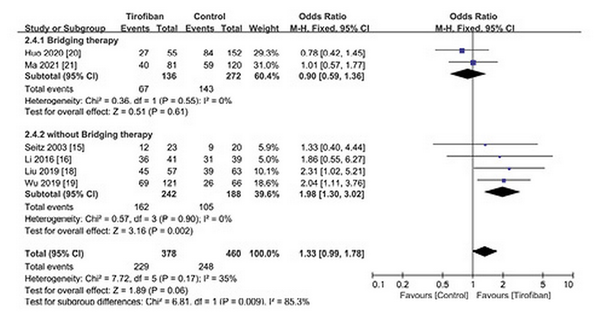
Fig. 5
Subgroup analysis of favorable functional outcome for tirofiban.
Sensitivity Analysis and Publication Bias
Through influence analysis, the results showed that none of the studies significantly influenced the results for any endpoints (online suppl. Fig. 1). Funnel plots showed no significant publication bias in safety and efficacy outcomes (online suppl. Fig. 2).
Discussion
This study is the first meta-analysis of the safety and efficacy of IVT combined with tirofiban for AIS. In this meta-analysis, we found that the use of intravenous tirofiban in patients with AIS after IVT, with or without EVT, was safe and did not increase the risk of sICH, any ICH, or mortality. Subgroup analysis showed that intravenous tirofiban may improve favorable functional outcomes, especially for patients undergoing IVT only.
Currently, tirofiban combined with heparin is commonly used in acute coronary syndromes and percutaneous coronary intervention. Tirofiban is still an off-label drug for AIS, but it has been applied to some selected patients with AIS in clinical practice. The SaTIS (Safety of Tirofiban in Acute Ischemic Stroke) trial showed that tirofiban alone did not increase the risk of cerebral infarction hemorrhage or parenchymal hemorrhage and significantly reduced mortality rates []. Tirofiban could reduce the rate of ICH and mortality even when compared with standard dual antiplatelet therapy [].
One RCT evaluated the efficacy of tirofiban in the treatment of AIS at different time points within 24 h after IVT. The results showed that tirofiban did not increase the incidence of adverse events such as bleeding and death, and early administration of tirofiban was more beneficial. Tirofiban is often used as a salvage agent during or after EVT. In terms of safety, current research is still controversial. Two studies showed that tirofiban did not significantly reduce mortality rates in patients with AIS [, ]. However, a recent meta-analysis showed that tirofiban combined with EVT reduced 3-month mortality []. Similarly, there are different views on efficacy. Zhao et al. [] believed that EVT combined with tirofiban in AIS did not improve the favorable functional outcome of patients at 3 months, while Huo et al. [] reported the opposite. The above studies all included some patients with AIS who received bridging therapy. It is possible that the influence of IVT on the safety and efficacy outcomes led to the inconsistent results of these trials. The safety and efficacy of IVT combined with tirofiban may also be in question. A meta-analysis conducted by Zhou et al. [] showed that the combination of IVT and tirofiban for AIS was safe and did not increase the risk of ICH and mortality, but its efficacy was not analyzed. On this basis, we incorporated the recently published literature and analyzed the efficacy of IVT combined with tirofiban. The results of the safety outcomes were consistent with those of Zhou et al. [] and did not result in higher sICH, any ICH, or mortality, nor did it reduce mortality rates. Efficacy analysis showed that tirofiban use after IVT did not improve functional outcomes at 3 months. Since our meta-analysis also included patients with AIS after bridging therapy, we conducted a subgroup analysis of mortality. The results showed that bridging therapy combined with intravenous tirofiban reduced mortality rates. Although EVT largely completed vascular reconstruction, optical coherence tomography demonstrated the presence of residual red thrombus not seen on computed tomography, magnetic resonance imaging, or digital subtraction angiography []. Residual thrombi may occlude vital perforators and result in death []. The antiplatelet effect of tirofiban reduces the further accumulation of residual thrombi, thereby reducing mortality after EVT. A subgroup analysis of favorable functional outcomes shows that IVT combined with tirofiban achieved a better functional outcome than the control group. The reason for this result is that the control group interventions in trials conducted by Huo et al. and Ma et al. were IVT and EVT. Since EVT itself can observably improve the functional outcome by recanalizing the large vasculature, tirofiban cannot obtain a more positive result for patients treated with bridging therapy. We conjecture that tirofiban still has a positive effect on improving functional outcomes. IVT drugs include recombinant tissue plasminogen activator and urokinase. Liu et al. [] showed that early administration of tirofiban after urokinase-mediated IVT could reduce early neurological deterioration and improve neurological prognosis at 3 months for patients with AIS. This study was finally eliminated to ensure the consistency of IVT drugs and the accuracy of this meta-analysis.
