What was known before
In 2012, more than 10% of Canada’s gross domestic product was spent on health care. As our population ages, this expenditure is expected to increase. Limiting redundant, non–evidence-based, “low-value health care” is paramount to shielding patients from unnecessary harm, while maintaining a sustainable health care system.
What this adds
The Choosing Wisely Canada Nephrology list parallels the American Choosing Wisely model in guiding physicians and patients in minimizing 5 investigations, treatments, and procedures that have been proven to expose patients to unnecessary harm or stress. These evidence-based recommendations will foster honest conversations with patients and help promote the concept that more efficient medicine leads to higher quality care.
Background
In 2012, more than 10% of Canada’s gross domestic product was spent on health care., As our population ages, this expenditure is expected to increase. In parallel, approximately one-third of medical spending in the United States is superfluous. In addition to driving increased costs, this “low-value health care” puts patients at unnecessary risk. Overuse of interventions that are redundant, not evidence-based, or supply-driven may delay appropriate treatment and expose patients to harm in the form of adverse reactions or iatrogenic complications., Addressing this situation through judicious resource stewardship is paramount to a safe and sustainable public health care system. Evidence-based guidelines and honest conversations with patients will help promote the concept that more efficient medicine leads to better care.
The national Choosing Wisely Canada campaign parallels the American Choosing Wisely model in its aim to guide physicians and patients in minimizing investigations, treatments, and procedures that may expose patients to unnecessary harm or stress, thus ensuring more effective and high-quality care. To date, 26 Canadian medical societies representing a broad spectrum of physicians, patient organizations, and accrediting bodies have developed specialty-specific lists of 5 items that should be questioned.
It is with this mind-set that the Canadian Society of Nephrology (CSN) joined the Choosing Wisely Canada campaign with an aim to compile a list of 5 tests, procedures, or therapies that have proven to be overused or misused in nephrology.
Methods
A working group was formed from the CSN Clinical Practice Guidelines Committee and tasked with establishing a list of recommendations using a multistage Delphi methodology. The working group first contacted all members of the CSN via e-mail to participate in an online survey using the FluidSurveys platform (Fluidware Inc, Ottawa, Ontario, Canada). The survey consisted of open-ended questions asking for their list of 5 interventions that they felt were most inappropriately or ineffectively used, or most potentially harmful. Heads of nephrology divisions across Canada were asked to assist with dissemination of this survey. Twenty-two percent of nephrologists nationwide responded to the survey. Responses were collated into themes by 1 member of the working group (R.M.) and ordered by frequency of occurrence.
