INTRODUCTION
Projections indicate that the current number of people living with dementia will triplicate by 2050 . This increase will be mainly due to the rising life expectancy of low- and middle-income countries, however the age-standardized prevalence of dementia is predicted to remain stable in both sexes . Epidemiological studies have estimated a population attributable fraction (PAF) for dementia of 30–50%, suggesting that up to half dementia cases could be prevented if those risk factors were eliminated from the population . In cancer research, the term “exposome” was coined to describe the cumulative lifelong experiences and exposures that can impact disease risk . An analogous concept has been proposed for dementia, comprised of exogenous (e.g., head trauma, infections) and endogenous (e.g., hypertension) exposures . Here we will critically review new developments and controversies regarding some potentially modifiable risk factors of the dementia exposome, including exogenous such as air pollution, microbial agents, and traumatic brain injury, as well as endogenous such as cardiovascular risk factors and hearing loss. We will use the broader term Alzheimer's disease and related dementias (ADRD) to account for the frequent co-occurrence of multiple brain pathologies contributing to cognitive decline and for the fact that most studies lack biomarker and autopsy data to ascertain the neuropathological substrate(s) of dementia. For each risk factor, we will examine the epidemiological studies supporting the association between the exposure and ADRD risk, the experimental evidence from mouse models supporting a causal pathophysiological link and, whenever available, the results of clinical trials targeting those risk factors.
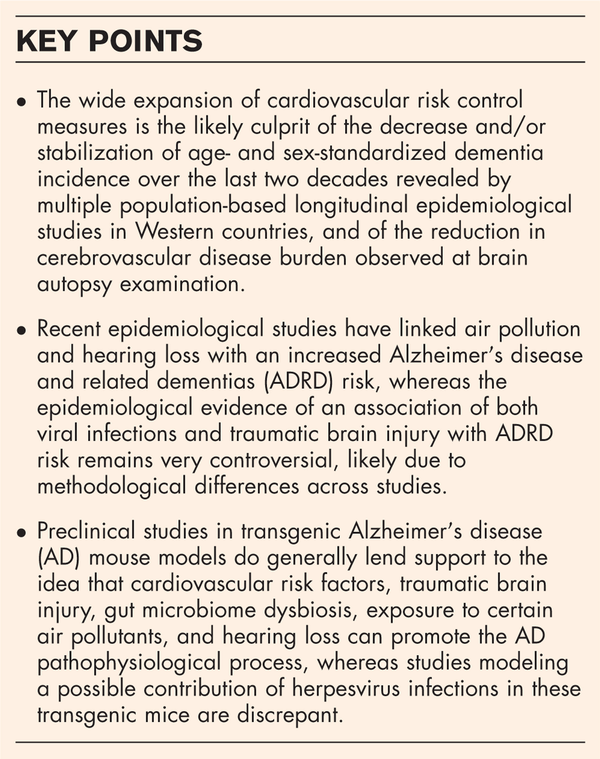
Box 1
no caption available
CARDIOVASCULAR RISK FACTORS
Epidemiological evidence
The importance of mid-life cardiovascular factors in the risk of developing dementia later in life is underscored by several epidemiological observations. First, cardiovascular risk factors (e.g., hypertension, obesity, and sedentarism) rank at the top of all modifiable risk factors by PAF across all ethno-racial groups . Second, population-based clinic-pathological studies have revealed that mixed AD and cerebrovascular disease is the most common pathological substrate underlying dementia in community-dwelling individuals . Third, age-adjusted measures of ADRD incidence and prevalence are decreasing or stabilizing in Western countries , possibly thanks to the expansion of cardiovascular risk screening, prevention, and treatment (e.g., statins, antihypertensive, antidiabetic, and antiplatelet drugs), together with the stricter recommendations to consider hypertension, diabetes mellitus, and hypercholesterolemia adequately controlled. Lastly, and supporting this idea, neuropathological studies have confirmed that the frequency of severe cerebrovascular disease at autopsy has dramatically decreased over the last decades .
Evidence from preclinical studies
A plethora of preclinical studies have shown that, besides their pro-atherosclerosis effects, hypertension and high fat diet can promote the accumulation of Aβ plaques and tau neurofibrillary tangles and worsen cognitive deficits in AD transgenic mouse models, whereas antihypertensive drugs, statins, and exercise improve these AD phenotypes (reviewed in ). The importance of exercise in preventing ADRD has been strengthened by new evidence implicating brain derived neurotrophic factor (BDNF) and irisin in the exercise-induced amelioration of the cognitive deficits observed in AD mice. Both BDNF and irisin promote hippocampal synaptic plasticity and neurogenesis, and irisin additionally reduces Aβ levels through enhancing the secretion of neprilysin – one of the main Aβ-degrading enzymes – by astrocytes .
Evidence from clinical trials
This strong epidemiological and preclinical evidence supporting a synergistic effect of cardiovascular risk factors to promote ADRD has led to the design of clinical trials to test the efficacy of multidomain lifestyle interventions (i.e., targeting exercise, diet, cognitive stimulation, and vascular risk control) and cardiovascular drugs at preventing cognitive decline in elderly people at risk for dementia (Table 1). Although the Finnish FINGER trial revealed the benefits of such multidomain lifestyle interventions on cognition , the French MAPT trial failed to do so . Moreover, a clinical trial testing the MIND diet has recently failed to slow down cognitive decline, brain atrophy, and white matter hyperintensities in participants without cognitive impairment but at risk of dementia . Clinical trials with similar design to the FINGER trial are underway worldwide to shed light on these conflicting outcomes , including the US POINTER (NCT03688126). In the SPRINT-MIND trial, intensive blood pressure control with antihypertensive drugs (goal systolic < 120 mmHg) significantly reduced the risk of MCI and MCI/probable dementia combined diagnoses over the 5-year follow-up compared to standard control (goal systolic < 140 mmHg) in nondemented individuals who had hypertension and increased cardiovascular risk, but no diabetes mellitus or stroke history . Secondary analyses have shown that intensive blood pressure control increases (rather than reduces) cerebral perfusion and slows down white matter damage , however slightly accelerates total brain and AD-like hippocampal volume loss . Data on plasma AD biomarkers would be very informative to determine whether this strategy has any impact on the AD pathophysiological process, but are not currently available. Conversely, in the TOMMORROW trial, low dose of the antidiabetic peroxisome proliferator receptor gamma (PPARγ) agonist pioglitazone failed to delay the onset of MCI due to AD relative to placebo in cognitively intact individuals who were deemed to be at high risk of developing AD based on their age as well as APOE and TOMM40 genotypes .
Table 1
Clinical trials testing cardiovascular preventative interventions with cognition as primary outcome
| Reference | Trial name | Intervention | Trial design | Participants | Primary endpoints | Secondary endpoints | Results |
| Ngandu T et al. 2015 | Finnish Geriatric Intervention Study to Prevent Cognitive Impairment and Disability (FINGER) (NCT01041989) | Multidomain (591) vs. control (599) Multidomain: nutritional advice (diet rich in fruits and vegetables, wholegrain cereals, low-fat milk and meat, fish ≥ 2x/wk, limit sucrose <50 g/d, avoid butter), physical exercise program (muscle strength 1–3x/wk, cardio 2–5x/wk, balance), cognitive training (group educational/cognitive skills sessions and individual web-based computer sessions), social activities (group sessions). Control: regular health advice. | 2-year, multicenter, randomized, double-blind, controlled | Age 60–77 y, community-dwelling, non-demented but at risk for dementia based on CAIDE score ≥6 and cognitive screening | Change in global cognition from baseline over 2 years | Change in cognitive domain-specific z-scores | Significantly slower cognitive decline in intervention vs. placebo groups, particularly in executive function and processing speed |
| Andrieu S et al. 2017 | Multidomain Alzheimer Preventive Trial (MAPT) (NCT00672685) | Multidomain + ω3 PUFA (374) vs. Multidomain + placebo (390) vs. ω3 PUFA (381) vs. placebo (380) Multidomain: cognitive training (group reasoning and memory skills sessions), physical exercise (advice on walking ≥30 min 5x/wk and tailored home-based program), nutritional advice (based on France national guidelines). ω3 PUFA: 2 caps/d, each containing 400 mg DHA and 112.5 mg EPA. | 3-year, multicenter, randomized, placebo-controlled superiority (double-blind regarding ω3 PUFA only) | Age ≥70 y, community-dwelling, non-demented but at risk of dementia based on spontaneous memory complaint to PCP, limitation in one ADL, or slow gait | Change in cognition from baseline over 3 years | • Change in cognitive domain-specific z-scores • CDR-SoB, ADL, physical performance, frailty, and depression scales | No difference of any intervention vs. placebo |
| Barnes LL et al. 2023 | MIND Diet Intervention and Cognitive Decline (MIND) (NCT02817074) | MIND diet (301) vs. control (303) diet (both with mild caloric restriction) MIND diet: increase MIND foods (e.g., skinless, not fried chicken/turkey, olive oil, green leafy and other vegetables, fish, whole-grain cereals, bread and pasta, beans/legumes, berries, nuts). Control diet: focus on portion control, calorie intake, behavioral strategies to lose weight, without changing diet structure. | 3-year, 2-center, randomized, controlled | Age ≥65 y, with overweight (BMI ≥25) and suboptimal diet (MIND-diet score ≤8), non-demented (MoCA ≥22) but with family history of dementia in 1st degree relative | Change in global and cognitive-domain specific cognitive scores from baseline over 3 years | MRI-based brain volumes and WMH | No difference of MIND diet vs. control diet |
| Williamson JD et al. 2019 ; Nasrallah IM et al. 2019 and 2021 ; Dolui S et al. 2022 | Systolic Blood Pressure Intervention Trial (SPRINT-MIND) (NCT01206062) | Intensive (SBP < 120 mmHg, 4278) vs. standard (SBP < 140 mmHg, 4285) treatment with major anti-hypertensive drug classes | 5-year, multicenter, randomized | Age ≥50 y, with hypertension and increased cardiovascular risk but no diabetes or history of stroke, non-demented | Rate of probable dementia diagnosis over 5 years | • Rate of MCI and combined MCI + probable dementia diagnoses over 5 years • MRI-based cerebral blood flow, brain volumes, and WMH | Significantly lower rate of MCI and combined MCI + dementia diagnoses, lower increase in WMH, and greater cerebral blood flow, but also greater total brain and hippocampal atrophy in intensive vs. standard treatment |
| Burns DK et al. 2021 | Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of AD (TOMMORROW) (NCT01931566) | Pioglitazone 0.8 mg/d sustained release (1430) vs. placebo (1406) | 3.5-year, multicenter, randomized, double-blind, placebo-controlled | Age 65–83, community-dwelling, cognitively intact, at high risk of AD (based on age and APOE/TOMM40 genotype) | Time to diagnosis of MCI due to AD | Change in cognition and ADL | No difference in pioglitazone vs. placebo |
BACTERIAL DYSBIOSIS
Epidemiological evidence
Both oral and intestinal bacterial dysbiosis – a dysregulation of the commensal bacterial flora – have emerged as potential risk factors for the development of dementia. Oral bacterial dysbiosis, such as that occurring in bacterial periodontitis, has been associated with AD through inflammatory mediators , but whether this association is due to a causal link between the oral microbiome and the AD pathophysiological process or just reflecting reverse causality (i.e., poor oral health as a result of cognitive decline) remains controversial. Biomarkers offer a unique opportunity to resolve the directionality of this association; for example, a cross-sectional study found a higher oral dysbiosis index (measured as a healthy/unhealthy bacteria genome ratio via DNA sequencing) in cognitively unimpaired old individuals positive for Aβ (i.e., with low Aβ CSF levels), suggesting that oral microbial dysbiosis may precede cognitive decline and contribute to AD progression . Similarly, AD has been associated with reduced diversity and altered composition of the fecal microbiome . Interestingly, these changes precede cognitive decline , cannot be explained by the changes in diet, caloric intake, and/or nutrition status observed in AD , and correlate with CSF AD biomarker levels in both cognitively unimpaired individuals and patients with AD dementia , suggesting a pathophysiological link between gut microbiome dysbiosis and AD. Longitudinal prospective studies with serial AD biomarkers in cognitively unimpaired individuals are needed to confirm this association and unequivocally rule out reverse causality.