In clinical treatment, tirofiban is still used sparingly and is not as widely used as aspirin and clopidogrel. We believe that tirofiban, administered with a bolus of 0.4 μg/kg/min for 30 min and followed by a continuous infusion of 0.1 μg/kg/min for 24 h, could be used intravenously after IVT to improve functional outcomes. Tirofiban is often used by interventional neurologists as a rescue when thrombectomy is not successful. Previous studies have shown that arterial low-dose tirofiban is safe during EVT [-]. Yi et al. [] conducted a subgroup analysis and showed that low-dose tirofiban was also safe even for patients who received IVT before EVT. Combined with this meta-analysis, we believe that intraoperative arterial low-dose tirofiban (0.25–1 mg) and intraoperative (or postoperative) intravenous doses immediately pumped at 0.1 μg/kg/min for 24 h can be used in cases of mechanical thrombectomy failure or unsatisfactory recanalization of responsible vessels, whether IVT was administered before EVT or not [].
There are several limitations in this meta-analysis. First, our review only included seven studies, including only one RCT. This may increase the risk of bias due to insufficient random sequence generation and blinding. Second, most trials included in this meta-analysis were performed in China and may not be applicable to all ethnic groups. Third, the included studies reported several different IVT strategies, including different dosing methods and dosages. Fourth, there were both anterior and posterior circulation patients in five of these studies. Finally, only two studies were included in the bridging therapy combined with the tirofiban group, and different EVT methods were used, including mechanical thrombectomy, stenting, and balloon angioplasty, so the results should be interpreted with caution. More RCTs are needed to fully clarify the safety and efficacy of tirofiban in patients with AIS receiving IVT.
Conclusion
Intravenous tirofiban could be safe for patients with AIS undergoing IVT, regardless of whether they are also receiving bridging therapy. Intravenous tirofiban may reduce mortality rates for patients undergoing bridging therapy. It also could increase the likelihood of a favorable functional outcome, especially for patients receiving IVT only. Further confirmation by RCTs with larger sample sizes is required.
Acknowledgments
We are particularly grateful to all the people who have given us help on our article.
Statement of Ethics
Ethical approval and consent were not required as this study was based on publicly available data.
Conflict of Interest Statement
The authors declare that they have no competing interests.
Funding Sources
This work was supported by the Fund Program for the Scientific Activities of Selected Returned Overseas Professionals in Shanxi Province (No. 2018-1059-13), Research Project Supported by the Shanxi Scholarship Council of China (No. HGKY2019096), Scientific Research Project Youth Fund of Shanxi Provincial Health Commission (No. 2022011) and Talent introduces research launches of Shanxi Bethune Hospital (No. 2020RC007).
Author Contributions
Heng Shi, Miao-Miao Hou, and Bo Sun: conception and design of the research; Ze-Fan He: acquisition of data; Xiao-Lei Liu and Gang Ren: analysis and interpretation of the data; Gang Ren and Ze-Fan He: statistical analysis; Miao-Miao Hou and Xiao-Lei Liu: obtaining financing; Heng Shi and Miao-Miao Hou: writing of the manuscript; Bo Sun and Xin-Yi Li: critical revision of the manuscript for intellectual content. All authors read and approved the final draft.
