1. Introduction
Cardiovascular disease (CVD) is the leading cause of pre-mature death with ∼17.8 million deaths worldwide in 2017. With approximately half a billion people afflicted by CVD globally, it also constitutes a major socioeconomic burden, accruing substantial direct costs to healthcare systems as well as indirect costs of reduced productivity and days of work lost to illness.
The heart and vasculature are primarily composed of non-haematopoietically derived cell types including cardiomyocytes, fibroblasts, endothelial cells (ECs), smooth muscle cells, pericytes, and stroma. However, the presence of leucocytes within cardiovascular tissue has long been acknowledged and all the major immune cell subtypes, including lymphocyte and myeloid-derived populations, are found within cardiovascular tissues., Some of these populations are resident cells, while infiltrating immune cells contribute to changes in the structural and functional properties of the heart and vasculature and may amplify CVD progression. Over the last two decades, the broad roles played by cells of the immune response in cardiovascular biology have begun to be appreciated. These range from roles in heart development, to maintenance of vascular homoeostasis and steady state physiology through to critical roles in directing and protecting from disease-related pathology. Interested readers are directed to specialized recent reviews on the field, provided by Swirski and Nahrendorf and Strassheim et al.
Understanding the critical regulators of immune cell activity and function is key to appreciate how immune responses which underlie or exacerbate certain diseases might be corrected to cure disease. MicroRNAs (miRNAs) are among the most important regulators of immune cellular function and identity. This review aims to provide an up-to-date discussion of available literature, which investigates immune cell-derived miRNAs and their roles in regulating immune responses in the setting of CVD and its associated risk factors. We additionally highlight putative roles played by immune system-derived miRNAs delivered to non-immune cardiovascular cell types via extracellular vesicles (EVs). Finally, we discuss miRNA regulation of regulatory T cells (TREGS) and suggest that the manipulation of miRNA pathways in these cells may prove beneficial in the context of TREGS immunotherapy and CVD. A reference for abbreviations used throughout this manuscript can be found in Table 1.
2. Immune system enriched miRNAs: roles in immune cell development and function
MicroRNAs play essential roles in governing immune system development, homoeostasis, and activation. The canonical pathways of miRNA biogenesis are well recognized but more recently, non-canonical biogenesis pathways and novel miRNA regulatory mechanisms have also been described (Figure 1). Dysregulated expression of miRNAs during disease settings including CVD, autoimmunity, and tumourigenesis has been extensively reported,, and alterations to the expressions of many of these miRNAs may serve useful roles as biomarkers for diagnostic purposes and/or for monitoring disease progression. Many individual miRNAs highly expressed by immune system cells—the so-called ‘immuno-miRNAs’, play unique roles in directing immune system responses and additional novel players are often described. However, some of these serve particularly critical roles and warrant a brief introduction.
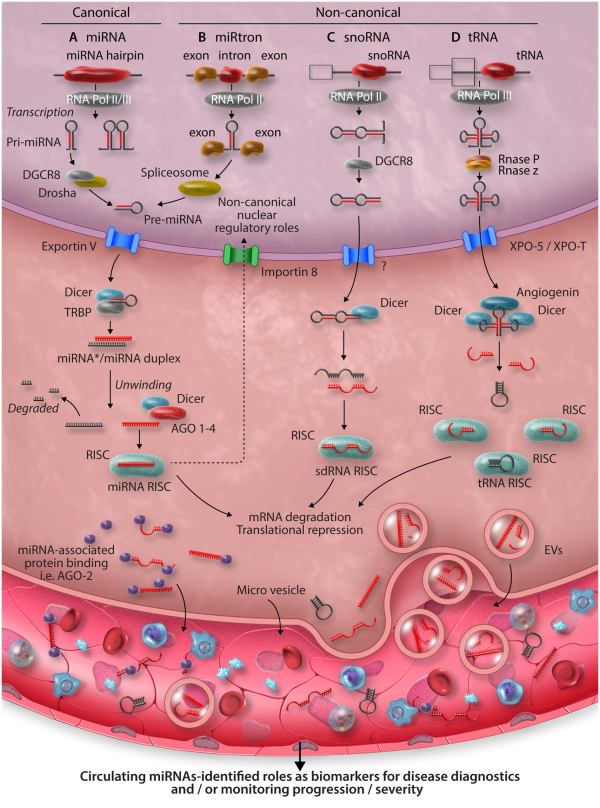
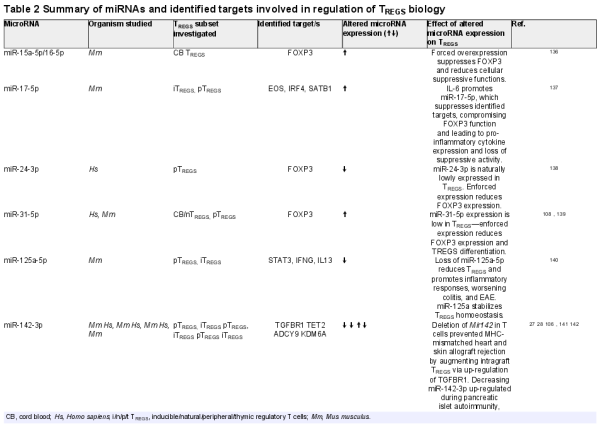
Figure 1
Overview of mammalian miRNA biogenesis pathways and functions. (A) Canonically, microRNAs are generated following RNA polymerase II/III dependant transcription as primary transcripts (pri-miRNAs). Cleavage of the pri-miRNA to a stem-loop, pre-miRNA follows within the nucleus, orchestrated by the microprocessor complex comprising the RNAse-III like enzyme, Drosha, and a dsDNA binding co-factor—DiGeorge syndrome chromosomal region 8 (DGCR8). Next, pre-miRNAs are translocated to the cytoplasm via the RAS-related nuclear protein-guanosine-5′-triphosphate-ase (Ran-GTP) dependant, Exportin V nuclear transporter. A final cleavage of pre-miRNA to a duplex containing the mature microRNA sequences is undertaken by Dicer. Dicer also integrates the duplex within an Argonaut protein family member (AGO1-4 in mammals) wherein, one strand of the duplex is typically degraded, while the other is selected to remain AGO-associated, forming the microRNA-induced silencing complex (miRISC). It is the miRISC, which facilitates localization and binding to mRNA target sequences. Finally, following formation of the miRISC: mRNA complex, enzymatic degradation of the mRNA transcript or its direct translational inhibition occurs, epigenetically altering gene expression by modifying the dynamics of protein synthesis. While all mature microRNAs are thought to be generated within the cytoplasm, nuclear import of AGO2 loaded with microRNA can occur in an Importin 8-dependant manner and described nuclear functions of microRNAs includes post-transcriptional gene-silencing, transcriptional gene-silencing, and transcriptional gene activation, via binding to recently transcribed RNAs, or through direct chromatin interactions with gene promotor or enhancer regions. (B) MiRtrons are derived from sequences spliced within the introns of coding genes. In the nucleus, processing of miRtrons bypasses the requirement for conventional Drosha/DGCR8 cleavage. The pre-miRNA is derived from spliceosome activity., (C) Small nucleolar RNAs undergo splicing and debranching in the nucleus before being exported to the cytoplasm via an unknown nuclear transporter. Here, they undergo final cleavage by Dicer before being incorporated into the RISC. (D) RNA polymerase III transcribed tRNA undergo processing by RNases, which cleave the 5′ and 3′ ends allowing its transport into the cytoplasm via Exportin-t. tRNAs undergo further cleavage by Dicer and Angiogenin before becoming incorporated into the tRNA RISC. Mature miRNAs can be released into the circulation via the excretion of EVs, exosomes, budding of microvesicles, or via proteins. In the circulation they can be readily detected in biological fluids, such as blood, urine, and saliva, thus acting as biomarkers of disease, creating unique miRNA signatures in response to disease.
2.1 miRNA-17-92 cluster
The cluster encodes six unique miRNAs: miR-17, miR-18a, miR-19a, miR-20a, miR-19b-1, and miR-92a-1 and plays multiple, non-redundant roles in B cell development, supportive of its actions as an oncogene promoting B cell lymphomas. Other critical roles include regulation of T cell activation, maturation and development of T-helper subsets (Th) and TREGS, and additional important functions in monocyte differentiation to macrophages and macrophage activation pathways.
2.2 miRNA-21
Expressed in multiple cell types in addition to leukocytes, up-regulation of miR-21 (referring to the predominantly expressed mature strand miR-21-5p) is associated with exposure to inflammatory stimuli and immune cell activation. Additionally, miR-21 functions as an oncogene and its overexpression results in B cell lymphoma in mice, supporting the association of miR-21 with the development of many cancers. Aging is associated with increased expression of miR-21 in T cells, resulting in failure to generate memory T cells, contributing to aging-related immune system dysfunction (immunosenescence).