Evidence from preclinical studies in mouse models
Preclinical studies support the idea that gut microbiota may impact Aβ and pTau accumulation. For example, a decrease in Aβ plaque accumulation has been described in AD transgenic mice raised in germ-free vs. conventional conditions or treated with an antibiotic cocktail to deplete the gut microbiome . Similarly, tauopathy mice bred in germ-free conditions or treated with broad spectrum antibiotics exhibit a reduction in pTau levels and pTau-mediated neurodegeneration compared to tauopathy mice raised in conventional conditions or treated with vehicle. Of note, these effects were modulated by sex and in the case of pTau also by the APOE genotype . Mechanistically, these studies have implicated gut microbiome-induced changes in the peripheral immune system and/or microglial function , possibly mediated by secreted short-chain fatty acids (SCFAs) – a major by-product of fermentation . However, further studies are needed to dissect the mechanisms by which the gut microbiota and their metabolites may interact with the peripheral immune system and/or microglia, and impact ADRD pathophysiology.
Evidence from clinical trials
Several randomized, double-blind, placebo-controlled clinical trials have evaluated the efficacy of probiotics in patients with MCI with mixed results . In addition, the safety and feasibility of oral fecal microbiota transplant is being evaluated .
VIRUS
Epidemiological evidence
In a revival of the viral hypothesis of AD , the possible implication of certain viral infections in ADRD risk is receiving increasing attention, particularly the reactivation of latent neurotropic viruses of the Herpesviridae family, including herpes simplex virus 1 and 2 (HSV-1/2), varicella-zoster virus (VZV), and Epstein-Barr virus (EBV). Indeed, numerous epidemiological studies in the last few years have tried to address this question but yielded conflicting results (Table 2) . Reasons for these mixed findings are likely methodological, including differences in study design (population-based longitudinal cohort vs. electronic health records or claims data), ascertainment of viral exposure (positive IgM or IgG serology vs. ICD codes and/or medical records of antiviral treatment) and of dementia and/or AD diagnosis (ICD codes vs. expert diagnosis), and length of follow-up (a shorter follow-up is prone to reporting bias, thus overestimating the link between viral infection and dementia). Similarly, neuropathological studies examining the frequency of herpesvirus genome detection in postmortem AD vs. control brains have rendered mixed results . The 2019 SARS-CoV2 pandemic has been associated with an increased risk of cognitive decline and ADRD , however it is still unclear whether these findings are due to neuroinvasive disease leading to neuropathological changes, reporting bias, or unmasking of a preexisting ADRD caused by the systemic inflammatory milieu; ongoing longitudinal cohort studies will eventually elucidate the long-term impact of SARS-CoV-2 infection on ADRD risk. Studies incorporating imaging and/or fluid biomarkers and APOE genotype (a potential major confounder) are much needed but scarce .
Table 2
Recent epidemiological studies on the association between viral infections and ADRD risk
| Reference | Risk factor/exposure | Comparator group | Study design | Location | Outcome | Follow-up length (y) | HR | OR | β | 95% CI |
| Herpes Simplex Virus (HSV) | ||||||||||
| Linard M et al. 2021 | Positive serum HSV IgG | Negative serum HSV IgG | Population-based longitudinal cohort | Bordeaux, Dijon, Montpellier (Southwest France) | Incident AD (NINCDS-ADRDA) | 6.8 ± 2.6 | 1.19 | N.A. | N.A. | 0.81, 1.77 |
| Murphy MJ et al. 2021 | Positive serum HSV1 IgG | Negative serum HSV1 IgG | Population-based longitudinal cohort | Rotterdam (The Netherlands) | Incident dementia (DSM-III-R) | 9.1 ± 3.4 | 1.18 | N.A. | N.A. | 0.83, 1.68 |
| Incident AD (NINCDS-ADRDA) | 1.13 | N.A. | N.A. | 0.77, 1.66 | ||||||
| Global cognition (MMSE) | N.A. | N.A. | -0.12 | -0.24, 0.002 | ||||||
| Serum HSV1 IgG antibody titer | N.A. | Global cognition (MMSE) | N.A. | N.A. | −0.06 | −0.11, −0.01 | ||||
| Shim Y et al. 2022 | Diagnosis of symptomatic HSV infection (ICD) | Controls with no HSV (or VZV) diagnosis | National insurance claim data, matched-cohort | South Korea | Incident dementia (ICD) | Up to 10 | 1.18 | N.A. | N.A. | 1.16, 1.20 |
| Incident AD (ICD) | 1.121 | N.A. | N.A. | 1.183, 1.239 | ||||||
| Varicella-Zoster Virus (VZV) | ||||||||||
| Chen VC-H et al. 2018 | Herpes zoster diagnosis (ICD) | Controls with no VZV diagnosis | National insurance claim data, matched-cohort | Taiwan | Incident dementia (ICD) | Up to 17 | 1.11 | N.A. | N.A. | 1.04, 1.17 |
| Johannesdottir Schmidt SA et al. 2022 | Incident herpes zoster (ICD) or antiviral treatment | No history of herpes zoster or antiviral treatment | National EHR data, matched-cohort | Denmark | Incident dementia (ICD) or antidementia drug | 6 (3–11), range 1–21 | 0.93 | N.A. | N.A. | 0.90, 0.95 |
| Incident AD (ICD) or antidementia drug | 0.93 | N.A. | N.A. | 0.90, 0.97 | ||||||
| Herpes zoster with cranial nerve involvement (ICD) | No history of herpes zoster or antiviral treatment | Incident dementia (ICD) or antidementia drug | 1.07 | N.A. | N.A. | 0.79, 1.45 | ||||
| Herpes zoster with CNS involvement (ICD) | No history of herpes zoster | Incident dementia (ICD) or antidementia drug | 1.94 | N.A. | N.A. | 0.78, 4.80 | ||||
| Shim Y et al. 2022 | Diagnosis of symptomatic VZV infection (ICD) | Controls with no VZV (or HSV) diagnosis | National insurance claim data, matched-cohort | South Korea | Incident dementia (ICD) | Up to 10 | 1.09 | N.A. | N.A. | 1.07, 1.11 |
| Incident AD (ICD) | 1.106 | N.A. | N.A. | 1.081, 1.131 | ||||||
| Epstein-Barr virus (EBV) | ||||||||||
| Torniainen-Holm M et al. 2018 | Positive serum EBV IgG | Negative serum EBV IgG | National health survey | Finland | Incident dementia (ICD) | Up to 13 | 1.74 | N.A. | N.A. | 0.51, 5.92 |
| Cytomegalovirus (CMV) | ||||||||||
| Barnes LL et al. 2015 | Positive serum CMV IgG | Negative serum CMV IgG | Longitudinal cohort (ROS, MAP, and MARS) | Chicago area (USA) | Incident AD (NINCDS-ADRDA) | 5.0 | 2.41 | N.A. | N.A. | 1.53–3.78 |
| Torniainen-Holm M et al. 2018 | Positive serum CMV IgG | Negative serum CMV IgG | National health survey | Finland | Incident dementia (ICD) | Up to 13 | 0.85 | N.A. | N.A. | 0.57, 1.27 |
| Antiviral treatment | ||||||||||
| Chen VC-H et al. 2018 | Antiviral drug after VZV diagnosis | Controls with no VZV diagnosis | National insurance claim data, matched-cohort | Taiwan | Incident dementia (ICD) | Up to 17 | 0.55 | N.A. | N.A. | 0.40–0.77 |
| Hemmingsson E-S et al. 2021 | Positive serum HSV1 IgG with antiviral drug treatment | Positive serum HSV1 IgG without antiviral drug treatment | Population-based, nested case-control | Umea, Sweden (Betula cohort study) | Incident AD (DSM-IV) | Up to 29 | N.A. | 0.287 | N.A. | 0.102, 0.809 |
| Lopatko Lindman K et al. 2021 | Antiviral treatment, irrespective of herpes diagnosis (ICD) | No history of antiviral treatment or herpes diagnosis (ICD) | National EHR and drug prescription data, matched-cohort | Umeâ, Sweden | Incident dementia (ICD) | Up to 12 | 0.89 | N.A. | N.A. | 0.86, 0.92 |
| Herpes diagnosis (ICD) with antiviral treatment | No history of antiviral treatment or herpes diagnosis (ICD) | 0.90 | N.A. | N.A. | 0.82, 0.98 | |||||
| Herpes diagnosis (ICD) without antiviral treatment | No history of antiviral treatment or herpes diagnosis (ICD) | 1.50 | N.A. | N.A. | 1.29, 1.74 | |||||
| Herpes diagnosis (ICD) with antiviral treatment | Herpes diagnosis (ICD) without antiviral treatment | 0.75 | N.A. | N.A. | 0.68–0.83 | |||||
| Schnier C et al. 2021 | History of oral antiherpetic medication | No history of oral antiherpetic medication | National EHR data | Denmark | Incident dementia | 7.4 (3.8–12.2) × 10 000 person-year | 0.91 | N.A. | N.A. | 0.89, 0.93 |
| History of oral antiherpetic medication | No history of oral antiherpetic medication | National EHR data | Scotland | Incident dementia | 2.7 (1.4–4.2) × 10 000 person-year | 0.98 | N.A. | N.A. | 0.64, 1.49 | |
| History of herpes treated with oral antiherpetic drugs | No history of herpes or oral antiherpetic medication | National EHR data | Wales | Incident dementia | 6.7 (3.3–11.3) x 10 000 person-year | 0.91 | N.A. | N.A. | 0.86, 0.97 | |