Data Availability Statement
All data generated or analyzed during this study are included in this article and its online supplementary material files. Further inquiries can be directed to the corresponding author.
References
- 1. Powers WJ, Rabinstein AA, Ackerson T, Adeoye OM, Bambakidis NC, Becker K, et al. Guidelines for the early management of patients with acute ischemic stroke: 2019 update to the 2018 guidelines for the early management of acute ischemic stroke: a guideline for healthcare Professionals from the American heart association/American stroke association. Stroke. 2019;50(12):e344–418. https://doi.org/10.1161/STR.0000000000000211.
- 2. Wahlgren N, Ahmed N, Dávalos A, Ford GA, Grond M, Hacke W, et al. Thrombolysis with alteplase for acute ischaemic stroke in the Safe Implementation of Thrombolysis in Stroke-Monitoring Study (SITS-MOST): an observational study. Lancet. 2007;369(9558):275–82. https://doi.org/10.1016/S0140-6736(07)60149-4.
- 3. Rha JH, Saver JL. The impact of recanalization on ischemic stroke outcome: a meta-analysis. Stroke. 2007;38(3):967–73. https://doi.org/10.1161/01.str.0000258112.14918.24.
- 4. Alexandrov AV, Grotta JC. Arterial reocclusion in stroke patients treated with intravenous tissue plasminogen activator. Neurology. 2002;59(6):862–7. https://doi.org/10.1212/wnl.59.6.862.
- 5. Seners P, Turc G, Tisserand M, Legrand L, Labeyrie MA, Calvet D, et al. Unexplained early neurological deterioration after intravenous thrombolysis: incidence, predictors, and associated factors. Stroke. 2014;45(7):2004–9. https://doi.org/10.1161/strokeaha.114.005426.
- 6. Zinkstok SM, Roos YBARTIS investigators. Early administration of aspirin in patients treated with alteplase for acute ischaemic stroke: a randomised controlled trial. Lancet. 2012;380(9843):731–7. https://doi.org/10.1016/s0140-6736(12)60949-0.
- 7. Jeong HG, Kim BJ, Yang MH, Han MK, Bae HJ, Lee SH. Stroke outcomes with use of antithrombotics within 24 hours after recanalization treatment. Neurology. 2016;87(10):996–1002. https://doi.org/10.1212/wnl.0000000000003083.
- 8. Karathanos A, Lin Y, Dannenberg L, Parco C, Schulze V, Brockmeyer M, et al. Routine glycoprotein IIb/IIIa inhibitor therapy in ST-segment elevation myocardial infarction: a meta-analysis. Can J Cardiol. 2019 Nov;35(11):1576–88. https://doi.org/10.1016/j.cjca.2019.05.003.
- 9. Roffi M, Chew DP, Mukherjee D, Bhatt DL, White JA, Moliterno DJ, et al. Platelet glycoprotein IIb/IIIa inhibition in acute coronary syndromes. Gradient of benefit related to the revascularization strategy. Eur Heart J. 2002 Sep;23(18):1441–8. https://doi.org/10.1053/euhj.2002.3160.
- 10. Shamseer L, Moher D, Clarke M, Ghersi D, Liberati A, Petticrew M, et al. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015: elaboration and explanation. BMJ. 2015 Jan 2;349:g7647. https://doi.org/10.1136/bmj.g7647.
- 11. Hacke W, Kaste M, Bluhmki E, Brozman M, Dávalos A, Guidetti D, et al. Thrombolysis with alteplase 3 to 4.5 hours after acute ischemic stroke. N Engl J Med. 2008 Sep 25;359(13):1317–29. https://doi.org/10.1056/nejmoa0804656.