The resultant list was reviewed independently by 3 members of the working group (R.M., B.H., S.K.), and each derived a top 10 list of recommendations from the survey responses. A priori criteria were established to prioritize items based on (1) potential for patient harm, (2) strength of evidence in the literature, (3) frequency, (4) potential for nephrology to control the item/issue, and (5) cost. These 3 lists were reviewed by the working group, and through a modified Delphi consensus process, a top 10 list was derived. This draft list of 10 items was presented at the CSN annual general meeting in Vancouver in April 2013, where members voted electronically on their agreement with each recommendation. Five final items were selected based on ranking by the members’ vote, strength of evidence, and potential for meaningful impact. The steps to derive the top 5 items are outlined in Figure 1. In addition, the American Society of Nephrology (ASN) provided the literature review for their parallel nephrology specific enumeration of 5 achievable practice changes to improve patient health through better treatment choices. Recommendations 1, 2, and 5 were adapted with permission from the ASN list. A CSN-elected item dealing with renovascular disease screening was not included, in favor of an ASN recommendation for erythropoiesis-stimulating agent (ESA) prescription (recommendation 1). Indeed, the current Canadian approach to interventional nephrology with respect to renal artery stenting is for the most part already quite conservative, as strongly supported in the literature., A recommendation on ESA prescription was therefore deemed to have a greater potential for changing practice and reducing risks and costs. ASN items dealing with cancer screening and peripherally inserted central catheter (PICC) line insertion in patients with chronic kidney disease (CKD) were discarded as less relevant, given the established Canadian cancer screening guidelines- and Fistula First campaign.,
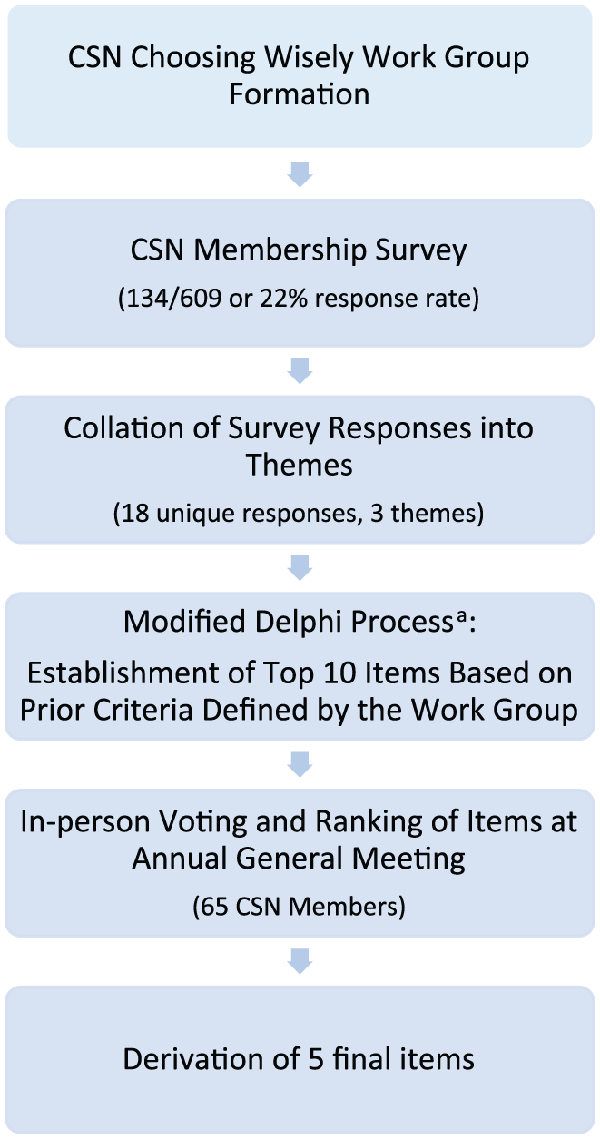
Figure 1
Method of derivation of the final 5 items for the Choosing Wisely campaign.
Note. CSN = Canadian Society of Nephrology; ASN = American Society of Nephrology; ESA = erythropoiesis-stimulating agent; CKD = chronic kidney disease.
aRecommendations 1, 2, and 5 were adapted with permission from the ASN Choosing Wisely list. In addition, elements were included based on relevance to the local Canadian context. Indeed, in view of current evidence-based, conservative uses of interventional radiology for the treatment of hypertension,, a CSN-elected item dealing with renovascular disease screening was rejected, in favor of an ASN recommendation for ESA prescription (recommendation 1), which has greater potential for changing practice and reducing risks and costs. Furthermore, ASN items dealing with cancer screening and PICC line insertion in patients with CKD were discarded as less relevant, given the established Canadian cancer screening guidelines- and Fistula First campaign.,
Patient collaborators then partnered with the CSN to translate the recommendations into lay language accessible to patients and the public.
Results
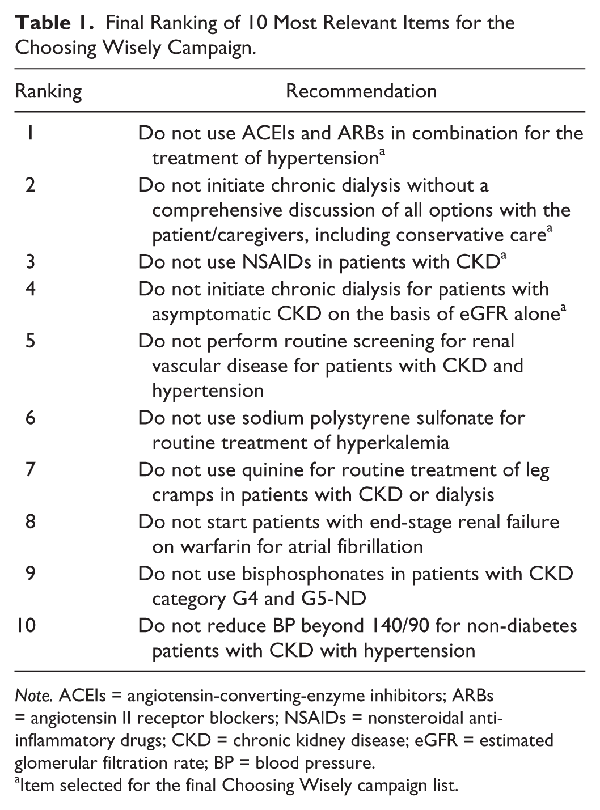
A total of 134 of 609 nephrologists contacted (22%) completed the online survey. From their responses, 18 unique items were collated under 3 themes: tests, treatments, and services. Three reviewers generated individual top 10 lists, from which the 10 most relevant items were selected by consensus. These 10 items were then submitted to in-person voting by 65 CSN members attending the Annual General Meeting, ultimately ranking the 10 items by order of importance (Table 1). The top 5 items from this final list were selected for the Choosing Wisely campaign (Figure 2).