| History of herpes treated with oral antiherpetic drugs | No history of herpes or oral antiherpetic medication | National EHR data | Germany | Incident dementia | 8.8 (4.5–14.5) × 10 000 person-year | 1.08 | N.A. | N.A. | 0.98, 1.20 | |
| Schnier C et al. 2022 | VZV vaccination | Non-vaccinated without shingles | National EHR data | Wales | Incident dementia (ICD) | Up to 6 | 0.72 | N.A. | N.A. | 0.69, 0.75 |
| Incident AD (ICD) | 0.81 | N.A. | N.A. | 0.77, 0.86 | ||||||
| Incident vascular dementia (ICD) | 0.66 | N.A. | N.A. | 0.61. 0.71 | ||||||
| Severe acute respiratory syndrome coronavirus 2 (SARS-CoV2) | ||||||||||
| Wang L et al. 2022 | COVID-19 infection (ICD) | Non-infection | National EHR data, matched-cohort | USA | Incident AD (ICD) | 1 | 1.69 | N.A. | N.A. | 1.53, 1.72 |
| Taquet M et al. 2022 | COVID-19 infection (ICD) | Other respiratory tract infections (ICD) | International EHR data, matched- cohort | International | Incident dementia (ICD) | 0.5 | 1.33 | N.A. | N.A. | 1.26, 1.41 |
| Liu Y-H et al. 2022 | Severe COVID-19 infection (WHO) | Non-infection | Longitudinal cohort | Wuhan, China | Early-onset cognitive decline (TICS40, IQCODE) | 1 | N.A. | 4.87 | N.A. | 3.30, 7.20 |
| Late-onset cognitive Decline (TICS40, IQCODE) | N.A. | 7.58 | N.A. | 3.58, 16.03 | ||||||
| Progressive cognitive decline (TICS40, IQCODE) | N.A. | 19.00 | N.A. | 9.14, 39.51 | ||||||
Evidence from preclinical studies in mouse models
Studies in AD transgenic mice have investigated whether viral agents, particularly HSV-1 and HHV-6, can induce Aβ plaque deposition. It has been reported that HSV-1 viral particles can interact with Aβ and induce Aβ seeding into plaques, and that Aβ plaques improve survival from HSV-1 encephalitis due to putative antiviral properties of the Aβ peptide . However, other studies have shown that HSV-1 and murine roseolovirus (MRV, the mouse homolog of HHV-6) do not induce Aβ plaque deposition, and that the presence of Aβ plaques does not protect from HSV-1 neurotoxicity or prevents MRV brain invasion . It is noteworthy that, in the absence of viral infection, microglia exhibit a prominent antiviral interferon type I response in both Aβ and tauopathy AD transgenic mice and human AD brains due to the activation of the cGAS-STING pathway . While the cGAS-STING pathway is canonically induced by the presence of viral double-stranded DNA (dsDNA) in the cytosol, other sources of cytosolic dsDNA can be circular mitochondrial DNA (mtDNA) and DNA double-strand breaks, which can leak into the cytosol from mitochondria and nucleus, respectively, due to oxidative stress-mediated damage of mitochondrial membranes and nuclear envelope .
Evidence from clinical trials
Clinical trials to test whether antiviral drugs (e.g., valacyclovir) can slow down cognitive decline in patients with AD are underway and may help elucidate if there is a link between these viruses and the AD pathophysiological process (NCT03282916) .
TRAUMATIC BRAIN INJURY
Epidemiological evidence
Epidemiological research on the association between traumatic brain injury (TBI) and ADRD has been reignited by the delineation of chronic traumatic encephalopathy (CTE) – a neurodegenerative disease neuropathologically defined by a neuronal and astrocytic tauopathy preferentially located in perivascular areas in the depth of cortical sulci, clinically manifested with progressive cognitive decline and prominent behavioral disturbances, and typically affecting professional athletes of contact sports who sustain repetitive head impacts, both concussions and nonconcussive . However, whether one or more TBIs in mid-life can increase the risk of late-onset ADRD remains controversial, partly due to substantial heterogeneity in study design. Overall, studies relying on a self-reported history of TBI have been inconsistent at finding an association between TBI and late-onset dementia, whereas those using medical records and/or claims data to ascertain TBI and dementia have found such association (Table 3). Similarly, cross-sectional biomarker and neuropathological studies investigating a link between a remote history of TBI and AD pathophysiology have failed to establish such association. Longitudinal studies measuring multimodal AD biomarkers closely after a well documented TBI and serially thereafter would help answer this longstanding question.
Table 3
Recent epidemiological studies on the association between TBI and ADRD risk
| Reference | Risk factor/exposure | Comparator | Study design | Location | Outcome | Follow-up length (y) | HR | OR | β | 95% CI |
| Lee Y-K et al. 2013 | Diagnosis of mild TBI (ICD) | No diagnosis of TBI | National insurance claim data | Taiwan | Incident dementia (ICD) or antidementia drug | Up to 5 | 3.26 | N.A. | N.A. | 2.69, 3.94 |
| Crane PK et al. 2016 | TBI with LOC > 1 h (self-reported) | No TBI | Longitudinal cohort (ROS-MAP) | Chicago area (USA) | Incident dementia | 4.7 (2.0–8.0) | 0.84 | N.A. | N.A. | 0.44, 1.57 |
| Incident AD (NINCDS-ADRDA) | 0.82 | N.A. | N.A. | 0.43, 1.59 | ||||||
| Population-based, longitudinal cohort (ACT study) | Seattle area (USA) | Incident dementia (DSM-IV) | 6.2 (3.9–11.1) | 1.18 | N.A. | N.A. | 0.77, 1.78 | |||
| Incident AD (NINCDS-ADRDA) | 1.16 | N.A. | N.A. | 0.72, 1.85 | ||||||
| Raj R et al. 2017 | Diagnosis of moderate-severe TBI (ICD) with length of stay ≥ 3 days | Diagnosis of mild TBI (ICD) with length of stay ≤ 1 day | National EHR data | Finland | Incident dementia (ICD) | 10.0 (4.0–17.0), up to 28 | 1.9 | N.A. | N.A. | 1.6, 2.2 |
| Fann JR et al. 2018 | Diagnosis of TBI (ICD) | No diagnosis of TBI | National EHR data | Denmark | Incident dementia (ICD) or antidementia drug | 9.89 ± 5.10 | 1.24 | N.A. | N.A. | 1.21, 1.27 |
| Incident AD (ICD) | 1.16 | N.A. | N.A. | 1.12, 1.22 | ||||||
| Nordström A and Nordström P 2018 | Diagnosis of TBI (ICD) | No diagnosis of TBI | National EHR data, matched-cohort, prospective | Sweden | Prevalence AD (ICD) | 15.3 (range 0–49) | N.A. | 1.81 | N.A. | 1.75, 1.86 |
| National EHR data, sibling pairs cohort, prospective | N.A. | 1.89 | N.A. | 1.62, 2.22 | ||||||
| National EHR data, case-control, retrospective | N.A. | 1.71 | N.A. | 1.66, 1.76 | ||||||
| Barnes DE et al. 2018 | Diagnosis of mild TBI without LOC (ICD + CTBIE) | No diagnosis of TBI | National EHR data (Veterans Health Administration), matched-cohort | USA | Incident dementia (ICD) | 4.2 ± 3.4 | 2.36 | N.A. | N.A. | 2.10, 2.66 |
| Diagnosis of mild TBI with LOC (ICD + CTBIE) | 2.51 | N.A. | N.A. | 2.29, 2.76 | ||||||
| Diagnosis of moderate-severe TBI (ICD + CTBIE) | 3.77 | N.A. | N.A. | 3.63, 3.91 | ||||||
| Schneider ALC et al. 2021 | History of TBI (self-reported + ICD) | No history of TBI | Community-based, longitudinal cohort (ARIC Study) | Minnesota, Maryland, North Carolina, Mississippi (USA) | Incident dementia (in-person and phone evaluation, and ICD) | 25.0 (17.9–28.2) | 1.44 | N.A. | N.A. | 1.32, 1.57 |
| Plassman BL et al. 2022 | Twins with history of TBI (self-reported) | Twins with no history of TBI | Longitudinal cohort (Duke Twins Study of Memory in Aging, male WWII veterans) | USA | Incident dementia (DSM-III-R) | 39.02 ± 22.42 | 1.44 | N.A. | N.A. | 0.97, 2.14 |
| Incident AD (NINCDS-ADRDA) | 1.23 | N.A. | N.A. | 0.76, 2.00 | ||||||
| Incident non-AD dementia | 2.00 | N.A. | N.A. | 0.97, 4.12 | ||||||
| Grasset L et al. 2023 | History of TBI with LOC (self-reported) | No history of TBI with LOC | Population-based, longitudinal cohort (3C-Dijon study) | Dijon (France) | Incident dementia (DSM-IV) | Up to 12 | 0.90 | N.A. | N.A. | 0.60, 1.36 |
| Incident AD (NINCDS-ADRDA) | 1.03 | N.A. | N.A. | 0.69, 1.52 |
Evidence from preclinical studies in mouse models
While there is considerable heterogeneity in both TBI paradigm (single vs. multiple repetitive mild impacts vs. blast injury) and mouse models used, multiple studies from different labs have shown a link between TBI and AD pathophysiology, specifically both Aβ and pTau accumulation. TBI leads to an acute increase in the amyloidogenic processing of the amyloid-β precursor protein (AβPP) and inhibition of this pathway has been reported to be neuroprotective in this scenario . Additionally, TBI leads to tau hyperphosphorylation, which is not just downstream Aβ generation , and may also promote seeding and propagation of a transmissible pTau form . Some authors, however, have questioned the relationship between TBI and AD . More reproducible TBI models that reflect the diversity of injury biomechanics and evaluate APOE genotype, age, and sex as relevant biological variables are needed to understand the link between TBI and ADRD .