- 12. von Kummer R, Broderick JP, Campbell BC, Demchuk A, Goyal M, Hill MD, et al. The Heidelberg bleeding classification: classification of bleeding events after ischemic stroke and reperfusion therapy. Stroke. 2015 Oct;46(10):2981–6. https://doi.org/10.1161/strokeaha.115.010049.
- 13. Stang A. Critical evaluation of the Newcastle-Ottawa scale for the assessment of the quality of nonrandomized studies in meta-analyses. Eur J Epidemiol. 2010 Sep;25(9):603–5. https://doi.org/10.1007/s10654-010-9491-z.
- 14. Cumpston M, Li T, Page MJ, Chandler J, Welch VA, Higgins JP, et al. Updated guidance for trusted systematic reviews: a new edition of the Cochrane Handbook for Systematic Reviews of Interventions. Cochrane Database Syst Rev. 2019 Oct 3;10:ED000142. https://doi.org/10.1002/14651858.ED000142.
- 15. Seitz RJ, Hamzavi M, Junghans U, Ringleb PA, Schranz C, Siebler M. Thrombolysis with recombinant tissue plasminogen activator and tirofiban in stroke: preliminary observations. Stroke. 2003;34(8):1932–5. https://doi.org/10.1161/01.str.0000080535.61188.a6.
- 16. Li W, Lin L, Zhang M, Wu Y, Liu C, Li X, et al. Safety and preliminary efficacy of early tirofiban treatment after alteplase in acute ischemic stroke patients. Stroke. 2016 Oct;47(10):2649–51. https://doi.org/10.1161/strokeaha.116.014413.
- 17. Cheng Z, Geng X, Gao J, Hussain M, Moon SJ, Du H, et al. Intravenous administration of standard dose tirofiban after mechanical arterial recanalization is safe and relatively effective in acute ischemic stroke. Aging Dis. 2019 Oct 1;10(5):1049–57. https://doi.org/10.14336/ad.2018.0922.
- 18. Liu J, Shi Q, Sun Y, He J, Yang B, Zhang C, et al. Efficacy of tirofiban administered at different time points after intravenous thrombolytic therapy with alteplase in patients with acute ischemic stroke. J Stroke Cerebrovasc Dis. 2019 Apr;28(4):1126–32. https://doi.org/10.1016/j.jstrokecerebrovasdis.2018.12.044.
- 19. Wu C, Sun C, Wang L, Lian Y, Xie N, Huang S, et al. Low-dose tirofiban treatment improves neurological deterioration outcome after intravenous thrombolysis. Stroke. 2019 Dec;50(12):3481–7. https://doi.org/10.1161/strokeaha.119.026240.
- 20. Huo X, Yang M, Ma N, Gao F, Mo D, Li X, et al. Safety and efficacy of tirofiban during mechanical thrombectomy for stroke patients with preceding intravenous thrombolysis. Clin Interv Aging. 2020 Jul 23;15:1241–8. https://doi.org/10.2147/cia.s238769.
- 21. Ma G, Li S, Jia B, Mo D, Ma N, Gao F, et al. Safety and efficacy of low-dose tirofiban combined with intravenous thrombolysis and mechanical thrombectomy in acute ischemic stroke: a matched-control analysis from a nationwide registry. Front Neurol. 2021 Jun 10;12:666919. https://doi.org/10.3389/fneur.2021.666919.
- 22. Siebler M, Hennerici MG, Schneider D, von Reutern GM, Seitz RJ, Röther J, et al. Safety of tirofiban in acute ischemic stroke: the SaTIS trial. Stroke. 2011 Sep;42(9):2388–92. https://doi.org/10.1161/strokeaha.110.599662.
- 23. Tao C, Zhu Y, Zhang C, Song J, Liu T, Yuan X, et al. Association between tirofiban monotherapy and efficacy and safety in acute ischemic stroke. BMC Neurol. 2021 Jun 24;21(1):237. https://doi.org/10.1186/s12883-021-02268-8.
