*Division of Gastroenterology, Department of Medicine, University of California San Diego, La Jolla, California
‡Division of Gastroenterology, University of Calgary, Calgary, Alberta, Canada
§Knowledge and Evaluation Research Unit, Mayo Clinic, Rochester, Minnesota
¶Division of Gastroenterology, University of Western Ontario, London, Ontario, Canada
*Reprint requests Address requests for reprints to: Siddharth Singh, MD, MS, Division of Gastroenterology, University of California San Diego, 9452 Medical Center Drive, ACTRI 1W501, La Jolla, California 92093. fax: (858) 657-7259.
E-mail: [email protected]
Conflicts of interest These authors disclose the following: Christopher Ma has received consulting fees from AbbVie, Janssen, Takeda, Pfizer, Roche, and Robarts Clinical Trials, Inc, and speaker's fees from AbbVie, Janssen, Takeda, and Pfizer; Parambir S. Dulai has received research support from Takeda, Pfizer, AbbVie, Janssen, Polymedco, ALPCO, Buhlmann, and Prometheus, and consulting fees from Takeda, Pfizer, AbbVie, and Janssen; Samuel Eisenstein has received consulting fees from Auris Health, Inc; Brian G. Feagan has received grant/research support from Millennium Pharmaceuticals, Merck, Tillotts Pharma AG, AbbVie, Novartis Pharmaceuticals, Centocor, Inc, Elan/Biogen, UCB Pharma, Bristol-Myers Squibb, Genentech, ActoGenix, and Wyeth Pharmaceuticals, Inc, has received consulting fees from Millennium Pharmaceuticals, Merck, Centocor, Inc, Elan/Biogen, Janssen-Ortho, Teva Pharmaceuticals, Bristol-Myers Squibb, Celgene, UCB Pharma, AbbVie, Astra Zeneca, Serono, Genentech, Tillotts Pharma AG, Unity Pharmaceuticals, Albireo Pharma, Given Imaging, Inc, Salix Pharmaceuticals, Novonordisk, GSK, Actogenix, Prometheus Therapeutics and Diagnostics, Athersys, Axcan, Gilead, Pfizer, Shire, Wyeth, Zealand Pharma, Zyngenia, GiCare Pharma, Inc, and Sigmoid Pharma, and speaker’s fees from UCB, AbbVie, and J&J/Janssen; Vipul Jairath has received consulting fees from AbbVie, Eli Lilly, GlaxoSmithKline, Arena Pharmaceuticals, Genetech, Pendopharm, Sandoz, Merck, Takeda, Janssen, Robarts Clinical Trials, Topivert, and Celltrion, and speaker’s fees from Takeda, Janssen, Shire, Ferring, AbbVie, and Pfizer; William J. Sandborn has received research grants from Atlantic Healthcare Limited, Amgen, Genentech, Gilead Sciences, AbbVie, Janssen, Takeda, Lilly, Celgene/Receptos, Pfizer, and Prometheus Laboratories (now Prometheus Biosciences), has received consulting fees from AbbVie, Allergan, Amgen, Arena Pharmaceuticals, Avexegen Therapeutics, BeiGene, Boehringer Ingelheim, Celgene, Celltrion, Conatus, Cosmo, Escalier Biosciences, Ferring, Forbion, Genentech, Gilead Sciences, Gossamer Bio, Incyte, Janssen, Kyowa Kirin Pharmaceutical Research, Landos Biopharma, Lilly, Oppilan Pharma, Otsuka, Pfizer, Progenity, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Reistone, Ritter Pharmaceuticals, Robarts Clinical Trials (owned by Health Academic Research Trust), Series Therapeutics, Shire, Sienna Biopharmaceuticals, Sigmoid Biotechnologies, Sterna Biologicals, Sublimity Therapeutics, Takeda, Theravance Biopharma, Tigenix, Tillotts Pharma, UCB Pharma, Ventyx Biosciences, Vimalan Biosciences, and Vivelix Pharmaceuticals, and stock or stock options from BeiGene, Escalier Biosciences, Gossamer Bio, Oppilan Pharma, Prometheus Biosciences (merger of Precision IBD and Prometheus Laboratories), Progenity, Ritter Pharmaceuticals, Ventyx Biosciences, and Vimalan Biosciences; and Siddharth Singh has received research grants from AbbVie and Janssen.
Funding Supported by an American Gastroenterological Association Career Development Award (P.S.D.), a National Institute of Diabetes and Digestive and Kidney Diseases–funded San Diego Digestive Diseases Research Center (P30 DK120515) (W.J.S.), National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (K23DK117058), an American College of Gastroenterology Junior Faculty Development Award (144271), and a Crohn’s and Colitis Foundation Career Development Award (404614) (S.S.).
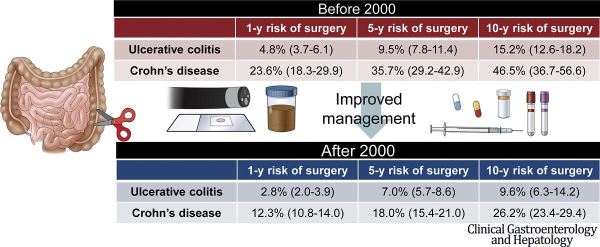
This article has an accompanying continuing medical education activity, also eligible for MOC credit, on page e103. Learning Objectives: Upon completion of the CME activity, successful learners will be able to: 1. List the changes in risk for surgery in patients with newly diagnosed ulcerative colitis and Crohn’s disease in the biologic era; 2. List the 5- and 10-year risk of repeat surgery in patients with Crohn’s disease; 3. List 3 factors that may have contributed to the observed decrease in risk of surgery in patients diagnosed with IBD after 2000.
Siddharth Singh, Section Editor