Figure 2
Summary of the 5 items for the Choosing Wisely campaign.
Note. ESAs = erythropoiesis-stimulating agents; CKD = chronic kidney disease; NSAIDs = nonsteroidal anti-inflammatory drugs; COX-2 = cyclooxygenase type 2; ACEIs = angiotensin-converting-enzyme inhibitors; ARBs = angiotensin II receptor blockers; eGFR = estimated glomerular filtration rate.
A summary of key guidelines, systematic reviews, and supporting literature for the top 5 items is outlined here.
Do not initiate ESAs in patients with CKD with hemoglobin levels greater than or equal to 100 g/L without symptoms of anemia.
Supported by the 2012 CSN and 2012 Kidney Disease: Improving Global Outcomes (KDIGO) Clinical Practice Guidelines
For more than 20 years, ESAs have been used for the treatment of anemia in patients with CKD. However, the ideal hemoglobin target has fluctuated over time. In the early 1990s, small studies demonstrated that “normalizing” anemia with ESAs to target a hemoglobin greater than 130 g/L in patients with CKD improved quality of life and exercise tolerance while minimizing transfusion requirements., Over the following 2 decades, large, randomized controlled trials studied the longer term impact of this higher hemoglobin target on mortality and adverse outcomes.- In 2006, both the Correction of Hemoglobin and Outcomes in Renal Insufficiency (CHOIR) trial and the Cardiovascular Risk Reduction by Early Anemia Treatment with Epoetin Beta (CREATE) trial reported no significant difference in survival between patients receiving ESAs for a target hemoglobin above 130 mg/L as compared with a conservative target hemoglobin between 100 and 110 mg/L., Subsequent studies, most notably the Trial to Reduce Cardiovascular Events with Aranesp Therapy (TREAT) trial, demonstrated a trend toward increased adverse cardiovascular events, in particular stroke, with a more aggressive ESA strategy. In addition, Tonelli et al determined the incremental cost per quality-adjusted life year gained to be $50 000 to $60 000 less when targeting a hemoglobin of 95 to 105 mg/L as compared with a higher target of 110 to 120 mg/L. In view of these landmark trials, the current CSN and KDIGO guidelines recommend that ESAs be prescribed to patients with CKD when hemoglobin levels fall between 90 and 100 mg/L, altogether aiming for a hemoglobin between 100 and 110 mg/L. Aggressive therapy, defined as initiation of ESAs in patients with asymptomatic anemia and hemoglobin levels surpassing 110 mg/L, and/or maintenance of ESAs despite hemoglobin levels beyond 135 mg/L, is strongly discouraged. Current data do not support continuing ESAs once hemoglobin levels are maintained above 115 mg/L, but individualized therapy may be considered in symptomatic patients to optimize quality of life and minimize transfusions.,
Do not prescribe nonsteroidal anti-inflammatory drugs (NSAIDs) in individuals with hypertension or heart failure or CKD of all causes, including diabetes.