- 24. Pan X, Zheng D, Zheng Y, Chan PWL, Lin Y, Zou J, et al. Safety and efficacy of tirofiban combined with endovascular treatment in acute ischaemic stroke. Eur J Neurol. 2019 Aug;26(8):1105–10. https://doi.org/10.1111/ene.13946.
- 25. Zhao L, Jian Y, Li T, Wang H, Lei Z, Sun M, et al. The safety and efficiency of tirofiban in acute ischemic stroke patients treated with mechanical thrombectomy: a multicenter retrospective cohort study. Biochem Res Int. 2020 Apr 27;2020:20205656173. https://doi.org/10.1155/2020/5656173.
- 26. Fu Z, Xu C, Liu X, Wang Z, Gao L. Safety and efficacy of tirofiban in acute ischemic stroke patients receiving endovascular treatment: a meta-analysis. Cerebrovasc Dis. 2020;49(4):442–50. https://doi.org/10.1159/000509054.
- 27. Huo X, Raynald, Wang A, Mo D, Gao F, Ma N, et al. Safety and efficacy of tirofiban for acute ischemic stroke patients with large artery atherosclerosis stroke etiology undergoing endovascular therapy. Front Neurol. 2021 Feb 11;12:630301. https://doi.org/10.3389/fneur.2021.630301.
- 28. Zhou J, Gao Y, Ma QL. Safety and efficacy of tirofiban in acute ischemic stroke patients not receiving endovascular treatment: a systematic review and meta-analysis. Eur Rev Med Pharmacol Sci. 2020;24(3):1492–503. https://doi.org/10.26355/eurrev_202002_20208.
- 29. Pasarikovski CR, Ramjist J, da Costa L, Black SE, Yang V. Optical coherence tomography imaging after endovascular thrombectomy for basilar artery occlusion: report of 3 cases. J Neurosurg. 2019:1–6. https://doi.org/10.3171/2019.5.JNS191252.
- 30. Seners P, Turc G, Oppenheim C, Baron JC. Incidence, causes and predictors of neurological deterioration occurring within 24 h following acute ischaemic stroke: a systematic review with pathophysiological implications. J Neurol Neurosurg Psychiatry. 2015;86(1):87–94. https://doi.org/10.1136/jnnp-2014-308327.
- 31. Liu B, Zhang H, Wang R, Qu H, Sun Y, Zhang W, et al. Early administration of tirofiban after urokinase-mediated intravenous thrombolysis reduces early neurological deterioration in patients with branch atheromatous disease. J Int Med Res. 2020 May;48(5):030006052092629. https://doi.org/10.1177/0300060520926298.
- 32. Zhang S, Hao Y, Tian X, Zi W, Wang H, Yang D, et al. Safety of intra-arterial tirofiban administration in ischemic stroke patients after unsuccessful mechanical thrombectomy. J Vasc Interv Radiol. 2019 Feb;30(2):141–7.e1. https://doi.org/10.1016/j.jvir.2018.08.021.
- 33. Yi HJ, Sung JH, Lee DH. Safety and efficacy of intra-arterial tirofiban injection during mechanical thrombectomy for large artery occlusion. Curr Neurovasc Res. 2020;16(5):416–24. https://doi.org/10.2174/1567202616666191023154956.
- 34. Yang M, Huo X, Gao F, Wang A, Ma N, Shi H, et al. Low-dose rescue tirofiban in mechanical thrombectomy for acute cerebral large-artery occlusion. Eur J Neurol. 2020 Jun;27(6):1056–61. https://doi.org/10.1111/ene.14170.
- 35. Fu QH, Yovitania V, Pei J, Zhou H. Neuroprotective effect of electroacupuncture against acute ischemic stroke via PI3K-Akt-mTOR pathway-mediated autophagy. World J Tradit Chin Med. 2022;8(3):339–49. https://doi.org/10.4103/2311-8571.333712.
Heng Shi and Miao-Miao Hou contributed equally to this study.