2.3 miRNA-142
miRNA-142 is a highly evolutionarily conserved miRNA, widely considered to be haematopoietic-lineage specific or enriched., Expression of mature isoforms of this miRNA from both arms of the miR-142 precursor (miR-142-3p and miR-142-5p) has been extensively validated. Multiple roles in the development of haematopoietic lineages and immune system function are attributed to these molecules. The regulation of lineage-enriched expression of miR-142 isoforms may be related to the association of MIR142 promotor activity with super-enhancers which are predominantly active in haematopoietic and immune cell types and epigenetic factors, such as lineage and/or context-specific differences in DNA methylation state of the MIR142 promotor.,
2.4 miRNA-146a
miRNA-146a is a powerful negative regulator of both innate and adaptive immune responses. Expression of miR-146a is driven by the NF-kB signalling pathway and is correlated with immune cell activation via T cell receptor signalling or Toll-like receptor activation. Critically, miR-146a-5p functions as a negative feedback regulator, down-regulating NF-kB signalling transducers IRAK1 and TRAF6 to limit cellular activation and its own expression levels.,
2.5 miRNA-155
miRNA-155 is a master regulator of immune system responses. Mice deficient for miR-155 have severely defective lymphoid and myeloid cell development and homoeostasis., Activation of immune cells is additionally promoted by miR-155. Often, this has been linked to repression of pathways involving the canonical miR-155-5p target, suppressor of cytokine signalling 1 (SOCS1), a potent inhibitor of Janus kinase (JAK)-signal transducer and activator of transcription (STAT) signalling pathways.
2.6 miRNA-181
Demonstration that miR-181 is highly and specifically expressed in haematopoietic progenitor cells gave one of the first indications that miRNAs might play critical roles in immune cell lineage differentiation. Subsequently, miR-181 family members (miR-181a-1, miR-181a-2, miR-181b-1, miR-181b-2, miR-181c, and miR-181d) have been shown to play critical roles in lymphoid and myeloid lineage differentiation and act as a metabolic rheostat for the immune system. Down-regulation of miR-181 family member expression may underlie reduced or dysregulated immune system function associated with aging and immunosenescence.,
2.7 miRNA-223
Particularly critical in the regulation of innate immunity,, expression of miR-223 within the immune system is restricted largely to myeloid-derived cell types, in which miR-223-3p acts as a critical mediator of both myeloid cell differentiation and activation.
3 miRNA-mediated regulation of immunity and CVD risk factors
3.1 Hypertension
While immune cell contribution to hypertension was discovered many years ago, it was not until the mid-2000s that a ground-breaking study by Guzik et al. showed that Rag1−/− mice lacking T and B cells were protected against angiotensin II (Ang-II) and deoxycorticosterone acetate salt-induced hypertension. Moreover, the adoptive transfer of T but not B cells was able to restore the hypertensive abnormalities seen in the control groups, suggesting that T cells are critical mediators of hypertension.
In a murine model of Ang-II-induced hypertension, levels of the miR-199/214 family member miR-214-3p were up-regulated eight-fold in perivascular tissue of Ang-II-infused mice compared to wild type (WT) mice. Moreover, under Ang-II infusion, miR-214−/− mice showed blunted hypertension, vascular stiffening, and perivascular fibrosis when compared with WT controls. These effects mapped specifically to changes in the T cell transcriptome in the miR-214 −/− mice, which led to blunted T cell activation and infiltration into perivascular adipose tissue (AT). To assess the translational relevance of this, it was found that plasma miR-214-3p expression levels were increased in hypertensive patients. In another murine study of Ang-II-induced hypertension, the knockout of miR-31 (miR-31−/−), which maps specifically to Treg and Th17 cells led to blunted hypertension and decreased cardiac hypertrophy and fibrosis. Furthermore, miR-31 ablation in mice resulted in a decrease of kidney infiltrating CD45+F4/80+ macrophages and an increase in kidney specific CD4+FoxP3+TREGS. As a result, miR-31−/− mice had reduced vascular and renal damage. These differences mapped specifically to miR-31−/− mediated post-transcriptional regulation of its direct target protein phosphatase 6c.
3.2 Obesity and epicardial AT
Obesity and diabetes are major risk factors for CVD. AT is an endocrine organ of paramount importance in the development of systemic inflammation, which affects cardiovascular cell metabolism, function, and disease progression. Furthermore, the immune system plays critical roles in the development and function of AT and obesity is increasingly considered to be an immunometabolic disease. Thickening of epicardial adipose tissue (EAT), which surrounds the heart and major vessels is associated with coronary artery disease (CAD). In patients with sudden-CAD associated death, levels of miR-34a-3p and miR-34a-5p in EAT were up-regulated. This is of particular interest as miR-34a has been associated with obesity-induced systemic inflammation via the suppression of anti-inflammatory ‘M2’ macrophage polarization and increase in pro-inflammatory ‘M1’ macrophages and cytokines within visceral AT of murine models of diet-induced obesity. Furthermore, whole-genome sequencing on EAT from CAD patients, shows that genes involved in antigen presentation, chemokine signalling, and systemic inflammation are up-regulated, whilst levels of miR-103-3p, which regulates Th2 chemokines, like CCL13, were down-regulated. Interestingly, the presence of EAT around the left atria (LAEAT) has been linked to the prevalence of atrial fibrillation. Using data from the miRhythm study and bioinformatic platforms, Tran et al. analysed miRNA levels associated with LAEAT from AF patients. Levels of miR-155-5p and miR-302a-3p were associated with LAEAT in AF. Both miRNAs are involved in the regulation of IL-8, which has been linked to leukocyte trafficking and activation., Furthermore, downstream predicted genes of both miRNAs were associated with cardiac hypertrophy and adipogenesis.
4. miRNA-mediated regulation of immune cells during CVD and associated complications
4.1 Atherosclerosis
Atherosclerosis is considered to be an inflammatory, immune-mediated disease. Of the immune cells associated with atherosclerosis pathogenesis, macrophages play a central role in plaque formation and disease progression. As LDL retained beneath the endothelial wall becomes oxidized (oxLDL), it activates ECs and promotes the recruitment and infiltration of monocytes/macrophages. Uptake of oxLDL and internalization of apoptotic cell fragments by macrophages results in lipid accumulation and foam cell (FC) formation. The roles of miRNAs in the regulation of macrophages during atherosclerosis have been extensively investigated. Recent work has continued to uncover novel miRNA-mediated mechanisms of macrophage regulation and their athero-protective and atherogenic effects (Figure 2). Utilizing publicly available miRNA array data, Zheng et al. postulated that miR-133b-3p expression in macrophages serves a pro-atherogenic role by modulating expression of mastermind-like protein 1 (MAML1), tentatively supporting studies which highlight a role for NOTCH signalling in plaque leukocyte influx and atherosclerosis progression. However, although the authors convincingly demonstrated regulation of MAML1 by miR-133b-3p, given the well-appreciated role of MAML1 as a transcriptional co-activator of NOTCH signalling, it is not immediately clear how de-repression of MAML1 expression via down-regulation of miR-133b-3p might promote the associated down-regulation of various NOTCH pathway components—as implied by the authors. Therefore, further work which clarifies the exact relevance of the miR-133b–3p/MAML1 interaction in macrophages during atherosclerosis is required.
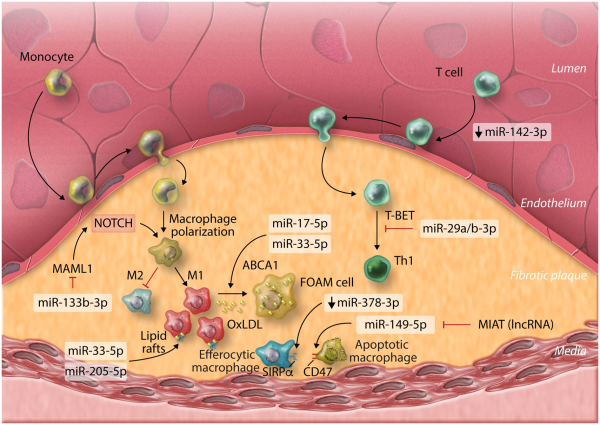
Figure 2
MicroRNAs involved with plaque-associated immune cells in atherosclerosis and their functions. OxLDL, oxidized low-density lipoprotein; lncRNA, long non-coding ribonucleic acid; MIAT, myocardial infarction associated transcript.
miRNA-17-5p—a potentially useful, circulating biomarker for monitoring atherosclerosis severity, has also been implicated in the regulation of inflammation and macrophage lipid accumulation during atherosclerosis, through the direct regulation of ATP-binding cassette transporter A1 (ABCA1), a critical component of cellular reverse cholesterol transport/lipid efflux. Down-regulation of ABCA1 leads to accumulation of intracellular lipids, promoting FC generation during atherosclerosis. miRNA-17-5p joins several additional miRNAs demonstrated to regulate ABCA1 in macrophages, thereby limiting reverse cholesterol transport,, including the miR-33 family., miRNA-33-5p also regulates macrophage expression of peroxisome proliferator-activated receptor gamma co-activator 1-alpha, a master regulator of mitochondrial biogenesis and energy metabolism—therefore limiting cholesterol efflux via ABCA1 by restricting production of ATP. Inhibition of cholesterol efflux can promote formation of lipid-rich cell membrane rafts, promoting pro-inflammatory extracellular signalling pathways and inflammation. Alongside enhanced plaque instability, miR-205-5p was recently shown to direct lipid raft formation in macrophages, promoting pro-inflammatory macrophage phenotypes, expanding the understanding of miRNA-mediated control of macrophage lipid transport and its importance during atherosclerosis.