Evidence from clinical trials
Efforts to investigate the efficacy of antitau therapies in patients with chronic traumatic encephalopathy syndrome have just begun with immunotherapy using antitau monoclonal antibodies (NCT03658135).
AIR POLLUTION
Epidemiological evidence
A number of reports have suggested that living in urban and polluted areas (e.g., close to major roads) is associated with an increased risk of ADRD . Specifically, exposure to both nitrogen dioxide (NO2) emissions from combustion engine vehicle emissions and pollution with particulate matter less than 2.5 μm in diameter (PM2.5) have been implicated in this association ; however, they do not fully account for it , suggesting one or more as-yet-unknown additional mediators. PM2.5 exposure has also been correlated with faster cognitive decline and poorer health outcomes (i.e., higher number of hospital admissions) in people living with ADRD . Conversely, a higher chronic residential exposure to green areas may reduce the risk of ADRD and have positive effects on cognition . Importantly, these findings could not be explained by differences in socioeconomic status, education attainment, or health comorbidities (Table 4).
Table 4
Recent epidemiological studies on air pollution as risk factor for ADRD
| Reference | Risk factor/Exposure | Comparator | Study design | Location | Outcome | Follow-up length (y) | HR | OR | β | 95% CI |
| Chen H et al. 2017 | Living <50 m from main road | Living >300 m from main road | National insurance and prescription claim data, zip codes | Ontario (Canada) | Incident dementia (ICD) | Up to 12 | 1.07 | N.A. | N.A. | 1.06, 1.08 |
| Distance from main roada | N.A. | 0.91 | N.A. | N.A. | 0.89, 0.92 | |||||
| Shi L et al. 2020 | Any exposure to PM2.5 | N.A. | Medicare claims and US EPA air quality data | USA | Cause-specific hospital admission for ADRD (ICD) | Up to 17 | 1.13c | N.A. | N.A. | 1.12, 1.14 |
| Low exposure to PM2.5 (<12 μg/m3) | N.A. | 1.18c | N.A. | N.A. | 1.15, 1.21 | |||||
| Grande G et al. 2021 | Low exposure to PM2.5 (≤8.6 μg/m3) 10 years prior | Median exposure level in the entire population | Population-based, longitudinal cohort (SNAC-K) and air quality data | Kungsholmen, Stockholm (Sweden) | Fast cognitive declineb (MMSE) | Up to 10 | N.A. | 1.46 | N.A. | 1.06, 2.01 |
| High exposure to PM2.5 (>8.6 μg/m3) 10 years prior | Median exposure level in the entire population | N.A. | 0.87 | N.A. | 0.76–1.01 | |||||
| Aitken WW et al. 2021 | Highest greenness tertiled | Lowest greenness tertile | Cross-sectional, Medicare claims and NVDI data | Miami-Dade County, Florida (USA) | AD diagnosis (ICD) | N.A. | N.A. | 0.94 | N.A. | 0.88, 1.00 |
| ADRD diagnosis (ICD) | N.A. | N.A. | 0.93 | N.A. | 0.88, 0.99 | |||||
| Non-AD dementia diagnosis (ICD) | N.A. | N.A. | 1.01 | N.A. | 0.93, 1.08 | |||||
| Jimenez MP et al 2022 | Higher green space exposure quintiled | Lower green space exposure quintiled | Cross-sectional, Nurses’ Health Study II and NVDI data | USA | Global cognition (Cogstate) | N.A. | N.A. | N.A. | 0.05 | 0.02, 0.07 |
Evidence from preclinical studies in mouse models
There is a growing body of evidence from preclinical studies supporting a direct effect of air pollutants on AD pathophysiology. Chronic exposure to PM2.5 can accelerate AD phenotypes in transgenic mouse models including Aβ plaque deposition , microglial reactivity and inflammation , tau phosphorylation , and neuronal loss , relative to filtered air. Ozone—a pollutant that causes lung injury and asthma—has been shown to increase Aβ plaque burden and plaque-associated dystrophic neurites as well as impair cholinergic neurotransmission, possibly through altering microglial response to plaques via cross-talk with the peripheral immune system . NO2 inhalation has been shown to accelerate Aβ plaque deposition and impair cognition , whereas carbon monoxide (CO) inhalation actually reduces Aβ generation and improves cognitive deficits in transgenic AD mice . More experimental studies are needed to dissect the effect of each pollutant on cognition and on each AD neuropathological feature.
HEARING LOSS
Epidemiological evidence
Presbycusis (a.k.a. age-related sensorineural hearing loss) affects 40% of people above 65 years old . Together with cardiovascular risk factors, hearing loss is one of the top modifiable risk factors for dementia, with PAF estimations across multiple ethno-racial groups and countries ranging from 5 to 17% . Numerous epidemiological studies in the last 3 years have evaluated the effect of hearing loss on dementia risk and the impact of hearing aids usage, with considerable heterogeneity in the methodology of hearing loss ascertainment (i.e., self-reported vs. informant-reported vs. audiometry test-based) (Table 5) . Overall, studies relying on self or proxy reports have shown an association with an increased incidence of MCI and/or dementia as well as faster rate of cognitive decline. However, reverse causality may have confounded some of these studies since memory loss is often initially masqueraded as hearing loss and hearing impairment can affect the performance on cognitive testing. Regarding ascertainment of hearing impairment by audiometry, speech-in-noise hearing impairment may be a better predictor of incident dementia than impaired pure-tone average hearing level by revealing an alteration in central auditory processing. However, a recent meta-analysis of pure-tone audiometry longitudinal studies did find a significant association between age-related hearing loss and an incident dementia diagnosis as well as the rate of decline in multiple cognitive domains, but not with a diagnosis of AD or vascular dementia . Moreover, another meta-analysis has also found that the usage of hearing aids and/or cochlear implants can reduce the long-term risk of any cognitive decline by 19%, relative to uncorrected hearing loss . Interestingly, older nondemented adults with hearing loss exhibit lower glucose metabolism in the auditory pathway and reduced white matter microstructure integrity specifically in the temporal lobe , suggesting deleterious central effects. Also, noteworthy, dual (visual and hearing) sensory impairment has an additive effect on rate of cognitive decline and ADRD risk over single sensory impairment (visual or hearing) .