Supported by the 2014 Joint National Committee 8 (JNC 8) and 2012 KDIGO Clinical Practice Guidelines
An aging population has led to an abundance of musculoskeletal ailments, which are most commonly treated by over-the-counter NSAIDs, including cyclooxygenase type 2 (COX-2) inhibitors. Unfortunately, patients are often unaware of their deleterious renal side effects, and primary care physicians may not screen for them assiduously., Indeed, NSAIDs facilitate the vasoconstriction of the afferent renal arteriole, leading to acute kidney injury and hyperkalemia. This is often potentiated by the concomitant use of renin-angiotensin-aldosterone system inhibitors. An National Health Service (NHS) study of 78 379 patients with stable renal function (eGFR > 30 mL/min/1.73 m2) and on an angiotensin-converting-enzyme inhibitor (ACEI), angiotensin II receptor blocker (ARB), and/or diuretic demonstrated that prescribing NSAIDs increased the probability of developing acute kidney injury (AKI) by 66%. Notable risk factors were age older than 75 years, eGFR less than 60 mL/min/1.73 m2, or concomitant use of a diuretic and aldosterone antagonist. Fortunately, this acute kidney injury is reversible. In a population-based cohort study involving 1522 patients with CKD, Wei et al report sustained and statistically significant improvement of renal function 3 months after discontinuation of NSAIDs. However, this effect was most pronounced in patients with CKD category G5-ND (13.9 mL/min/1.73 m2) than in those with lesser categories of CKD (1.0 mL/min/1.73 m2 and 3.2 mL/min/1.73 m2 for CKD categories G3 and G4, respectively). NSAIDs may also reduce GFR by acute allergic interstitial nephritis, or, in the longer term, nephrotic syndrome. The long-term nephrotoxicity of NSAIDs has been well documented. Hsu et al demonstrated a 32% increased risk of developing CKD in a nationwide study of patients with isolated hypertension, after more that 90 days on NSAIDs. In 2007, Gooch et al showed that chronic NSAID exposure in elderly patients with CKD of all causes led to a 13% decline in eGFR, with patients subjected to high cumulative exposure to NSAIDs at higher risk. Furthermore, NSAIDs amplify the risk of new or worsened high blood pressure by impairing production of natriuretic prostaglandin E2. The resultant sodium retention renders antihypertensive drugs such as ACEIs and ARBs less effective. It also puts patients with heart failure at risk of volume overload and cardiac decompensation., In this context, the KDIGO guidelines identify patients with hypertensive and/or non–end-stage renal disease (ESRD) CKD on regular NSAIDs to be at risk of progressive CKD. The current JNC 8 clinical practice guidelines discourage daily use of NSAIDs other than low-dose aspirin in patients at risk of CKD and in patients with a history of hypertension or heart failure. Rather, prescription of equally effective but safer alternative medical therapies, such as acetaminophen, is preferred.,
Do not prescribe ACEIs in combination with ARBs for the treatment of hypertension, diabetic nephropathy, or heart failure.
Supported by the 2014 JNC 8 and 2014 Canadian Hypertension Education Program (CHEP) guidelines
Several landmark studies have demonstrated that ACEIs or ARBs alone decrease the rate of proteinuria and major adverse cardiovascular events in patients with hypertension, diabetic nephropathy, or heart failure., However, subsequent rigorous randomized control trials have failed to show a similar benefit for dual renin angiotensin system (RAS) blockade. In Ongoing Telmisartan Alone and in Combination with Ramipril Global Endpoint Trial (ONTARGET), combination therapy in patients at high risk of vascular disease effectively reduced blood pressure but did not affect overall survival or doubling of creatinine. Of note, adverse events such as symptomatic hypotension, hyperkalemia, and renal dysfunction were more common., These same side effects led to the early termination of Veterans Affairs Nephropathy in Diabetes (VA NEPHRON D). A 34% reduction in the risk of predefined reductions in eGFR, ESRD, or death with combination therapy as compared with losartan monotherapy in type 2 diabetics with overt nephropathy could not be shown to be clinically significant. Concerns of acute kidney injury and hyperkalemia again limited the use of dual RAS blockade in patients with symptomatic left ventricular dysfunction, as evidenced by a large meta-analysis including 17 337 patients. Despite a significant reduction in hospital admissions for heart failure, no conclusive survival advantage was shown. As such, the current JNC 8 and CHEP guidelines recommend initiation of an ACEI or an ARB for the treatment of hypertension in all adult patients regardless of diabetes status or race, but suggest avoiding an ACEI and ARB combination.,
Do not initiate chronic dialysis without ensuring a shared decision-making process between patients, their families, and their nephrology health care team.
Supported by the 2014 CSN Clinical Practice Guidelines
In patients with ESRD, chronic dialysis may increase survival and may ameliorate signs and symptoms attributable to fluid overload and uremia. However, all dialysis modalities are time-intensive and require a significant amount of personal investment. In-center hemodialysis implies 12 hours of treatment weekly. Home dialysis involves up to 8 hours of treatment 4 to 7 days a week. Peritoneal dialysis requires a daily commitment. This may prove especially burdensome for elderly patients, the fastest expanding subgroup in the ESRD population. The survival advantage of dialysis is lost in patients with ESRD over the age of 75 years with significant comorbidities, notably ischemic heart disease., Strikingly, the 1-year mortality rate after initiation of dialysis is 46% in patients above 80 years old. Furthermore, observational data detected a significant decline in functional status of nursing home residents in the year following the initiation of dialysis, with only 1 in 8 maintaining their predialysis capacity. In contrast, quality of life seems to be maintained in patients opting for conservative management, despite a significant survival disadvantage. Hence, it is essential to foster an open dialogue between the nephrology team, the patient, and their family on the prognosis of ESRD with or without dialysis to establish a care plan that prioritizes the patient’s preferences and values.