Defective macrophage clearance of other apoptotic macrophages and FCs (efferocytosis) may also underlie inflammation and atherosclerosis progression. Down-regulation of miR-378a-3p in aortic macrophages of hyperlipidemic Apoe−/− mice was shown to result in de-repression of signal regulatory protein α (SIRPα), the transmembrane signalling receptor for CD47 (integrin-associated protein). The CD47: SIRPα axis act as a don’t eat me signal and up-regulation of this pathway prevents clearance of apoptotic cells within plaque lesions, accelerating atherosclerosis progression. Dysregulation of the CD47: SIRPα axis could also underlie atherosclerosis progression in humans, as was implied by the observation that the long non-coding RNA (lncRNA) myocardial infarction associated transcript (MIAT) was significantly higher in the circulating sera of atherosclerosis patients and those with symptoms of vulnerable atherosclerotic plaque, compared to controls, indicating that MIAT may also serve as a useful biomarker of plaque stability and atherosclerosis severity. In this study, the authors suggest that MIAT acts as a competing endogenous RNA (ceRNA) for miR-149-5p in macrophages and contains complementary miR-149-5p binding sequences which allow MIAT to ‘sponge’ miR-149-5p away from CD47, de-repressing CD47 expression. This study appears to convincingly demonstrate a functional role for this interaction, using ceRNA overexpression systems and in vitro miRNA manipulation to probe the nature of this dynamic in macrophage cell lines. However, the degree to which ceRNAs truly act to regulate endogenous miRNA activity through modulation of bioavailability for molecular target interaction is controversial (reviewed by Thomson and Dinger). In their article, the authors do not investigate the limitations that physiological expression levels of both molecules may impose upon the outcome of the interaction during the real-world context of disease. This general criticism applies to many attempts to characterize cell-endogenous physiological regulators of miRNA activity. However, the concept presented in this work highlights the importance of considering the complex components of networks that regulate the functional activity of specific miRNAs in a given cell type.
Additional leukocytes also serve critical roles in the pathophysiology of atherosclerosis. T cells and particularly the CD4+ lineage are important players in atherosclerosis pathogenesis and the influx of CD4+ T cells to the vascular walls may be regulated by alterations to cytoskeleton dynamics as a result of aberrant expression of miR-142-3p. CD4+ Th1 responses, directed by the Th1 master T-box transcriptional regulators T-BET and (to some extent) Eomesodermin (EOMES) are the most prevalent Th subset in atherosclerotic plaques and promote atherosclerosis progression and severity. miRNA-29 seed-family members miR-29a-3p and miR-29b-3p act to restrict T-BET and EOMES expression in T cells and may therefore putatively alleviate atherosclerosis and enhance plaque stability by directly inhibiting Th1 development. However, evidence that global antagonism of miR-29 members using locked nucleic acids (LNAs) decreases atherosclerotic lesion size and promotes plaque remodelling and stability in mice, suggests that in certain cell types, miR-29 family members may actually contribute to disease progression. However, in this study, the impact of LNA-miR-29 on the development of Th1 responses was not specifically addressed. More studies are required to fully define the contribution of T-cell-derived miR-29 family members to the promotion of plaque-associated Th1 development, both during human and murine disease, in order to guide potential therapeutic strategies.
4.2 Myocardial infarction
Monocytes and macrophages are critical mediators of inflammation and promote cardiomyocyte apoptosis during the early inflammatory response post-MI, but also contribute to later stages of left ventricular remodelling and dilation, eventually leading to HF. miRNA-21-5p may be a critical endogenous mediator, which limits the polarization of pro-inflammatory macrophages in the heart, post-MI. miRNA-21-5p expression was up-regulated rapidly in infarct areas of murine cardiac tissue, however, MI-induction in mice deficient for miR-21 (miR-21−/−) resulted in worse survival rates and greater infarct size and scarring. Macrophage expression of miR-21-5p directly restricted inflammatory activation and cytokine production by targeting Kelch repeat and BTB (POZ) domain containing 7 (KBTBD7), a transcriptional activator involved in mitogen-activated protein kinase signalling. In the absence of miR-21-5p, KBTBD7 overexpression enhanced macrophage activation by promoting p38 and NF-kB activation. Promotion of miR-21 regulatory mechanisms through intravenous delivery of nanoparticles (NPs) containing miR-21-5p mimics, targeted to cardiac macrophages shortly after MI-induction may therefore be a therapeutic strategy to limit pro-inflammatory/promote reparative, wound healing macrophage phenotypes, leading to attenuation of pathological remodelling. Therapeutically targeting miR-375-3p in macrophages may be an additional means of modulating pro-inflammatory macrophage development post-MI, via activation of 3-phosphoinositide-dependent protein kinase 1 and reduction of pro-inflammatory cytokine expression. Additionally, expression of a superoxide producing enzyme, NADPH oxidase 2 (NOX2) is enhanced in cardiomyocytes and macrophages post-MI and promotes macrophage inflammatory polarization and cytokine expression and is associated with adverse cardiac remodelling post-MI. In both human and murine macrophages, miR-106b-5p, miR-148b-3p, and miR-204-5p were shown to suppress NOX2 and targeted up-regulation of these miRNAs through pH-sensitive polyketal NP-specific delivery to macrophages significantly improved infarct size and cardiac function post-MI in mice, highlighting another potential therapeutic axis for management of MI (Figure 3A).
Figure 3
MicroRNAs involved in immune-regulation of diseases of the heart: depicting (A) myocardial infarction, (B) myocarditis, (C) cardiac hypertrophy, and (D) heart failure.
4.3 Myocarditis
Myocarditis is an important cause of sudden cardiac death and heart failure, particularly in young, otherwise healthy patients. A breakdown in tolerogenic immune mechanisms is apparent during myocarditis and may particularly underpin autoimmune-mediated myocarditis. For example, miR-98-5p expression is enhanced in B cells during experimental autoimmune myocarditis (EAM) of mice and acts to suppress regulatory IL-10 expression. Likewise, polarization of tolerogenic dendritic cells, which promote induction of TREGS, may be inhibited in EAM as a result of miR-223-3p down-regulation, de-repressing NLRP3 inflammasome expression. However, the study of DCs in this work relies entirely on the use of murine bone marrow-derived dendritic cells generated through in vitro culture, potentially limiting conclusions regarding translational relevance in humans. None-the-less, TREGS are generally thought to be cardioprotective during myocarditis while effector CD4+ T cells are critical players in myocarditis pathogenesis. Th17 effectors play a particularly pathogenic role in later or chronic stages of autoimmune-related myocarditis progression and in the progression to dilated cardiomyopathy (DCM), while the balance of Th17 to TREG cells also serves to modulate myocarditis severity, with reduced TREGS leading to more profound Th17 responses and more severe disease. As reported for inflammatory conditions of other solid organs, miR-155-5p plays a critical role in regulating Th17/TREG balance during murine EAM, in which up-regulation of miR-155-5p in cardiac CD4+ T cells following induction of EAM promotes Th17 responses, while inhibiting generation and responses of TREGS. Additional evidence from Coxsackievirus-B3 murine models of viral myocarditis also supports a pathogenic role for up-regulated miR-155-5p in dysfunctional activation of T cells during acute myocarditis, as well as in promoting monocyte/macrophage cardiac infiltration and inflammatory ‘M1’ cardiac macrophage polarization., Moreover, up-regulated expression of miR-155-5p, as well as the immune-related miRNAs miR-146b-5p and miR-21-5p were observed in human ventricular septal biopsies of acute viral myocarditis patients. Targeting miR-155-5p expression may therefore hold therapeutic promise for the treatment of myocarditis in certain settings. However, a pathogenic role for miR-155-5p during myocarditis may not be absolute. Natural Killer (NK) cells are innate mediators of viral clearance and are protective in viral myocarditis, with roles in restricting autoimmune inflammation during EAM also reported. NK cell activation is positively correlated with expression of miR-155-5p, and promotes NK cell expression of IFNγ, partially through repression of the inositol phosphatase SHIP1. During murine viral myocarditis, increased NK expression of miR-155-5p and enhanced NK cell activation is apparent—promoting viral clearance and reducing cardiac immune cell infiltration and inflammatory cytokine production (Figure 3B).