Table 5
Recent epidemiological studies on hearing loss as risk factor for ADRD
| Reference | Risk factor/Exposure | Comparator | Study design | Location | Outcome | Follow-up length (y) | HR | OR | β | 95% CI |
| Hwang PH et al. 2020 | Hearing loss (self-reported) | No hearing loss (self-reported) | Population-based clinical trial cohort (GEM) | USA | Incident all-cause dementia (DSM-IV) | Up to 8 years | 1.20 | N.A. | N.A. | 0.88, 1.63 |
| Incident AD (NINCDS-ADRDA) | Up to 8 years | 1.31 | N.A. | N.A. | 0.92, 1.89 | |||||
| Incident VaD (ADDTC) | Up to 8 years | 0.90 | N.A. | N.A. | 0.48, 1.69 | |||||
| Byeon G et al. 2021 | Hearing loss (self-reported) | Normal hearing (self-reported) | Population-based longitudinal cohort (KLOSCAD) | South Korea | Prevalent dementia (DSM-IV) | Up to 6 | N.A. | 1.15 | N.A. | 0.35, 3.79 |
| Incident dementia (DSM-IV) | Up to 6 | 0.93 | N.A. | N.A. | 0.26, 3.30 | |||||
| Cognitive decline (change in Korean CERAD total score) | Up to 6 | N.A. | N.A. | −0.38 | −1.01, 0.26 | |||||
| Bucholc M et al. 2021 | MCI with hearing loss using aids (informant-reported) | MCI with hearing loss not using aids (informant-reported) | Longitudinal retrospective cohort (NACC) | USA (ADRCs) | Incident dementia (DSM-IV) | Up to 14 | 0.73 | N.A. | N.A. | 0.61, 0.89 |
| Dementia with hearing loss using aids (informant-reported) | Dementia with hearing loss not using aids (informant-reported) | Longitudinal retrospective cohort study (NACC) | USA (ADRCs) | Death | Up to 14 | 0.98 | N.A. | N.A. | 0.78, 1.24 | |
| Nedelec T et al. 2022 | Hearing loss (ICD code) | No hearing loss (ICD code) | National EHR data | France | Incident AD (ICD code) | Up to 10 | N.A. | 1.51 | N.A. | 1.01, 2.26 |
| UK | Incident AD (ICD code) | Up to 10 | N.A. | 1.19 | N.A. | 1.04, 1.36 | ||||
| Stevenson JS et al. 2022 | Insufficient speech-in-noise hearing (audiometry) | Normal speech-in-noise hearing (audiometry) | National EHR data | UK | Incident dementia (ICD code) | Median 10.1 | 1.61 | N.A. | N.A. | 1.41, 1.84 |
| Poor speech-in-noise hearing (audiometry) | Normal speech-in-noise hearing (audiometry) | National EHR data | UK | Incident dementia (ICD code) | Median 10.1 | 1.91 | N.A. | N.A. | 1.55, 2.36 | |
| Bucholc M et al. 2022 | Normal cognition with hearing loss (self-reported) | Normal cognition with no hearing loss (self-reported) | Longitudinal retrospective cohort (NACC) | USA (ADRCs) | Incident MCI (Petersen) | 4.0 ± 2.8 | 2.58 | N.A. | N.A. | 1.73, 3.84 |
| Normal cognition with hearing loss using aids (self-reported) | Normal cognition with hearing loss not using aids (self-reported) | Longitudinal retrospective cohort (NACC) | USA (ADRCs) | Incident MCI (Petersen) | 4.0 ± 2.8 | 0.47 | N.A. | N.A. | 0.29, 0.74 | |
| Normal cognition with hearing loss using aids (self-reported) | Normal cognition with no hearing loss (self-reported) | Longitudinal retrospective cohort (NACC) | USA (ADRCs) | Incident MCI (Petersen) | 4.0 ± 2.8 | 0.86 | N.A. | N.A. | 0.56, 1.34 | |
| Hwang PH et al. 2022 | Hearing loss (self-reported) | No hearing loss (self-reported) | Population-based longitudinal study (CHSCS) | USA | Incident all-cause dementia (DSM-IV) | Up to 10 | 1.53 | N.A. | N.A. | 1.20, 1.97 |
| Incident AD (NINCDS-ADRDA) | Up to 10 | 1.54 | N.A. | N.A. | 1.09, 2.18 | |||||
| Incident VaD (ADDTC) | Up to 10 | 1.66 | N.A. | N.A. | 1.16, 2.38 | |||||
| Marinelli JP et al. 2022 | Hearing loss (audiometry pure-tone threshold) | N.A. | Population based longitudinal cohort | Olmsted County, Minnesota (USA) | Incident dementia | Up to 7.0 ± 3.7 | 0.99a | N.A. | N.A. | 0.89, 1.12 |
| Hearing loss (informant-reported) | No hearing loss (informant-reported) | Population based longitudinal cohort | Olmsted County, Minnesota (USA) | Incident dementia | Up to 7.0 ± 3.7 | 1.95 | N.A. | N.A. | 1.45, 2.62 | |
| Huang AR et al. 2023 | Hearing loss (self-reported) | No hearing loss (self-reported) | Longitudinal population-based, Medicare beneficiaries (NHATS) | USA | Change in 10-word list immediate recall | Up to 8 | N.A. | N.A. | −0.22 | −0.36, −0.09 |
| Change in 10-word list delayed recall | Up to 8 | N.A. | N.A. | −0.27 | −0.44, −0.10 | |||||
| Change to fair/poor self-reported memory | Up to 8 | N.A. | N.A. | 0.88 | 0.71, 1.10 |
Evidence from preclinical studies in mouse models
Several lines of preclinical evidence support a link between hearing loss and the AD pathophysiological process. First, several studies using various neurophysiological assessments have reported increased age-related hearing deficits in several AD mouse models, involving both peripheral (including cochlear hair cell loss) and central mechanisms . Second, hearing impairment in AD transgenic mice, modeled either by chronic noise exposure or by chronic perforation of the tympanic membrane (conductive) , accelerates cognitive deficits in these mice, likely through enhancing synaptic loss and dysfunction . Third, a mouse model of sensorineural hearing impairment based on treatment with high doses of the ototoxic drugs kanamycin and furosemide exhibits hippocampal AD-like phenotypes such as increased hyperphosphorylated tau, neuronal loss, reduced neurogenesis, and memory deficits . Last, auditory stimulation at 40 Hz has been shown to ameliorate Aβ plaque burden and tau phosphorylation and seeding in the auditory cortex and hippocampus of AD transgenic mice, possibly through effects in blood vessels and microglia .
Evidence from clinical trials
In a randomized clinical trial comparing the effect of hearing aids vs. a health education control intervention on the rate of cognitive decline over 3 years in individuals with audiometry-proven hearing loss but no substantial cognitive impairment, those at high risk of dementia wearing hearing aids exhibited a 48% slower cognitive decline than those in the control intervention, suggesting that hearing aids may help prevent dementia or delay dementia onset . On the other hand, a smaller and shorter clinical trial in individuals with audiometry-proven hearing loss and mild-to-moderate AD dementia failed to slow down cognitive decline, mitigate neuropsychiatric manifestations, or improve quality of life over the 6-month duration of the trial, suggesting little clinical benefit of hearing loss treatment at the dementia stage . Interpretation of these clinical trials should be cautious, however, because unmasking of the hearing aids intervention may have been suboptimal and performance on cognitive testing partly relies on auditory function. Larger and longer clinical trials with adequate masking of hearing therapy are needed to confirm the clinical benefit of this intervention on cognitively unimpaired individuals with hearing loss and at risk of dementia.
CONCLUSION
We are entering an exciting new era in which epidemiology, genetics, biomarkers, and basic science have the potential to expand our understanding of the complex genetic-environmental interactions explaining ADRD risk . Eventually, it may be possible to develop a robust exposome risk score (ERS) to be used in combination with a polygenic risk score (PRS) to improve the accuracy of ADRD risk prediction at the individual level . Meanwhile, the modifiable risk factors comprising the dementia exposome could represent a window of opportunity to reduce ADRD incidence and prevalence at the population level via health screenings, and education and health policies.
Acknowledgements
We would like to thank patients and families involved in research at the Massachusetts Alzheimer's Disease Research Center (MADRC).
Financial support and sponsorship
M.J. was supported by the Martin L. and Sylvia Seevak-Hoffman Fellowship for Alzheimer's Research; C.M.-C. was supported by the Real Colegio Complutense at Harvard University Research Fellowship and the V Plan Propio US-Acceso Universidad de Sevilla; AS-P was supported by the National Institute on Aging (K08AG064039), the Karen Toffler Charitable Trust, the Jack Satter Foundation, and the Harrison Gardner Jr. Innovation Award.
Conflicts of interest
There are no conflicts of interest.
REFERENCES AND RECOMMENDED READING
Papers of particular interest, published within the annual period of review, have been highlighted as:
▪ of special interest
▪▪ of outstanding interest
REFERENCES
1
Adrait A, Perrot X, Nguyen M-F, et al. ADPHA study group. Do hearing aids influence behavioral and psychological symptoms of dementia and quality of life in hearing impaired Alzheimer's disease patients and their caregivers? J Alzheimers Dis 2017; 58:109–121.2
Aitken WW, Lombard J, Wang K, et al. Relationship of neighborhood greenness to Alzheimer's disease and non-Alzheimer's dementia among 249,405 U.S. medicare beneficiaries. J Alzheimers Dis 2021; 81:597–606.3
Allnutt MA, Johnson K, Bennett DA, et al. Human herpesvirus 6 detection in Alzheimer's disease cases and controls across multiple cohorts. Neuron 2020; 105:1027–1035. e2.4
Andrieu S, Guyonnet S, Coley N, et al. MAPT Study Group. Effect of long-term omega 3 polyunsaturated fatty acid supplementation with or without multidomain intervention on cognitive function in elderly adults with memory complaints (MAPT): a randomised, placebo-controlled trial. Lancet Neurol 2017; 16:377–389.5
Asaoka D, Xiao J, Takeda T, et al. Effect of probiotic bifidobacterium breve in improving cognitive function and preventing brain atrophy in older patients with suspected mild cognitive impairment: results of a 24-week randomized, double-blind, placebo-controlled trial. J Alzheimers Dis 2022; 88:75–95.6
Barnes DE, Byers AL, Gardner RC, et al. Association of mild traumatic brain injury with and without loss of consciousness with dementia in US military veterans. JAMA Neurol 2018; 75:1055–1061.7
Barnes DE, Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol 2011; 10:819–828.8
Barnes LL, Capuano AW, Aiello AE, et al. Cytomegalovirus infection and risk of Alzheimer disease in older black and white individuals. J Infect Dis 2015; 211:230–237.9▪▪
Barnes LL, Dhana K, Liu X, et al. Trial of the MIND diet for prevention of cognitive decline in older persons. N Engl J Med 2023; 389:602–611.In this clinical trial, the MIND diet did not slow down the rate cognitive decline compared to control diet in elderly cognitively unimpaired people with overweight, suboptimal diet, and a first degree relative affected with AD. These results will inform ongoing clinical trials with multidomain lifestyle interventions.