Do not initiate dialysis in outpatients with CKD Category G5-ND in the absence of clinical indications.
Supported by the 2014 CSN Clinical Practice Guidelines
As the number of patients with CKD category G5-ND increases, the need for judicious management of dialysis resources is ever more relevant. However, the threshold at which the benefits of dialysis outweigh its risks remains difficult to pinpoint. In 2010, the Initiating Dialysis Early and Late (IDEAL) trial randomized patients with an eGFR of less than 15 mL/min/1.73 m2 to an intent-to-start-early group (which was dialyzed at an eGFR 10-14 mL/min/1.73 m2) and an intent-to-defer group (which was dialyzed at an eGFR 5-7 mL/min/1.73 m2). Despite a 5- to 6-month lag time to dialysis start between both groups, there was no statistically significant difference between groups in survival or in quality of life. Both groups were regularly monitored by nephrologists. Not surprisingly, later dialysis starts were associated with substantial cost savings of at least $10 777 less per patient,, approximately one-third of which ($3610) was attributed to transportation costs. Thus, the CSN clinical practice guidelines recommend close nephrology follow-up of patients with CKD category G5-ND from all causes and deferral of the initiation of dialysis until the eGFR declines below 6 mL/min/1.73 m2 or symptoms of uremia, hypervolemia, refractory acidosis, or hyperkalemia arise.
Discussion
The CSN undertook a formal approach to identify a list of 5 items for nephrology health care professionals and their patients to reevaluate based on evidence that they are overused or misused. These 5 items represent achievable practice changes in our approach to prevent and treat advanced renal disease. Patient with CKD often carry a complex burden of comorbidities, and the benefit associated with an intervention must be weighed against its risks and its impact on each individual patient. Our suggested practice changes aim to optimize adherence and therapeutic effect through patient involvement, education, and personalization of care. There are always exceptions to standardized care, especially when dealing with medically complex renal patients. Our list is not intended to override clinical reasoning but will ideally lead to better, safer, and higher value treatment choices.
This is Canada’s first Choosing Wisely campaign. The method of selection of recommendations was established a priori and followed a formal, rigorous process involving nephrologists nationwide. Early stakeholder involvement was crucial to allowing early and widespread dissemination of recommendations. Although physicians generally appreciate the value of eliminating low-value care, societies often struggle to streamline specialty-specific revenue-generating interventions. During the American Choosing Wisely campaign, many specialties overlooked their own procedural services, despite evidence of redundancy in the care offered. The current Canadian Choosing Wisely Nephrology list includes 3 items specifically dealing with dialysis or the management of advanced chronic renal disease. The remaining 2 items apply to a broader group of patients served by both nephrologists and primary care providers.
Some limitations apply to our campaign. The survey only included nephrologists, and response rates were modest, restricting buy-in to the project. Though a more comprehensive consensus would have been preferred, the focus of the Choosing Wisely project was to generate discussion on areas for potential quality improvement. In fact, response rates in other Choosing Wisely campaigns were similar, ranging from 6% to 36%., Other specialties did not report this value, possibly indicating equally low values., Some American campaigns have even eschewed general polling in favor of discussion within designated expert committees.,- Future study of variations in regional as well as academic and community practices will be required.
The current recommendations apply to the practice of general nephrology, directed to general practitioners. They do not delve into subspecialized areas such as transplantation or glomerulonephritis. More refined recommendations are forthcoming but may be more challenging to compile due to rapidly evolving treatments and a smaller pool of evidence.
A natural extension of this campaign will be to methodically collect feedback from patients and allied health care professionals, in addition to nephrologists. Subsequently, measurement of outcomes and adherence will provide a system-level performance baseline. This, in addition to regular reviews of emerging new evidence, will drive further iterations of the Delphi process, toward updated recommendations.
Conclusion
Collaboration between the patient and the physician has always been central in the care of patients with kidney disease given the complexity and chronicity of kidney disease. This is an opportunity for nephrology health care professionals to once again assert their leadership and advocate for their patients. Choose wisely.