4.4 Cardiac hypertrophy
Leukocyte infiltration into cardiac tissue exacerbates cardiovascular dysfunction in in vivo models of pressure overload-induced hypertrophy. miRNA-155-5p was increased in cardiac biopsies of hypertrophic aortic stenosis patients and correlated negatively with cardiac function yet positively with wall thickness relative to non-hypertrophic controls undergoing coronary bypass artery grafting. Furthermore, in two different murine models of pressure overload, genetic ablation, or pharmacological inhibition of miR-155-5p led to a decrease in cardiac infiltrating leukocytes, particularly CD68+CD11b+F4/80+ monocytes/macrophages. This was accompanied by preserved cardiac function and the absence of hypertrophy, both at the physiological and gene level. These affects were shown to be macrophage specific, as adenoviral-mediated inhibition of miR-155-5p in cardiomyocytes of WT mice did not prevent hypertrophy, whilst in bone marrow adoptive transfer experiments, the presence of miR-155−/− macrophages in WT mice was able to protect against hypertrophy. SOCS1 was the main target of miR-155-5p in macrophages postulated to drive hypertrophy; in the absence of miR-155-5p, SOCS1 overexpression inhibited the JAK/STAT signalling axis and specifically inhibited STAT3 signalling, suppressing the macrophage inflammatory response and release of paracrine mediators driving myocyte hypertrophy. See Figure 3C.
4.5 Heart failure
An ongoing inflammatory response is associated with chronic HF. HF patients have elevated levels of circulating pro-inflammatory cytokines including IL-6, IL-1β, and TNFα, and recent analysis of the results of the CANTOS phase III clinical trial revealed that use of the anti-IL-1β monoclonal antibody canakinumab (Ilaris), reduced hospitalizations and mortality related to HF in MI-patients. However, other phase III trials aimed at modulating HF with anti-inflammatory interventions have had less successful outcomes, suggesting that inflammatory responses may only drive HF in certain patient cohorts. In DCM patients, CD4+ T cell over-activation and proliferation is associated with down-regulation of miR-451a-5p and increased expression of its target Myc, marking the importance of T cell responses and their regulation by miRNAs in the development of HF. Immunosenescence is also related to the development of HF associated with aging. Ageing-associated miRNAs, such as miR-181 family members—which are important positive regulators of B-cell lymphopoiesis, are reduced in immune cells of aged patients and miR-34a-5p which inhibits B cell maturation and demonstrates enhanced expression in leucocytes with aging, may underlie these effects. Targeting these molecules may mitigate aging-associated HF progression by restoring lymphocyte function. See Figure 3D.
4.6 Vasculitis
Vasculitis covers a highly heterogeneous group of multi-system autoimmune, inflammatory conditions that affect blood vessels. Although rare, complications of vasculitides can include systemic emboli, thrombosis, aneurysms, sepsis, vessel occlusion, and major end-organ damage. In Wegener’s granulomatosis—the most common form of anti-neutrophil cytoplasmic autoantibody-associated vasculitis, accumulation of effector CD4+ T cells and a T-helper profile skewed towards IL-17 and IL-23 producing Th17 cells alongside a reduction in TREGS suppressive capacity, is thought to critically regulate disease pathogenesis. In this setting, aberrantly up-regulated expression of miR-142-3p may underlie defective TREGS suppressive activity, potentially driving pathogenic effector T cell responses. miRNA expression profiling of a circulating memory-like population of TREGS found to be enriched within the CD4+ T cell pool of patients relative to healthy controls revealed miR-142-3p to be significantly up-regulated alongside decreased expression of the miR-142-3p target adenylate cyclase 9 (ADCY9) and a corresponding reduction in intracellular cyclic adenosine monophosphate (cAMP)—a central mediator of TREG suppression of effector T cells, synthesized by ADCY9. Dysregulated TREGS activity alongside aberrant changes in TREGS miRNA expression has also been reported in Kawasaki’s disease (KD)—an acute form of systemic vasculitis, which can affect the coronary arteries. The proportion of circulating TREGS and TREGS expression of markers of maturation and suppressive function were significantly lower in KD patients (aged 3–54 months), while miR-155-5p and miR-21-5p expression was reduced and miR-31-5p expression was increased significantly. Putatively, these miRNA expression changes during KD lead to reduced proportion and activity of TREGS through de-repression of the miR-155-5p target SOCS1-inhibiting STAT5 signalling critical for TREGS homoeostasis and function, and restricted expression of the TREGS master transcription factor FOXP3.,
5. Immune cell-derived EVs and miRNAs—implications for CVD pathogenesis
EVs are a heterogeneous group of lipid-bilayer encapsulated particles, naturally shed into the cellular microenvironment from the cell membrane. Once considered merely a disposal route for unwanted cellular components and debris, it is now appreciated that although this may be the case for a subset of specialized EVs (apoptotic bodies), EVs (including exosomes and microvesicles) can also be packed with numerous forms of viable cargo derived from their parental cells, including intracellular proteins, DNA, and multiple RNA species, such as miRNAs. Following uptake by recipient cells, molecular cargo delivered within these packages can exert important biological functions, including post-transcriptional gene regulation by non-host cell-derived miRNAs, forming a critical component of intercellular communication and regulatory networks.
In the context of CVD, cardiovascular non-immune cells can release EVs loaded with miRNAs to influence intercellular communication and regulation of other cell types, affecting multiple facets of their responses including cell migration and tissue influx, and inflammation., However, immune cells also utilize these pathways to modulate the surrounding cardiovascular tissue environment (Figure 4). For instance, EVs derived from activated CD4+ T cells may be packaged with miR-142-3p. When delivered into mouse hearts following experimental MI, T-cell-derived EVs worsened post-MI cardiac dysfunction via the repression of the miR-142-3p target, adenomatous polyposis coli in cardiac fibroblasts, stimulating up-regulation of WNT-signalling, and pro-fibrotic pathways (Figure 4A). Platelet-derived EVs, thought to be responsible for the majority of circulating EVs, may also play an important role in CVD pathogenesis via miR-142-3p delivery to cardiovascular cell types. Previously, it has been demonstrated that miR-142-3p plays critical roles in generation of platelets, via the regulation of a network of actin cytoskeletal targets with critical roles to play during megakaryopoiesis. However, as for T cells, platelet-derived miR-142-3p can extend its regulatory actions beyond merely cell-endogenous effects; in the context of hypertension, ECs may take-up platelet-derived EVs, resulting in abnormal EC proliferation and apoptosis via regulation of BCL2-like1 and BCL2-associated transcription factor 1 by platelet-derived miR-142-3p (Figure 4B).
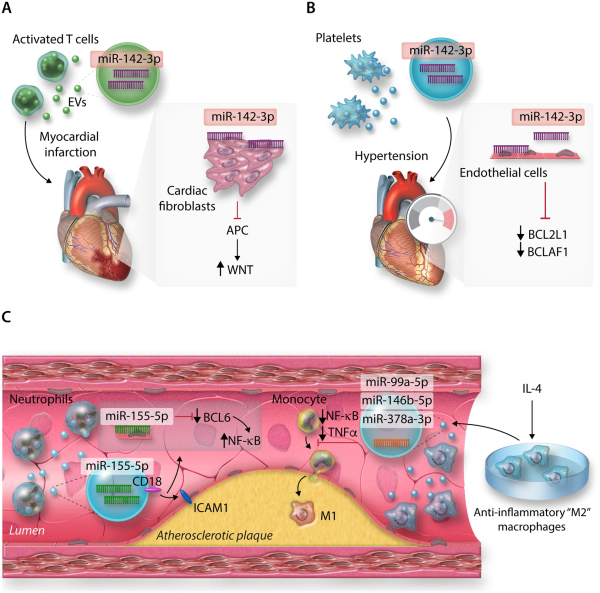
Figure 4
MicroRNAs from immune cell-derived extracellular vesicles (EVs) and their targets in (A) cardiac fibroblasts post-MI, (B) Endothelial cells (ECs) in hypertension, (C) Atherosclerotic plaques.