10
Bartos A, Weinerova J, Diondet S. Effects of human probiotics on memory and psychological and physical measures in community-dwelling older adults with normal and mildly impaired cognition: results of a bi-center, double-blind, randomized, and placebo-controlled clinical trial (CleverAge biota). Front Aging Neurosci 2023; 15:1163727.11
Bigley TM, Xiong M, Ali M, et al. Murine roseolovirus does not accelerate amyloid-β pathology and human roseoloviruses are not over-represented in Alzheimer disease brains. Mol Neurodegener 2022; 17:10.12
Bocharova O, Pandit NP, Molesworth K, et al. Alzheimer's disease-associated β-amyloid does not protect against herpes simplex virus 1 infection in the mouse brain. J Biol Chem 2021; 297:100845.13
Bocharova OV, Fisher A, Pandit NP, et al. Aβ plaques do not protect against HSV-1 infection in a mouse model of familial Alzheimer's disease, and HSV-1 does not induce Aβ pathology in a model of late onset Alzheimer's disease. Brain Pathol 2023; 33:e13116.14
Borelli WV, Formoso CR, Bieger A, et al. Race-related population attributable fraction of preventable risk factors of dementia: a Latino population-based study. Alzheimers Dement (Amst) 2023; 15:e12408.15
Boyle PA, Yu L, Wilson RS, et al. Person-specific contribution of neuropathologies to cognitive loss in old age. Ann Neurol 2018; 83:74–83.16
Brewster KK, Pavlicova M, Stein A, et al. A pilot randomized controlled trial of hearing aids to improve mood and cognition in older adults. Int J Geriatr Psychiatry 2020; 35:842–850.17
Bucholc M, Bauermeister S, Kaur D, et al. The impact of hearing impairment and hearing aid use on progression to mild cognitive impairment in cognitively healthy adults: an observational cohort study. Alzheimers Dement (N Y) 2022; 8:e12248.18
Bucholc M, McClean PL, Bauermeister S, et al. Association of the use of hearing aids with the conversion from mild cognitive impairment to dementia and progression of dementia: a longitudinal retrospective study. Alzheimers Dement (N Y) 2021; 7:e12122.19
Burns DK, Alexander RC, Welsh-Bohmer KA, et al. TOMMORROW study investigators. Safety and efficacy of pioglitazone for the delay of cognitive impairment in people at risk of Alzheimer's disease (TOMMORROW): a prognostic biomarker study and a phase 3, randomised, double-blind, placebo-controlled trial. Lancet Neurol 2021; 20:537–547.20
Byeon G, Oh GH, Jhoo JH, et al. Dual sensory impairment and cognitive impairment in the Korean longitudinal elderly cohort. Neurology 2021; 96:e2284–e2295.21
Chen H, Kwong JC, Copes R, et al. Living near major roads and the incidence of dementia, Parkinson's disease, and multiple sclerosis: a population-based cohort study. Lancet 2017; 389:718–726.22
Chen VC-H, Wu S-I, Huang K-Y, et al. Herpes zoster and dementia: a nationwide population-based cohort study. J Clin Psychiatry 2018; 79:16m11312.23
Chen X, Zhang W, Lin Z, et al. Preliminary evidence for developing safe and efficient fecal microbiota transplantation as potential treatment for aged related cognitive impairments. Front Cell Infect Microbiol 2023; 13:1103189.24
Choi SH, Bylykbashi E, Chatila ZK, et al. Combined adult neurogenesis and BDNF mimic exercise effects on cognition in an Alzheimer's mouse model. Science 2018; 361:eaan8821.25
Colombo AV, Sadler RK, Llovera G, et al. Microbiota-derived short chain fatty acids modulate microglia and promote Aβ plaque deposition. Elife 2021; 10:e59826.26
Crane PK, Gibbons LE, Dams-O’Connor K, et al. Association of traumatic brain injury with late-life neurodegenerative conditions and neuropathologic findings. JAMA Neurol 2016; 73:1062–1069.27
Croll PH, Vernooij MW, Reid RI, et al. Hearing loss and microstructural integrity of the brain in a dementia-free older population. Alzheimers Dement 2020; 16:1515–1523.28▪
Cummins TL, Doré V, Feizpour A, et al. Tau, β-amyloid, and glucose metabolism following service-related traumatic brain injury in vietnam war veterans: the Australian Imaging Biomarkers and Lifestyle Study of Aging-Veterans Study (AIBL-VETS). J Neurotrauma 2023; 40:1086–1097.This study failed to find increased levels of AD neuropathology in Vietnam war veterans with a remote history of TBI based on Aβ, tau and FDG PET imaging relative to those without TBI history.
29▪
Dams-O’Connor K, Awwad HO, Hoffman S, et al. Alzheimer's disease-related dementias summit 2022: national research priorities for the investigation of post-traumatic brain injury Alzheimer's disease and related dementias. J Neurotrauma 2023; 40:1512–1523.Consensus statement on the research priorities to investigate the relationship between TBI and ADRD risk.
30
Dodiya HB, Lutz HL, Weigle IQ, et al. Gut microbiota-driven brain Aβ amyloidosis in mice requires microglia. J Exp Med 2022; 219:e20200895.31
Dolui S, Detre JA, Gaussoin SA, et al. Association of intensive vs standard blood pressure control with cerebral blood flow: secondary analysis of the SPRINT MIND Randomized Clinical Trial. JAMA Neurol 2022; 79:380–389.32
Eimer WA, Vijaya Kumar DK, Navalpur Shanmugam NK, et al. Alzheimer's disease-associated β-amyloid is rapidly seeded by herpesviridae to protect against brain infection. Neuron 2018; 99:56–63. e3.33
Luo J, Thomassen JQ, Bellenguez C, et al. European Alzheimer's & Dementia Biobank Mendelian Randomization (EADB-MR) collaboration. Genetic associations between modifiable risk factors and Alzheimer disease. JAMA Netw Open 2023; 6:e2313734.34
Fann JR, Ribe AR, Pedersen HS, et al. Long-term risk of dementia among people with traumatic brain injury in Denmark: a population-based observational cohort study. Lancet Psychiatry 2018; 5:424–431.35▪▪
Ferreiro AL, Choi J, Ryou J, et al. Gut microbiome composition may be an indicator of preclinical Alzheimer's disease. Sci Transl Med 2023; 15:eabo2984.This study demonstrated reduced diversity and differences in composition in gut microbiome already in cognitively unimpaired participants with positive AD biomarkers, and how the gut microbiome profile correlates with Aβ and tau and improves the diagnostic performance of these in preclinical AD. These findings have potentially relevant implications for AD pathophysiology and early diagnosis.
36
Finch CE, Kulminski AM. The Alzheimer's disease exposome. Alzheimers Dement 2019; 15:1123–1132.37
Gates GA, Mills JH. Presbycusis. Lancet 2005; 366:1111–1120.38
GBD 2019 Dementia Forecasting Collaborators. Estimation of the global prevalence of dementia in 2019 and forecasted prevalence in 2050: an analysis for the Global Burden of Disease Study 2019. Lancet Public Health 2022; 7:e105–e125.39
Goldstein LE, Fisher AM, Tagge CA, et al. Chronic traumatic encephalopathy in blast-exposed military veterans and a blast neurotrauma mouse model. Sci Transl Med 2012; 4:134–160.40
Grande G, Ljungman PLS, Eneroth K, et al. Association between cardiovascular disease and long-term exposure to air pollution with the risk of dementia. JAMA Neurol 2020; 77:801–809.41▪
Grasset L, Power MC, Crivello F, et al. How traumatic brain injury history relates to brain health MRI markers and dementia risk: findings from the 3C Dijon cohort. J Alzheimers Dis 2023; 92:183–193.This epidemiological study failed to find a significant association between self-reported TBI and risk of dementia and AD.
42
Greve HJ, Dunbar AL, Lombo CG, et al. The bidirectional lung brain-axis of amyloid-β pathology: ozone dysregulates the peri-plaque microenvironment. Brain 2023; 146:991–1005.43▪▪
Grodstein F, Leurgans SE, Capuano AW, et al. Trends in postmortem neurodegenerative and cerebrovascular neuropathologies over 25 years. JAMA Neurol 2023; 80:370–376.This analysis of the ROSMAP neuropathological cohort over 25 years revealed that the frequency and severity of cerebrovascular neuropathologies are decreasing, whereas measures of neurodegenerative pathologies have remained stable, indicating that the decreasing incidence of dementia in Western countries is likely due to improved cardio- and cerebrovascular health and, perhaps, greater resilience to AD neuropathological changes.
44▪
Gu D, Ou S, Liu G. Traumatic brain injury and risk of dementia and Alzheimer's disease: a systematic review and meta-analysis. Neuroepidemiology 2022; 56:4–16.Systematic review and meta-analysis of epidemiological studies investigating the relationship between TBI and dementia and AD risk.
45
Hemmingsson E-S, Hjelmare E, Weidung B, et al. Antiviral treatment associated with reduced risk of clinical Alzheimer's disease – a nested case–control study. Alzheimers Dement (N Y) 2021; 7:e12187.46
Huang AR, Rebok GW, Swenor BK, et al. Concurrent hearing and vision impairment and 8-year memory decline in community-dwelling older adults. Alzheimers Dement 2023; 19:2307–2316.47
Hwang PH, Longstreth WT, Brenowitz WD, et al. Dual sensory impairment in older adults and risk of dementia from the GEM study. Alzheimers Dement (Amst) 2020; 12:e12054.48
Hwang PH, Longstreth WT, Thielke SM, et al. Longitudinal changes in hearing and visual impairments and risk of dementia in older adults in the United States. JAMA Netw Open 2022; 5:e2210734.49
Islam MR, Valaris S, Young MF, et al. Exercise hormone irisin is a critical regulator of cognitive function. Nat Metab 2021; 3:1058–1070.50
Jafari Z, Afrashteh N, Kolb BE, Mohajerani MH. Hearing loss and impaired short-term memory in an Alzheimer's disease mouse model of amyloid-beta pathology. Exp Neurol 2023; 365:114413.51▪
Jemimah S, Chabib CMM, Hadjileontiadis L, AlShehhi A. Gut microbiome dysbiosis in Alzheimer's disease and mild cognitive impairment: a systematic review and meta-analysis. PLoS One 2023; 18:e0285346.Systematic review and meta-analysis of cross-sectional and longitudinal studies linking gut bacterial dysbiosis with MCI.