ASN, American Society of Nephrology; ACEI, angiotensin converting enzyme inhibitor; ARB, angiotensin II receptor blocker; CSN, Canadian Society of Nephrology; CKD, chronic kidney disease; eGFR, estimated glomerular filtration rate; ESA, erythropoiesis-stimulating agent; ESRD, end-stage renal disease; JNC8, Joint National Committee 8; KDIGO, Kidney Disease: Improving Global Outcomes; NSAIDs, nonsteroidal anti-inflammatory drugs.
Author Contributions EC drafted the manuscript. All authors critically revised the manuscript and approved the final draft before submission.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. Canadian Institute for Health Information. Canada’s health care spending growth slows. https://www.cihi.ca/en/spending-and-health-workforce/spending/canadas-health-care-spending-growth-slows. Published , 2012. Accessed August 2015.
- 2. Canadian Institute for Health Information. Health spending in Canada in 2013. http://www.cihi.ca/CIHI-ext-portal/internet/en/document/spending+and+health+workforce/spending/release_29oct13_infogra1pg. Accessed August 2015.
- 3. Berwick DM, Hackbarth AD. Eliminating waste in US health care. JAMA. 2012;307(14):1513–1516.
- 4. Delaune J, Everett W. Waste and Inefficiency in the U.S. Healthcare System. Cambridge, MA: New England Healthcare Institute; 2008.
- 5. Olsen LA, Young PL. The Healthcare Imperative: Lowering Costs and Improving Outcomes: Workshop Series Summary. Washington, DC: National Academies Press; 2010.
- 6. Choosing Wisely: An initiative of the ABIM Foundation. http://www.choosingwisely.org. Published 2013. Accessed February 6, 2017.
- 7. Williams AW, Dwyer AC, Eddy AA, et al. Critical and honest conversations: the evidence behind the “Choosing Wisely” campaign recommendations by the American Society of Nephrology. Clin J Am Soc Nephro. 2012;7(10): 1664–1672.
- 8. Linstone HA, Turoff M. The Delphi Method: Techniques and Applications. MA: Addison-Wesley Educational; 1975.
- 9. Cooper CJ, Murphy TP, Cutlip DE, et al. Stenting and medical therapy for atherosclerotic renal-artery stenosis. N Engl J Med. 2014;370(1):13–22.
- 10. ASTRAL Investigators, Wheatley K, Ives N, et al. Revascularization versus medical therapy for renal-artery stenosis. N Engl J Med. 2009;361:1953–1962.
- 11. Canadian Task Force on Preventive Health Care, Bell N, Connor Gorber S, et al. Recommendations on screening for prostate cancer with the prostate-specific antigen test. CMAJ. 2014;186(16):1225–1234.
- 12. Canadian Task Force on Preventive Health Care, Bacchus CM, Dunfield L, et al. Recommendations on screening for colorectal cancer in primary care. CMAJ. 2016;188(5):340–348.
- 13. Canadian Task Force on Preventive Health Care, Dickinson J, Tsakonas E, et al. Recommendations on screening for cervical cancer. CMAJ. 2013;185(1):35–45.
- 14. Canadian Task Force on Preventive Health Care, Tonelli M, Connor Gorber S, et al. Recommendations on screening for breast cancer in average-risk women aged 40-74 years. CMAJ. 2011;183(17):1991–2001.
- 15. Canadian Society of Nephrology Guidelines. Chapter 4: vascular access. J Am Soc Nephrol. 2006;17:S18–S21.
- 16. Canadian Association of Nephrology Nurses and Technologists. Recommendations for the management of vascular access in adult hemodialysis patients (recommendation 1). CANNT J. 2016;25(supp 1):10–12.
- 17. Canadian Erythropoietin Study Group. Association between recombinant human erythropoietin and quality of life and exercise capacity of patients receiving haemodialysis. BMJ. 1990;300:573–578.
- 18. The US Recombinant Human Erythropoietin Predialysis Study Group. Double-blind, placebo-controlled study of the therapeutic use of recombinant human erythropoietin for anemia associated with chronic renal failure in predialysis patients. Am J Kidney Dis. 1991;18:50–59.
- 19. Besarab A, Bolton WK, Browne JK, et al. The effects of normal as compared with low hematocrit values in patients with cardiac disease who are receiving hemodialysis and epoetin. N Engl J Med. 1998;339:584–590.
- 20. Drueke TB, Locatelli F, Clyne N, et al. Normalization of hemoglobin level in patients with chronic kidney disease and anemia. N Engl J Med. 2006;355(20):2071–2084.
- 21. Singh AK, Szczech L, Tang KL, et al. Correction of anemia with epoetin alfa in chronic kidney disease. N Engl J Med. 2006;355(20):2085–2098.