As EVs are secreted into the extracellular environment and may be carried in the circulation, this also raises the possibility that immune cell types not overtly present in cardiovascular tissues in the context of inflammation, may significantly contribute to CVD progress. For example, neutrophils are not typically enriched within atherosclerotic plaques, but may still influence disease pathogenesis through the provision of integrin beta-2 (CD18)+ EVs containing neutrophil-derived miR-155-5p (among other miRs), which targets BCL-6 to promote pro-inflammatory NF-kB signalling in ECs at atheroprone arterial regions in a murine model of atherosclerosis (Figure 4C). Additionally, the therapeutic potential of utilizing immune cell-derived EVs to deliberately target pathways involved in CVD pathogenesis has recently become a point of interest., For example, EVs derived from macrophages polarized towards anti-inflammatory, ‘M2’ phenotypes by IL-4, enriched with miR-99a-5p, miR-146b-5p, and miR-378a-3p may restrict plaque development and pro-inflammatory polarization of circulating monocytes and macrophages in the Apoe−/− model of murine atherosclerosis, via down-regulation of NF-kB and pro-inflammatory TNF-α production (Figure 4C).
However, it is important to note that, despite the large number of studies that support a functional capacity for EV-delivered miRNAs to regulate critical cellular pathways, there persists a significant degree of controversy and debate surrounding these claims. For instance, the majority of EVs may actually hold very few copies of individual miRNAs, and the miRNA containing EVs represent only a very small fraction of the total circulating miRNA in plasma. As such, work in the EV field has been greatly criticized for not, in general, considering the physiological importance of establishing the stoichiometric requirements between the delivered miRNAs and the predicted targets of relevance, in order for a functional impact upon target expression levels to be seen. It is unlikely that EVs carrying very few copies of a specific miRNA will functionally impact the expression levels of a high abundance target in another cell type, unless these EVs are delivered and taken up by target cells in sufficiently large quantities to do so. Additionally, few, if any standardized methods have been agreed on by the field for conducting these studies.
6. TREGS immunotherapy and miRNAs: therapeutic potential of miRNA regulated pathways for the treatment of CVD
There has been significant investment in research to target and/or utilize TREGS for treatment of conditions with underlying immunological dysfunctions. This has included graft-versus-host disease (GvHD), autoimmune conditions, such as Crohn’s disease and diabetes,, and in the context of solid organ transplantation wherein induction of long-term tolerance to the allograft is a major clinical goal. As it has become clear that TREGS can serve many cardioprotective and vasoprotective roles during CVD, and accumulated evidence also implicates dysregulated TREGS numbers and/or suppressive functions in CVD pathogenesis,,,,, immunotherapy delivered by exogenously delivered TREGS or through targeted TREGS-specific therapies might be a novel therapeutic option.
Many studies have identified specific miRNAs, which critically regulate development, differentiation, and the suppressive actions of the spectrum of TREGS subsets identified—from thymus-derived ‘natural’ TREGS (tTREGS or nTREGS) to TREGS induced from naïve T cell precursors in the periphery (pTREGS) or differentiated in vitro (iTREGS), in addition to unique TREGS from specialized niches, such as those found in umbilical cord blood TREGS (Table 2). Of the miRNAs, which regulate TREGS, miR-142 isoforms particularly appear to regulate many facets of TREGS biology (Table 2 and Figure 5). A central mediator of TREGS suppression of effector T cells, cAMP production is critically regulated on several molecular levels by miR-142 isoforms in TREGS. Maintenance of ADCY9 expression through the specific down-regulation of miR-142-3p in TREGS constitutes one mechanism of ensuring cAMP production and TREGS suppressive activity., In addition, our group have also recently demonstrated that a major role for the miR-142-5p isoform in TREGS is to ensure complete suppression of the potent cAMP hydrolase, phosphodiesterase 3B (PDE3B). In the absence of TREGS-specific miR-142-restriction of PDE3B, a severe breakdown in peripheral immune tolerance occurs, resulting in multi-organ tissue damage and widespread immune cell activation in mice. Conversely, we also recently identified miR-142-3p as a critical regulator of TGF-β mediated TREGS development through the regulation of TGFBR1, and established that deletion of the Mir142 locus promotes indefinite tolerance to cardiac allografts in a major MHC-mismatched murine model of heterotopic heart transplantation.
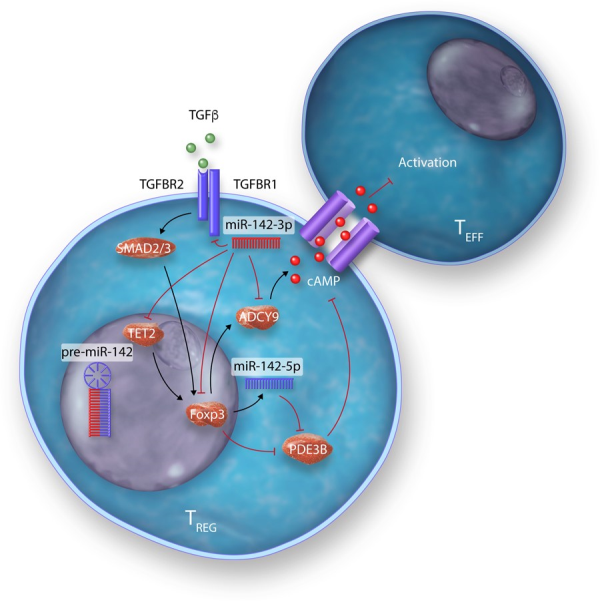
Figure 5
Modulation of multiple biological pathways in TREGS by miR-142 isoforms.
However, what these findings and much of the collective information regarding miRNA regulation of TREGS mean in the context of CVD largely remains to be investigated. Indeed, despite good evidence for protective functions of TREGS during CVD, studies that characterize the potential for TREGS therapy in major forms of CVD, such as atherosclerosis, are currently lacking–as recently outlined by Albany et al. The concept of using miRNA mimics and/or synthetic miRNA inhibitory molecules (i.e. antagomiRs) as therapeutic agents is highly topical., Candidates in early development aim to target a range of conditions including hepatitis C infection (Mirvirsen, RG-101), cancer (MesomiR, MRG-106), and ischaemia (MRG-110), with promising results in phase I and II clinical trials. Furthermore, pre-clinical data using antagomiR-treated TREGS in humanized mouse models of GvHD indicate that ex vivo miRNA modulation of TREGS is a promising avenue to enhance TREGS stability and suppressive activity during adoptive cell transfer immunotherapy. The prospect that targeted miRNA manipulation of TREGS might overcome some of the predicted limitations of adoptive transfer TREGS immunotherapy, such as stabilization of specific regulatory phenotypes or better control over trafficking and migration to desired sites, is particularly enticing both for CVD and other conditions. In addition, these points also raise the possibility that correction of dysfunctional TREGS in specific forms of CVD might also be achieved therapeutically by targeting aberrantly expressed miRNAs in ex vivo expanded populations of the patient’s cells. This may be particularly important when considering that, although interventions targeted to cardiovascular resident or infiltrating cell populations may be desirable for treatment of CVD, off-target, systemic suppression of immunity and inflammatory responses may theoretically result in unwanted general and/or opportunistic infections and other undesirable outcomes—despite any benefits to CVD treatment.
7. Concluding remarks
Attempts to characterize and better understand how immune system responses are regulated by miRNAs is a highly topical avenue of research. Unravelling the dynamics of these regulatory pathways and their functions in specific immune and inflammatory cell types is key to their therapeutic potential. With regards to CVD, it is critical to examine the dynamics of immune system regulation by miRNAs in each specific disease context, rather than make generalized inferences. Against this background, for certain well-known immuno-miRNAs, such as miR-155-5p, there is a robust body of literature, which implicates dysregulated expression in CVD pathogenesis. In our opinion, this molecule represents an obvious therapeutic candidate target of interest in several forms of CVD. However, for other immuno-miRNAs, there are notable gaps in the literature surrounding immune system regulation specifically in CVD. For example, the mature isoforms of miR-142 demonstrably influence key immune system developmental and cellular signalling pathways, and circulating levels are altered in CVD, but they have rarely been directly investigated in this setting.
As discussed in this review, among leucocytes, macrophages and CD4+ T cell responses are particularly important in CVD aetiology and progression. Targeting miRNAs and miRNA-regulated signalling networks in these cells—particularly those which regulate multiple aspects of immune cell biology, may prove to be especially useful in immunotherapeutic settings. However, how best to target these molecules in an effective, safe, yet cell type-specific manner is still unclear. The use of modified synthetic anti-sense oligonucleotides, such as antagomirs may be associated with off-target effects, particularly at higher therapeutic doses in vivo. Additionally, such effects could be relatively long-lasting, given the enhanced stability of these molecules. Packaging technologies, such as modified polymeric NPs may overcome some of the challenges the field faces, for instance, allowing for more controlled delivery or release of therapeutic molecules to target cells. As we have outlined, an alternative to this cell-targeted approach may be to turn to immunotherapy using miRNA modified TREGs to suppress dysregulated inflammation and immune responses underlying CVD pathogenesis. This area warrants further investigation and significant research effort.