52
Jimenez MP, Elliott EG, DeVille NV, et al. Residential green space and cognitive function in a large cohort of middle-aged women. JAMA Netw Open 2022; 5:e229306.53
Johannesdottir Schmidt SA, Veres K, Sørensen HT, et al. Incident herpes zoster and risk of dementia: a population-based Danish cohort study. Neurology 2022; 99:e660–668.54
Jørgensen K, Nielsen TR, Nielsen A, Waldemar G. Potential for prevention of dementia in Denmark. Alzheimers Dement 2023; 19:4590–4598.55
Kamer AR, Pushalkar S, Gulivindala D, et al. Periodontal dysbiosis associates with reduced CSF Aβ42 in cognitively normal elderly. Alzheimers Dement (Amst) 2021; 13:e12172.56
Katz DI, Bernick C, Dodick DW, et al. National institute of neurological disorders and stroke consensus diagnostic criteria for traumatic encephalopathy syndrome. Neurology 2021; 96:848–863.57▪
Kim E, Kim H, Jedrychowski MP, et al. Irisin reduces amyloid-β by inducing the release of neprilysin from astrocytes following downregulation of ERK-STAT3 signaling. Neuron 2023; 111:3619–3633.e8.This study demonstrated that the exercise-induced hormone irisin promotes Aβ degradation by the Aβ-degrading enzyme neprilysin secreted from astrocytes.
58
Kim HJ, Joe Y, Chen Y, et al. Carbon monoxide attenuates amyloidogenesis via down-regulation of NF-κB-mediated BACE1 gene expression. Aging Cell 2019; 18:e12864.59
Kim JS, Lee H-J, Lee S, et al. Conductive hearing loss aggravates memory decline in Alzheimer model mice. Front Neurosci 2020; 14:843.60
Kivipelto M, Mangialasche F, Snyder HM, et al. World-Wide FINGERS Network: A global approach to risk reduction and prevention of dementia. Alzheimers Dement 2020; 16:1078–1094.61
Lee M, Whitsel E, Avery C, et al. Variation in population attributable fraction of dementia associated with potentially modifiable risk factors by race and ethnicity in the US. JAMA Netw Open 2022; 5:e2219672.62
Lee S-H, Chen Y-H, Chien C-C, et al. Three month inhalation exposure to low-level PM2.5 induced brain toxicity in an Alzheimer's disease mouse model. PLoS One 2021; 16:e0254587.63
Lee Y-K, Hou S-W, Lee C-C, et al. Increased risk of dementia in patients with mild traumatic brain injury: a nationwide cohort study. PLoS One 2013; 8:e62422.64
Levy Nogueira M, Hamraz M, Abolhassani M, et al. Mechanical stress increases brain amyloid β, tau, and α-synuclein concentrations in wild-type mice. Alzheimers Dement 2018; 14:444–453.65▪▪
Lin FR, Pike JR, Albert MS, et al. ACHIEVE Collaborative Research Group. Hearing intervention versus health education control to reduce cognitive decline in older adults with hearing loss in the USA (ACHIEVE): a multicentre, randomised controlled trial. Lancet 2023; 402:786–797.This clinical trial found a significant slowing of cognitive decline over 3 years of follow-up in individuals with hearing loss at high risk of dementia who were randomized to hearing aids vs. the control group, but not in individuals at low risk for dementia.
66
Linard M, Baillet M, Letenneur L, et al. Herpes simplex virus, early neuroimaging markers and incidence of Alzheimer's disease. Transl Psychiatry 2021; 11:414.67
Liu Y, Fang S, Liu L-M, et al. Hearing loss is an early biomarker in APP/PS1 Alzheimer's disease mice. Neurosci Lett 2020; 717:134705.68
Liu Y-H, Chen Y, Wang Q-H, et al. One-year trajectory of cognitive changes in older survivors of COVID-19 in Wuhan, China: a longitudinal cohort study. JAMA Neurol 2022; 79:509–517.69
Livingston G, Huntley J, Sommerlad A, et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020; 396:413–446.70
Loane DJ, Pocivavsek A, Moussa CE-H, et al. Amyloid precursor protein secretases as therapeutic targets for traumatic brain injury. Nat Med 2009; 15:377–379.71
Lopatko Lindman K, Hemmingsson E-S, Weidung B, et al. Herpesvirus infections, antiviral treatment, and the risk of dementia-a registry-based cohort study in Sweden. Alzheimers Dement (N Y) 2021; 7:e12119.72
Loughrey DG, Kelly ME, Kelley GA, et al. Association of age-related hearing loss with cognitive function, cognitive impairment, and dementia: a systematic review and meta-analysis. JAMA Otolaryngol Head Neck Surg 2018; 144:115–126.73
Lourenco MV, Frozza RL, de Freitas GB, et al. Exercise-linked FNDC5/irisin rescues synaptic plasticity and memory defects in Alzheimer's models. Nat Med 2019; 25:165–175.74
Marinelli JP, Lohse CM, Fussell WL, et al. Association between hearing loss and development of dementia using formal behavioural audiometric testing within the Mayo Clinic Study of Aging (MCSA): a prospective population-based study. Lancet Healthy Longev 2022; 3:e817–e824.75
Martorell AJ, Paulson AL, Suk H-J, et al. Multisensory gamma stimulation ameliorates Alzheimer's-associated pathology and improves Cognition. Cell 2019; 177:256–271. e2.76
Ma’u E, Cullum S, Cheung G, et al. Differences in the potential for dementia prevention between major ethnic groups within one country: a cross sectional analysis of population attributable fraction of potentially modifiable risk factors in New Zealand. Lancet Reg Health West Pac 2021; 13:100191.77▪
McKee AC, Stein TD, Huber BR, et al. Chronic traumatic encephalopathy (CTE): criteria for neuropathological diagnosis and relationship to repetitive head impacts. Acta Neuropathol 2023; 145:371–394.Consensus statement with updated neuropathological diagnostic criteria for CTE.
78
Mei L, Liu L-M, Chen K, Zhao H-B. Early functional and cognitive declines measured by auditory-evoked cortical potentials in mice with Alzheimer's disease. Front Aging Neurosci 2021; 13:710317.79
Murphy MJ, Fani L, Ikram MK, et al. Herpes simplex virus 1 and the risk of dementia: a population-based study. Sci Rep 2021; 11:8691.80
Nakagawa Y, Reed L, Nakamura M, et al. Brain trauma in aged transgenic mice induces regression of established abeta deposits. Exp Neurol 2000; 163:244–252.81
Nasrallah IM, Gaussoin SA, Pomponio R, et al. SPRINT Research Group. Association of intensive vs standard blood pressure control with magnetic resonance imaging biomarkers of Alzheimer disease: secondary analysis of the SPRINT MIND Randomized Trial. JAMA Neurol 2021; 78:568–577.82
Nedelec T, Couvy-Duchesne B, Monnet F, et al. Identifying health conditions associated with Alzheimer's disease up to 15 years before diagnosis: an agnostic study of French and British health records. Lancet Digit Health 2022; 4:e169–e178.83
Ngandu T, Lehtisalo J, Solomon A, et al. A 2 year multidomain intervention of diet, exercise, cognitive training, and vascular risk monitoring versus control to prevent cognitive decline in at-risk elderly people (FINGER): a randomised controlled trial. Lancet 2015; 385:2255–2263.84
Nguyen M-F, Bonnefoy M, Adrait A, et al. ADPHA study group. Efficacy of hearing aids on the cognitive status of patients with Alzheimer's disease and hearing loss: a multicenter controlled randomized trial. J Alzheimers Dis 2017; 58:123–137.85
Nordström A, Nordström P. Traumatic brain injury and the risk of dementia diagnosis: a nationwide cohort study. PLoS Med 2018; 15:e1002496.86
Norton S, Matthews FE, Barnes DE, et al. Potential for primary prevention of Alzheimer's disease: an analysis of population-based data. Lancet Neurol 2014; 13:788–794.87
O’Leary TP, Shin S, Fertan E, et al. Reduced acoustic startle response and peripheral hearing loss in the 5xFAD mouse model of Alzheimer's disease. Genes Brain Behav 2017; 16:554–563.88
Paciello F, Rinaudo M, Longo V, et al. Auditory sensory deprivation induced by noise exposure exacerbates cognitive decline in a mouse model of Alzheimer's disease. Elife 2021; 10:e70908.89
Perez Garcia G, De Gasperi R, Tschiffely AE, et al. Repetitive low-level blast exposure improves behavioral deficits and chronically lowers Aβ42 in an Alzheimer disease transgenic mouse model. J Neurotrauma 2021; 38:3146–3173.90
Plassman BL, Chanti-Ketterl M, Pieper CF, Yaffe K. Traumatic brain injury and dementia risk in male veteran older twins-controlling for genetic and early life nongenetic factors. Alzheimers Dement 2022; 18:2234–2242.91▪
Pruntel SM, Van Munster BC, De Vries JJ, et al. Oral health as a risk factor for Alzheimer disease. J Prev Alz Dis 2023; doi: 10.14283/jpad.2023.82.Systematic review of the relationship between oral bacterial periodontitis and AD highlighting the role of oral pathogens, inflammatory mediators, the Aβ peptide, and the APOEε4 allele.