- 22. Pfeffer MA, Burdmann EA, Chen CY, et al. A trial of darbepoetin alfa in type 2 diabetes and chronic kidney disease. N Engl J Med. 2009;361(21):2019–2032.
- 23. Palmer SC, Navaneethan SD, Craig JC, et al. Meta-analysis: erythropoiesis-stimulating agents in patients with chronic kidney disease. Ann Intern Med. 2010;153:23–33.
- 24. Tonelli M, Winkelmayer WC, Jindal KK, Owen WF, Manns BJ. The cost-effectiveness of maintaining higher hemoglobin targets with erythropoietin in hemodialysis patients. Kidney Int. 2003;64(1):295–304.
- 25. Drüeke TB, Parfrey PS. Summary of the KDIGO guideline on anemia and comment: reading between the (guide)line(s). Kidney Int. 2012;82(9):952–960.
- 26. Moist LM, Troyanov S, White CT, et al. Canadian Society of Nephrology commentary on the 2012 KDIGO Clinical Practice Guideline for Anemia in CKD. Am J Kidney Dis. 2013;62(5):860–873.
- 27. Rothenberg RJ, Holcomb JP. Guidelines for monitoring of NSAIDs: who listened? J Clin Rheumatol. 2000;6(5):258–265.
- 28. Pugliese F, Cinotti GA. Nonsteroidal anti-inflammatory drugs (NSAIDs) and the kidney. Nephrol Dial Transplant. 1997;12:386–388.
- 29. Dreischulte T, Morales DR, Bell S, Guthrie B. Combined use of nonsteroidal anti-inflammatory drugs with diuretics and/or renin-angiotensin system inhibitors in the community increases the risk of acute kidney injury. Kidney Int. 2015;88(2):396–403.
- 30. Wei L, MacDonald TM, Jennings C, et al. Estimated GFR reporting is associated with decreased nonsteroidal anti-inflammatory drug prescribing and increased renal function. Kidney Int. 2013;84(1):174–178.
- 31. Hsu C-C, Wang H, Hsu YH, et al. Use of nonsteroidal anti-inflammatory drugs and risk of chronic kidney disease in subjects with hypertension: nationwide longitudinal cohort study. Hypertension. 2015;66(3):524–533.
- 32. Gooch K, Culleton BF, Manns BJ, et al. NSAID use and progression of chronic kidney disease. Am J Med. 2007;120(3):280.e1–280.e7.
- 33. Antman EM, Bennett JS, Daugherty A, Furberg C, Roberts H, Taubert KA; American Heart Association. Use of nonsteroidal antiinflammatory drugs: an update for clinicians: a scientific statement from the American Heart Association. Circulation. 2007;115:1634–1642.
- 34. Scottish Intercollegiate Guidelines Network (sponsored by NHS Quality Improvement Scotland). Management of Chronic Heart Failure: A National Clinical Guideline [Internet]. Edinburgh, UK: Scottish Intercollegiate Guidelines Network. http://www.sign.ac.uk/pdf/SIGN147.pdf. Published 2007. Accessed September 23, 2014.
- 35. National Heart, Lung, and Blood Institute, U.S. Department of Health and Human Services. The seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure [Internet]. National Kidney Foundation. https://www.nhlbi.nih.gov/files/docs/guidelines/jnc7full.pdf. Published 2004. Accessed September 23, 2014.
- 36. Stevens PE, Levin A. Evaluation and management of chronic kidney disease: synopsis of the Kidney Disease: Improving Global Outcomes 2012 clinical practice guideline. Ann Intern Med. 2013;158(11):825–830.
- 37. Phillips CO, Kashani A, Ko DK, Francis G, Krumholz HM. Adverse effects of combination angiotensin II receptor blockers plus angiotensin-converting enzyme inhibitors for left ventricular dysfunction: a quantitative review of data from randomized clinical trials. Arch Intern Med. 2007;167(18):1930–1936.
- 38. Mann JF, Gerstein HC, Pogue J, Bosch J, Yusuf S. Renal insufficiency as a predictor of cardiovascular outcomes and the impact of ramipril: the HOPE randomized trial. Ann Intern Med. 2001;134(8):629–636.
- 39. Mann JF, Schmieder RE, McQueen M, et al. Renal outcomes with telmisartan, ramipril, or both, in people at high vascular risk (the ONTARGET study): a multicentre, randomised, double-blind, controlled trial. Lancet. 2008;372(9638):547–553.