Finally, recent advances in single-cell sequencing and analytical technologies allow for a depth in the resolution of data concerning rare cardiac immune cell populations, previously not accessible to researchers. For instance, it has only recently come to light that a population of tissue-resident, helper-like innate immune cells (ILCs) displaying a predominantly group 2-commited profile (ILC2) exists within heart tissue in mice and humans. These cells may play important roles during CVD including myocarditis, cardiac ischaemia, and pericarditis, while populations observed in para-aortic AT and lymph nodes may also serve vital roles in directing progression of atherosclerosis. However, these cells have primarily been studied in mice thus far and the relevance to human CVD remains to be established. Although still in its infancy, our understanding of how miRNAs regulate development, homeostasis, and activity of ILCs is gradually being unravelled. Future studies should aim to identify how these molecules regulate these newly identified populations, both during health and disease. This may potentially reveal exciting new avenues of therapeutic promise for patients suffering from CVD.
Conflict of interest: A.M.S. is a consulting editor for Cardiovascular Research. G.M.L. has filed a patent regarding modulation of PDE3B by miR-142.
Funding
L.B.R. is supported by the National Institute for Health Research (NIHR) Biomedical Research Centre based at Guy's and St Thomas' NHS Foundation Trust and King's College London. P.K. is supported by a King’s British Heart Foundation (BHF) Centre PhD Studentship. J.K.H. is supported by the Medical Research Council (grant MR/K002996/1). A.M.S. is supported by the BHF (CH/1999001/11735, RE/18/2/34213), the NIHR at Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust (IS-BRC-1215-20006), and the Fondation Leducq. G.M.L. is supported by the Wellcome Trust (grant number 091009), the British Heart Foundation (award number PG/12/36/29444), the MRC (grant number MR/M003493/1), and the NIHR at Guy’s & St Thomas’ NHS Foundation Trust in partnership with King’s College London and King’s College Hospital NHS Foundation Trust.
References
- 1. Virani SS, Alonso A, Benjamin EJ, Bittencourt MS, Callaway CW, Carson AP, Chamberlain AM, Chang AR, Cheng S, Delling FN, Djousse L, Elkind MSV, Ferguson JF, Fornage M, Khan SS, Kissela BM, Knutson KL, Kwan TW, Lackland DT, Lewis TT, Lichtman JH, Longenecker CT, Loop MS, Lutsey PL, Martin SS, Matsushita K, Moran AE, Mussolino ME, Perak AM, Rosamond WD, Roth GA, Sampson UKA, Satou GM, Schroeder EB, Shah SH, Shay CM, Spartano NL, Stokes A, Tirschwell DL, VanWagner LB, Tsao CW; On behalf of the American Heart Association Council on Epidemiology and Prevention Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics—2020 update: a report from the American Heart Association. Circulation2020;141:e139–e596.
- 2. Swirski FK, Nahrendorf M. Cardioimmunology: the immune system in cardiac homeostasis and disease. Nat Rev Immunol2018;18:733–744.
- 3. Martini E, Kunderfranco P, Peano C, Carullo P, Cremonesi M, Schorn T, Carriero R, Termanini A, Colombo FS, Jachetti E, Panico C, Faggian G, Fumero A, Torracca L, Molgora M, Cibella J, Pagiatakis C, Brummelman J, Alvisi G, Mazza EMC, Colombo MP, Lugli E, Condorelli G, Kallikourdis M. Single-cell sequencing of mouse heart immune infiltrate in pressure overload-driven heart failure reveals extent of immune activation. Circulation2019;140:2089–2107.
- 4. Strassheim D, Dempsey EC, Gerasimovskaya E, Stenmark K, Karoor V. Role of inflammatory cell subtypes in heart failure. J Immunol Res2019;2019:1–9.
- 5. Mehta A, Baltimore D. MicroRNAs as regulatory elements in immune system logic. Nat Rev Immunol2016;16:279–294.
- 6. Liu H, Lei C, He Q, Pan Z, Xiao D, Tao Y. Nuclear functions of mammalian MicroRNAs in gene regulation, immunity and cancer. Mol Cancer2018;17:14.
- 7. Wei Y, Li L, Wang D, Zhang CY, Zen K. Importin 8 regulates the transport of mature microRNAs into the cell nucleus. J Biol Chem2014;289:10270–10275.
- 8. Zhang Y, Liu W, Chen Y, Liu J, Wu K, Su L, Zhang W, Jiang Y, Zhang X, Zhang Y, Liu C, Tao L, Liu B, Zhang H. A cellular microRNA facilitates regulatory T lymphocyte development by targeting the FOXP3 promoter TATA-box motif. J Immunol2018;200:1053–1063.
- 9. Berezikov E, Chung W-J, Willis J, Cuppen E, Lai EC. Mammalian mirtron genes. Mol Cell2007;28:328–336.
- 10. Ruby JG, Jan CH, Bartel DP. Intronic microRNA precursors that bypass Drosha processing. Nature2007;448:83–86.
- 11. Stavast CJ, Erkeland SJ. The non-canonical aspects of microRNAs: many roads to gene regulation. Cells2019;8:1465.
- 12. O'Connell RM, Rao DS, Chaudhuri AA, Baltimore D. Physiological and pathological roles for microRNAs in the immune system. Nat Rev Immunol2010;10:111–122.
- 13. Zampetaki A, Willeit P, Drozdov I, Kiechl S, Mayr M. Profiling of circulating microRNAs: from single biomarkers to re-wired networks. Cardiovasc Res2012;93:555–562.
- 14. Mogilyansky E, Rigoutsos I. The miR-17/92 cluster: a comprehensive update on its genomics, genetics, functions and increasingly important and numerous roles in health and disease. Cell Death Differ2013;20:1603–1614.
- 15. Kuo G, Wu CY, Yang HY. MiR-17-92 cluster and immunity. J Formos Med Assoc2019;118:2–6.
- 16. Sheedy FJ. Turning 21: induction of miR-21 as a key switch in the inflammatory response. Front Immunol2015;6:1–9.
- 17. Medina PP, Nolde M, Slack FJ. OncomiR addiction in an in vivo model of microRNA-21-induced pre-B-cell lymphoma. Nature2010;467:86–90.
- 18. Feng YH, Tsao CJ. Emerging role of microRNA-21 in cancer (Review). Biomed Rep2016;5:395–402.
- 19. Kim C, Hu B, Jadhav RR, Jin J, Zhang H, Cavanagh MM, Akondy RS, Ahmed R, Weyand CM, Goronzy JJ. Activation of miR-21-regulated pathways in immune aging selects against signatures characteristic of memory T cells. Cell Rep2018;25:2148–2162.E5.
- 20. Chen C-Z. MicroRNAs modulate hematopoietic lineage differentiation. Science2004;303:83–86.
- 21. Landgraf P, Rusu M, Sheridan R, Sewer A, Iovino N, Aravin A, Pfeffer S, Rice A, Kamphorst AO, Landthaler M, Lin C, Socci ND, Hermida L, Fulci V, Chiaretti S, Foà R, Schliwka J, Fuchs U, Novosel A, Müller R-U, Schermer B, Bissels U, Inman J, Phan Q, Chien M, Weir DB, Choksi R, De Vita G, Frezzetti D, Trompeter H-I, Hornung V, Teng G, Hartmann G, Palkovits M, Di Lauro R, Wernet P, Macino G, Rogler CE, Nagle JW, Ju J, Papavasiliou FN, Benzing T, Lichter P, Tam W, Brownstein MJ, Bosio A, Borkhardt A, Russo JJ, Sander C, Zavolan M, Tuschl T. A mammalian microRNA expression atlas based on small RNA library sequencing. Cell2007;129:1401–1414.
- 22. Sharma S. Immunomodulation: a definitive role of microRNA-142. Dev Comp Immunol2017;77:150–156.
- 23. Nimmo R, Ciau-Uitz A, Ruiz-Herguido C, Soneji S, Bigas A, Patient R, Enver T. MiR-142-3p controls the specification of definitive hemangioblasts during ontogeny. Dev Cell2013;26:237–249.
- 24. Kramer NJ, Wang W-L, Reyes EY, Kumar B, Chen C-C, Ramakrishna C, Cantin EM, Vonderfecht SL, Taganov KD, Chau N, Boldin MP. Altered lymphopoiesis and immunodeficiency in miR-142 null mice. Blood2015;125:3720–3730.
- 25. Rivkin N, Chapnik E, Mildner A, Barshtein G, Porat Z, Kartvelishvily E, Dadosh T, Birger Y, Amir G, Yedgar S, Izraeli S, Jung S, Hornstein E. Erythrocyte survival is controlled by microRNA-142. Haematologica2017;102:676–685.