92
Raj R, Kaprio J, Korja M, et al. Risk of hospitalization with neurodegenerative disease after moderate-to-severe traumatic brain injury in the working-age population: a retrospective cohort study using the Finnish national health registries. PLoS Med 2017; 14:e1002316.93▪▪
Rashid T, Li K, Toledo JB, et al. Association of intensive vs standard blood pressure control with regional changes in cerebral small vessel disease biomarkers: post hoc secondary analysis of the SPRINT MIND Randomized Clinical Trial. JAMA Netw Open 2023; 6:e231055.Secondary analysis of the SPRINT-MIND trial demonstrating that intensive blood pressure control (goal SBP < 120 mmHg) slows down the accumulation of white matter hyperintensities relative to standard blood pressure control (goal SBP < 140 mmHg) based on serial MRIs.
94
Readhead B, Haure-Mirande J-V, Funk CC, et al. Multiscale analysis of independent Alzheimer's cohorts finds disruption of molecular, genetic, and clinical networks by human herpesvirus. Neuron 2018; 99:64–82. e7.95
Sahu B, Mackos AR, Floden AM, et al. Particulate matter exposure exacerbates amyloid-β plaque deposition and gliosis in APP/PS1 mice. J Alzheimers Dis 2021; 80:761–774.96
Schneider ALC, Selvin E, Latour L, et al. Head injury and 25-year risk of dementia. Alzheimers Dement 2021; 17:1432–1441.97
Schnier C, Janbek J, Lathe R, Haas J. Reduced dementia incidence after varicella zoster vaccination in Wales. Alzheimers Dement (N Y) 2022; 8:e12293.98
Schnier C, Janbek J, Williams L, et al. Antiherpetic medication and incident dementia: observational cohort studies in four countries. Eur J Neurol 2021; 28:1840–1848.99
See RS, Thompson F, Russell S, et al. Potentially modifiable dementia risk factors in all Australians and within population groups: an analysis using cross-sectional survey data. Lancet Public Health 2023; 8:e717–e725.100▪▪
Seo D-O, O’Donnell D, Jain N, et al. ApoE isoform- and microbiota-dependent progression of neurodegeneration in a mouse model of tauopathy. Science 2023; 379:eadd1236.Study in tauopathy transgenic mice with humanized APOE gene revealing the importance of the gut microbiome, sex, and the APOEε allele in the progression of neurofibrillary tangles in the brain. These results lend support to the idea that the so-called gut-brain axis can impact AD pathophysiology.
101
Serrano-Pozo A, Aldridge GM, Zhang Q. Four decades of research in Alzheimer's disease (1975–2014): a bibliometric and scientometric analysis. J Alzheimers Dis 2017; 59:763–783.102
Serrano-Pozo A, Growdon JH. Is Alzheimer's disease risk modifiable? J Alzheimers Dis 2019; 67:795–819.103
Shen Y, Hu H, Fan C, et al. Sensorineural hearing loss may lead to dementia-related pathological changes in hippocampal neurons. Neurobiol Dis 2021; 156:105408.104
Shi L, Wu X, Danesh Yazdi M, et al. Long-term effects of PM2·5 on neurological disorders in the American Medicare population: a longitudinal cohort study. Lancet Planet Health 2020; 4:e557–e565.105
Shim Y, Park M, Kim J. Increased incidence of dementia following herpesvirus infection in the Korean population. Medicine (Baltimore) 2022; 101:e31116.106
Nasrallah IM, Pajewski NM, Auchus AP, et al. SPRINT MIND Investigators for the SPRINT Research Group. Association of intensive vs standard blood pressure control with cerebral white matter lesions. JAMA 2019; 322:524–534.107
Williamson JD, Pajewski NM, Auchus AP, et al. SPRINT MIND Investigators for the SPRINT Research Group. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA 2019; 321:553–561.108
Stevenson JS, Clifton L, Kuźma E, Littlejohns TJ. Speech-in-noise hearing impairment is associated with an increased risk of incident dementia in 82 039 UK Biobank participants. Alzheimers Dement 2022; 18:445–456.109
Sugarman MA, McKee AC, Stein TD, et al. Failure to detect an association between self-reported traumatic brain injury and Alzheimer's disease neuropathology and dementia. Alzheimers Dement 2019; 15:686–698.110▪▪
Taquet M, Sillett R, Zhu L, et al. Neurological and psychiatric risk trajectories after SARS-CoV-2 infection: an analysis of 2-year retrospective cohort studies including 1 284 437 patients. Lancet Psychiatry 2022; 9:815–827.This large international electronic health records-based study reveals the negative impact of COVID-19 on cognition and many other neurological and psychiatric disease outcomes over 2 years in different age groups. Studies evaluating the long-term impact of COVID-19 are warranted.
111
Torniainen-Holm M, Suvisaari J, Lindgren M, et al. Association of cytomegalovirus and Epstein-Barr virus with cognitive functioning and risk of dementia in the general population: 11-year follow-up study. Brain Behav Immun 2018; 69:480–485.112
Tran HT, LaFerla FM, Holtzman DM, Brody DL. Controlled cortical impact traumatic brain injury in 3xTg-AD mice causes acute intra-axonal amyloid-β accumulation and independently accelerates the development of tau abnormalities. J Neurosci 2011; 31:9513–9525.113▪
Udeochu JC, Amin S, Huang Y, et al. Tau activation of microglial cGAS-IFN reduces MEF2C-mediated cognitive resilience. Nat Neurosci 2023; 26:737–750.This study reported the deleterious activation of the antiviral cGAS-STING pathway by microglia in human AD brains and tauopathy transgenic mice, resulting in synaptic loss and cognitive impairment. These findings support cGAS-STING as a novel promising therapeutic target against AD.
114
Vergara RC, Zitko P, Slachevsky A, et al. Population attributable fraction of modifiable risk factors for dementia in Chile. Alzheimers Dement (Amst) 2022; 14:e12273.115
Vermeulen R, Schymanski EL, Barabási A-L, Miller GW. The exposome and health: where chemistry meets biology. Science 2020; 367:392–396.116
Vogt NM, Kerby RL, Dill-McFarland KA, et al. Gut microbiome alterations in Alzheimer's disease. Sci Rep 2017; 7:13537.117
Wang L, Davis PB, Volkow ND, et al. Association of COVID-19 with new-onset Alzheimer's disease. J Alzheimers dis 2022; 89:411–414.118
Weidung B, Hemmingsson E-S, Olsson J, et al. VALZ-Pilot: high-dose valacyclovir treatment in patients with early-stage Alzheimer's disease. Alzheimers Dement (N Y) 2022; 8:e12264.119
Weiner MW, Harvey D, Landau SM, et al. Alzheimer's Disease Neuroimaging Initiative and the Department of Defense Alzheimer's Disease Neuroimaging Initiative. Traumatic brain injury and posttraumatic stress disorder are not associated with Alzheimer's disease pathology measured with biomarkers. Alzheimers Dement 2022; doi: 10.1002/alz.12712.120
Welch GM, Boix CA, Schmauch E, et al. Neurons burdened by DNA double-strand breaks incite microglia activation through antiviral-like signaling in neurodegeneration. Sci Adv 2022; 8:eabo4662.121
Wozniak MA, Mee AP, Itzhaki RF. Herpes simplex virus type 1 DNA is located within Alzheimer's disease amyloid plaques. J Pathol 2009; 217:131–138.122
Xiao J, Katsumata N, Bernier F, et al. Probiotic bifidobacterium breve in improving cognitive functions of older adults with suspected mild cognitive impairment: a randomized, double-blind, placebo-controlled trial. J Alzheimers Dis 2020; 77:139–147.123▪
Xie J, Bruggeman A, De Nolf C, et al. Gut microbiota regulates blood-cerebrospinal fluid barrier function and Aβ pathology. EMBO J 2023; 42:e111515.This study described how the gut microbiota maintains the blood-CSF barrier integrity at the choroid plexus via secretion of short chain fatty acids (SCFA), which alter microglia phenotype and reduce Aβ plaque burden.
124▪
Xie X, Ma G, Li X, et al. Activation of innate immune cGAS-STING pathway contributes to Alzheimer's pathogenesis in 5 × FAD mice. Nat Aging 2023; 3:202–212.This study reported the deleterious activation of the antiviral cGAS-STING pathway by microglia in human AD brains and Aβ plaque-depositing transgenic mice, and how a STING inhibitor drug ameliorates Aβ plaque burden and neuroinflammation in these mice.
125
Yan W, Yun Y, Ku T, et al. NO2 inhalation promotes Alzheimer's disease-like progression: cyclooxygenase-2-derived prostaglandin E2 modulation and monoacylglycerol lipase inhibition-targeted medication. Sci Rep 2016; 6:22429.126▪▪
Yeo BSY, Song HJJMD, Toh EMS, et al. Association of hearing aids and cochlear implants with cognitive decline and dementia: a systematic review and meta-analysis. JAMA Neurol 2023; 80:134–141.This systematic review and meta-analysis found that hearing loss therapy with hearing aids or cochlear implants lowers both the risk of incident dementia and the rate of cognitive decline but did not find a significant effect on the risk of incident AD or vascular dementia.
127
Zainul Abidin FN, Scelsi MA, Dawson SJ, Altmann A. Alzheimer's Disease Neuroimaging Initiative. Glucose hypometabolism in the auditory pathway in age related hearing loss in the ADNI cohort. Neuroimage Clin 2021; 32:102823.128
Zanier ER, Bertani I, Sammali E, et al. Induction of a transmissible tau pathology by traumatic brain injury. Brain 2018; 141:2685–2699.
∗
These two authors contributed equally to this work.