- 40. Fried LF, Emanuele N, Zhang JH, et al. Combined angiotensin inhibition for the treatment of diabetic nephropathy. N Engl J Med. 2013;369(20):1892–1903.
- 41. Heran BS, Musini VM, Bassett K, Taylor RS, Wright JM. Angiotensin receptor blockers for heart failure. Cochrane Database Syst Rev. 2012;4:CD003040.
- 42. Jassal SV, Trpeski L, Zhu N, Fenton S, Hemmelgarn B. Changes in survival among elderly patients initiating dialysis from 1990 to 1999. CMAJ. 2007;177(9):1033–1038.
- 43. Chandna SM, Da Silva-Gane M, Marshall C, Warwicker P, Greenwood RN, Farrington K. Survival of elderly patients with stage 5 CKD: comparison of conservative management and renal replacement therapy. Nephrol Dial Transplant. 2011;26(5):1608–1614.
- 44. Murtagh FE, Marsh JE, Donohoe P, Ekbal NJ, Sheerin NS, Harris FE. Dialysis or not? a comparative survival study of patients over 75 years with chronic kidney disease stage 5. Nephrol Dial Transplant. 2007;22(7):1955–1962.
- 45. Kurella M, Covinsky KE, Collins AJ, Chertow GM. Octogenarians and nonagenarians starting dialysis in the United States. Ann Intern Med. 2007;146(3):177–183.
- 46. Kurella Tamura M, Covinsky KE, Chertow GM, Yaffe K, Landefeld CS, McCulloch CE. Functional status of elderly adults before and after initiation of dialysis. N Engl J Med. 2009;361(16):1539–1547.
- 47. Da Silva-Gane M, Wellsted D, Greenshields H, Norton S, Chandna SM, Farrington K. Quality of life and survival in patients with advanced kidney failure managed conservatively or by dialysis. Clin J Am Soc Nephrol. 2012;7(12):2002–2009.
- 48. Thorsteinsdottir B, Swetz KM, Albright RC. The ethics of chronic dialysis for the older patient: time to reevaluate the norms. Clin J Am Soc Nephrol. 2015;10:2094–2099.
- 49. Cooper BA, Branley P, Bulfone L, et al. A randomized, controlled trial of early versus late initiation of dialysis. N Engl J Med. 2010;363(7):609–619.
- 50. Harris A, Cooper BA, Li JJ, et al. Cost-effectiveness of initiating dialysis early: a randomized controlled trial. Am J Kidney Dis. 2011;57:707–715.
- 51. Nesrallah GE, Mustafa RA, Clark WF, et al. Canadian Society of Nephrology 2014 clinical practice guideline for timing the initiation of chronic dialysis. CMAJ. 2014;186(2):112–117.
- 52. Morden NE, Colla CH, Sequist TD, Rosenthal MB. Choosing wisely—the politics and economics of labeling low-value services. N Engl J Med. 2014;370(7):589–592.
- 53. Chow SL, Carter Thorne J, Bell MJ, et al. Choosing Wisely: the Canadian Rheumatology Association’s list of 5 items physicians and patients should question. J Rheumatol. 2015;42(4):682–689.
- 54. Onuoha OC, Arkoosh VA, Fleisher LA. Choosing wisely in anesthesiology: the gap between evidence and practice. JAMA Intern Med. 2014;174(8):1391–1395.
- 55. Mitera G, Earle C, Latosinsky S, et al. Choosing wisely Canada cancer list: ten low-value or harmful practices that should be avoided in cancer care. J Oncol Pract. 2015;11(3):e296–e303.
- 56. Canadian Cardiovascular Society and Choosing Wisely Canada. The road to creating a list of five things physicians and patients should question. Can J Cardiol. 2014;30(7):949–955.
- 57. Rouster-Stevens KA, Ardoin SP, Cooper AM, et al. Choosing wisely: the American College of Rheumatology’s top 5 for pediatric rheumatology. Arthritis Care Res. 2014;66(5):649–657.
- 58. Wiener RS, Ouellette DR, Diamond E, et al. An official American Thoracic Society/American College of Chest Physicians policy statement: the Choosing Wisely top five list in adult pulmonary medicine. Chest. 2014;145(6):1383–1391.
- 59. Halpern SD, Becker D, Curtis JR, et al. An official American Thoracic Society/American Association of Critical-Care Nurses/American College of Chest Physicians/Society of Critical Care Medicine policy statement: the Choosing Wisely® top 5 list in critical care medicine. Am J Respir Crit Care Med. 2014;190(7):818–826.