- 26. Anandagoda N, Willis JCD, Hertweck A, Roberts LB, Jackson I, Refik Gökmen M, Jenner RG, Howard JK, Lord GM. MicroRNA-142–mediated repression of phosphodiesterase 3B critically regulates peripheral immune tolerance. J Clin Invest2019;129:1257–1271.
- 27. Anandagoda N, Roberts LB, Willis JCD, Sarathchandra P, Xiao F, Jackson I, Hertweck A, Kapoor P, Jenner RG, Howard JK, Lord GM. Dominant regulation of long-term allograft survival is mediated by microRNA-142. Am J Transplant2020;20:2715–2727.
- 28. Scherm MG, Serr I, Zahm AM, Schug J, Bellusci S, Manfredini R, Salb VK, Gerlach K, Weigmann B, Ziegler AG, Kaestner KH, Daniel C. miRNA142-3p targets Tet2 and impairs Treg differentiation and stability in models of type 1 diabetes. Nat Commun2019;10:15.
- 29. Suzuki HI, Young RA, Sharp PA. Super-enhancer-mediated RNA processing revealed by integrative microRNA network analysis. Cell2017;168:1000–1014.E15.
- 30. Skårn M, Barøy T, Stratford EW, Myklebost O. Epigenetic regulation and functional characterization of microRNA-142 in mesenchymal cells. PLoS One2013;8:e79231.
- 31. Razak SRA, Baba Y, Nakauchi H, Otsu M, Watanabe S. DNA methylation is involved in the expression of miR-142-3p in fibroblasts and induced pluripotent stem cells. Stem Cells Int2014;2014:1–8.
- 32. Saba R, Sorensen DL, Booth SA. MicroRNA-146a: a dominant, negative regulator of the innate immune response. Front Immunol2014;5:1–11.
- 33. Rusca N, Monticelli S. MiR-146a in immunity and disease. Mol Biol Int2011;2011:1–7.
- 34. Yang L, Boldin MP, Yu Y, Liu CS, Ea CK, Ramakrishnan P, Taganov KD, Zhao JL, Baltimore D. miR-146a controls the resolution of T cell responses in mice. J Exp Med2012;209:1655–1670.
- 35. Taganov KD, Boldin MP, Chang KJ, Baltimore D. NF-κB-dependent induction of microRNA miR-146, an inhibitor targeted to signaling proteins of innate immune responses. Proc Natl Acad Sci USA2006;103:12481–12486.
- 36. Rodriguez A, Vigorito E, Clare S, Warren MV, Couttet P, Soond DR, Dongen S, van Grocock RJ, Das PP, Miska EA, Vetrie D, Okkenhaug K, Enright AJ, Dougan G, Turner M, Bradley A. Requirement of bic/microRNA-155 for normal immune function. Science2007;316:608–611.
- 37. Vigorito E, Perks KL, Abreu-Goodger C, Bunting S, Xiang Z, Kohlhaas S, Das PP, Miska EA, Rodriguez A, Bradley A, Smith KGC, Rada C, Enright AJ, Toellner KM, MacLennan ICM, Turner M. microRNA-155 regulates the generation of immunoglobulin class-switched plasma cells. Immunity2007;27:847–859.
- 38. Alivernini S, Gremese E, McSharry C, Tolusso B, Ferraccioli G, McInnes IB, Kurowska-Stolarska M. MicroRNA-155-at the critical interface of innate and adaptive immunity in arthritis. Front Immunol2018;8:1932.
- 39. Wang D, Tang M, Zong P, Liu H, Zhang T, Liu Y, Zhao Y. MiRNA-155 regulates the Th17/Treg ratio by targeting SOCS1 in severe acute pancreatitis. Front Physiol2018;9:1–10.
- 40. Lu L-F, Thai T-H, Calado DP, Chaudhry A, Kubo M, Tanaka K, Loeb GB, Lee H, Yoshimura A, Rajewsky K, Rudensky AY. Foxp3-dependent microRNA155 confers competitive fitness to regulatory T cells by targeting SOCS1 protein. Immunity2009;30:80–91.
- 41. Heymans S, Corsten MF, Verhesen W, Carai P, Leeuwen REW, Van Custers K, Peters T, Hazebroek M, Stöger L, Wijnands E, Janssen BJ, Creemers EE, Pinto YM, Grimm D, Schürmann N, Vigorito E, Thum T, Stassen F, Yin X, Mayr M, Windt LJ, De Lutgens E, Wouters K, Winther MPJ, De Zacchigna S, Giacca M, Bilsen M, Van Papageorgiou AP, Schroen B. Macrophage microRNA-155 promotes cardiac hypertrophy and failure. Circulation2013;128:1420–1432.
- 42. Williams A, Henao-Mejia J, Harman CCD, Flavell RA. miR-181 and metabolic regulation in the immune system. Cold Spring Harb Symp Quant Biol2013;78:223–230.
- 43. Henao-Mejia J, Williams A, Goff LA, Staron M, Licona-Limón P, Kaech SM, Nakayama M, Rinn JL, Flavell RA. The microRNA miR-181 is a critical cellular metabolic rheostat essential for NKT cell ontogenesis and lymphocyte development and homeostasis. Immunity2013;38:984–997.
- 44. Su R, Lin HS, Zhang XH, Yin XL, Ning HM, Liu B, Zhai PF, Gong JN, Shen C, Song L, Chen J, Wang F, Zhao HL, Ma YN, Yu J, Zhang JW. MiR-181 family: regulators of myeloid differentiation and acute myeloid leukemia as well as potential therapeutic targets. Oncogene2015;34:3226–3239.
- 45. Ye Z, Li G, Kim C, Hu B, Jadhav RR, Weyand CM, Goronzy JJ. Regulation of miR-181a expression in T cell aging. Nat Commun2018;9:11.
- 46. Seeger T, Haffez F, Fischer A, Koehl U, Leistner DM, Seeger FH, Boon RA, Zeiher AM, Dimmeler S. Immunosenescence-associated microRNAs in age and heart failure. Eur J Heart Fail2013;15:385–393.
- 47. Yuan X, Berg N, Lee JW, Le TT, Neudecker V, Jing N, Eltzschig H. MicroRNA miR-223 as regulator of innate immunity. J Leukoc Biol2018;104:515–524.
- 48. Roffel MP, Bracke KR, Heijink IH, Maes T. miR-223: a key regulator in the innate immune response in asthma and COPD. Front Med2020;7:1–16.
- 49. Guzik TJ, Hoch NE, Brown KA, McCann LA, Rahman A, Dikalov S, Goronzy J, Weyand C, Harrison DG. Role of the T cell in the genesis of angiotensin II-induced hypertension and vascular dysfunction. J Exp Med2007;204:2449–2460.
- 50. Nosalski R, Siedlinski M, Denby L, McGinnigle E, Nowak M, Cat AND, Medina-Ruiz L, Cantini M, Skiba D, Wilk G, Osmenda G, Rodor J, Salmeron-Sanchez M, Graham G, Maffia P, Graham D, Baker AH, Guzik TJ. T-cell-derived miRNA-214 mediates perivascular fibrosis in hypertension. Circ Res2020;126:988–1003.
- 51. Li X, Cai W, Xi W, Sun W, Shen W, Wei T, Chen X, Sun L, Zhou H, Sun Y, Chen W, Gao P, Wang H, Li Q. MicroRNA-31 regulates immunosuppression in Ang II (Aangiotensin II)-induced hypertension by targeting Ppp6C (protein phosphatase 6c). Hypertension2019;73:E14–E24.
- 52. Marí-Alexandre J, Barceló-Molina M, Sanz-Sánchez J, Molina P, Sancho J, Abellán Y, Santaolaria-Ayora ML, Giner J, Martínez-Dolz L, Estelles A, Braza-Boïls A, Zorio E. Thickness and an altered miRNA expression in the epicardial adipose tissue is associated with coronary heart disease in sudden death victims. Rev Española Cardiol (English Ed)2019;72:30–39.
- 53. Pan Y, Hui X, Chong Hoo RL, Ye D, Cheung Chan CY, Feng T, Wang Y, Ling Lam KS, Xu A. Adipocyte-secreted exosomal microRNA-34a inhibits M2 macrophage polarization to promote obesity-induced adipose inflammation. J Clin Invest2019;129:834–849.
- 54. Vacca M, Di Eusanio M, Cariello M, Graziano G, D'Amore S, Petridis FD, D'orazio A, Salvatore L, Tamburro A, Folesani G, Rutigliano D, Pellegrini F, Sabbà C, Palasciano G, Di Bartolomeo R, Moschetta A. Integrative miRNA and whole-genome analyses of epicardial adipose tissue in patients with coronary atherosclerosis. Cardiovasc Res2016;109:228–239.
- 55. Tran K, Van Majka J, Sanghai S, Sardana M, Lessard D, Milstone Z, Tanriverdi K, Freedman JE, Fitzgibbons TP, McManus D. Micro-RNAs are related to epicardial adipose tissue in participants with atrial fibrillation: data from the MiRhythm study. Front Cardiovasc Med2019;6:1–8.
- 56. Apostolakis S, Vogiatzi K, Amanatidou V, Spandidos DA. Interleukin 8 and cardiovascular disease. Cardiovasc Res2009;84:353–360.
- 57. Wei Y, Zhu M, Schober A. Macrophage microRNAs as therapeutic targets for atherosclerosis, metabolic syndrome, and cancer. Int J Mol Sci2018;19:1–20.
- 58. Zheng CG, Chen BY, Sun RH, Mou XZ, Han F, Li Q, Huang HJ, Liu JQ, Tu YX. miR-133b downregulation reduces vulnerable plaque formation in mice with AS through inhibiting macrophage immune responses. Mol Ther Nucleic Acids2019;16:745–757.
- 59. Nus M, Martínez-Poveda B, MacGrogan D, Chevre R, D’Amato G, Sbroggio M, Rodríguez C, Martínez-González J, Andrés V, Hidalgo A, de la Pompa JL. Endothelial Jag1-RBPJ signalling promotes inflammatory leucocyte recruitment and atherosclerosis. Cardiovasc Res2016;112:568–580.
- 60. Fukuda D, Aikawa E, Swirski FK, Novobrantseva TI, Kotelianski V, Gorgun CZ, Chudnovskiy A, Yamazaki H, Croce K, Weissleder R, Aster JC, Hotamisligil GS, Yagita H, Aikawa M. Notch ligand delta-like 4 blockade attenuates atherosclerosis and metabolic disorders. Proc Natl Acad Sci USA2012;109:E1868–E1877.
- 61. Liu ZJ, Tan Y, Beecham GW, Seo DM, Tian R, Li Y, Vazquez-Padron RI, Pericak-Vance M, Vance JM, Goldschmidt-Clermont PJ, Livingstone AS, Velazquez OC. Notch activation induces endothelial cell senescence and pro-inflammatory response: implication of Notch signaling in atherosclerosis. Atherosclerosis2012;225:296–303.
- 62. Chen J, Xu L, Hu Q, Yang S, Zhang B, Jiang H. MiR-17-5p as circulating biomarkers for the severity of coronary atherosclerosis in coronary artery disease. Int J Cardiol2015;197:123–124.
- 63. Tan L, Liu L, Jiang Z, Hao X. Inhibition of microRNA-17-5p reduces the inflammation and lipid accumulation, and up-regulates ATP-binding cassette transporterA1 in atherosclerosis. J Pharmacol Sci2019;139:280–288.
- 64. Feinberg MW, Moore KJ. MicroRNA regulation of atherosclerosis. Circ Res2016;118:703–720.
- 65. Horie T, Ono K, Horiguchi M, Nishi H, Nakamura T, Nagao K, Kinoshita M, Kuwabara Y, Marusawa H, Iwanaga Y, Hasegawa K, Yokode M, Kimura T, Kita T. MicroRNA-33 encoded by an intron of sterol regulatory element-binding protein 2 (Srebp2) regulates HDL in vivo. Proc Natl Acad Sci USA2010;107:17321–17326.
- 66. Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science2010;328:1566–1569.
- 67. Karunakaran D, Thrush AB, Nguyen M-A, Richards L, Geoffrion M, Singaravelu R, Ramphos E, Shangari P, Ouimet M, Pezacki JP, Moore KJ, Perisic L, Maegdefessel L, Hedin U, Harper M-E, Rayner KJ. Macrophage mitochondrial energy status regulates cholesterol efflux and is enhanced by anti-miR33 in atherosclerosis. Circ Res2015;117:266–278.
- 68. Sorci-Thomas MG, Thomas MJ. Microdomains, inflammation, and atherosclerosis. Circ Res2016;118:679–691.
- 69. Meng X, Yin J, Yu X, Guo Y. MicroRNA-205-5p promotes unstable atherosclerotic plaque formation in vivo. Cardiovasc Drugs Ther2020;34:25–39.
- 70. Chen W, Li X, Wang J, Song N, Zhu A, Jia L. miR-378a modulates macrophage phagocytosis and differentiation through targeting CD47-SIRPα axis in atherosclerosis. Scand J Immunol2019;90:10.
- 71. Ye Z, Ming Yang S, Xia Y, Peng Hu R, Ting Chen S, Li B, Wei Chen S, Li Luo X, Ying Mao L, Li Y, Jin H, Qin C, Hu B. LncRNA MIAT sponges miR-149-5p to inhibit efferocytosis in advanced atherosclerosis through CD47 upregulation. Cell Death Dis2019;10:138.
- 72. Thomson DW, Dinger ME. Endogenous microRNA sponges: evidence and controversy. Nat Rev Genet2016;17:272–283.
- 73. Liu J, Li W, Wang S, Wu Y, Li Z, Wang W, Liu R, Ou J, Zhang C, Wang S. MiR-142-3p attenuates the migration of CD4+T cells through regulating actin cytoskeleton via RAC1 and ROCK2 in arteriosclerosis obliterans. PLoS One2014;9:e95514.
- 74. Ji Q, Wei Guo M, Zheng J, Song Mao X, Bo Peng Y, Dong Li S, Nan Liang Z, Shan Dai Z, Yin Mao Y, Zeng Q. Tang Downregulation of T helper cell type 3 in patients with acute coronary syndrome. Arch Med Res2009;40:285–293.
- 75. Methe H, Brunner S, Wiegand D, Nabauer M, Koglin J, Edelman ER. Enhanced T-helper-1 lymphocyte activation patterns in acute coronary syndromes. J Am Coll Cardiol2005;45:1939–1945.
- 76. Buono C, Binder CJ, Stavrakis G, Witztum JL, Glimcher LH, Lichtman AH. T-bet deficiency reduces atherosclerosis and alters plaque antigen-specific immune responses. Proc Natl Acad Sci USA2005;102:1596–1601.
- 77. Steiner DF, Thomas MF, Hu JK, Yang Z, Babiarz JE, Allen CDC, Matloubian M, Blelloch R, Ansel KM. MicroRNA-29 regulates T-box transcription factors and interferon-γ production in helper T cells . Immunity 2011;35:169–181.
- 78. Ulrich V, Rotllan N, Araldi E, Luciano A, Skroblin P, Abonnenc M, Perrotta P, Yin X, Bauer A, Leslie KL, Zhang P, Aryal B, Montgomery RL, Thum T, Martin K, Suarez Y, Mayr M, Fernandez‐Hernando C, Sessa WC. Chronic miR‐29 antagonism promotes favorable plaque remodeling in atherosclerotic mice. EMBO Mol Med2016;8:643–653.
- 79. Peet C, Ivetic A, Bromage DI, Shah AM. Cardiac monocytes and macrophages after myocardial infarction. Cardiovasc Res2020;116:1101–1112.
- 80. Yang L, Wang B, Zhou Q, Wang Y, Liu X, Liu Z, Zhan Z. MicroRNA-21 prevents excessive inflammation and cardiac dysfunction after myocardial infarction through targeting KBTBD7. Cell Death Dis2018;9:1–14.
- 81. Bejerano T, Etzion S, Elyagon S, Etzion Y, Cohen S. Nanoparticle delivery of miRNA-21 mimic to cardiac macrophages improves myocardial remodeling after myocardial infarction. Nano Lett2018;18:5885–5891.
- 82. Garikipati VNS, Verma SK, Jolardarashi D, Cheng Z, Ibetti J, Cimini M, Tang Y, Khan M, Yue Y, Benedict C, Nickoloff E, Truongcao MM, Gao E, Krishnamurthy P, Goukassian DA, Koch WJ, Kishore R. Therapeutic inhibition of miR-375 attenuates post-myocardial infarction inflammatory response and left ventricular dysfunction via PDK-1-AKT signalling axis. Cardiovasc Res2017;113:938–949.
- 83. Krijnen PAJ, Meischl C, Hack CE, Meijer CJLM, Visser CA, Roos D, Niessen HWM. Increased Nox2 expression in human cardiomyocytes after acute myocardial infarction. J Clin Pathol2003;56:194–199.
- 84. Wu Q, Allouch A, Paoletti A, Leteur C, Mirjolet C, Martins I, Voisin L, Law F, Dakhli H, Mintet E, Thoreau M, Muradova Z, Gauthier M, Caron O, Milliat F, Ojcius DM, Rosselli F, Solary E, Modjtahedi N, Deutsch E, Perfettini JL. NOX2-dependent ATM kinase activation dictates pro-inflammatory macrophage phenotype and improves effectiveness to radiation therapy. Cell Death Differ2017;24:1632–1644.
- 85. Looi YH, Grieve DJ, Siva A, Walker SJ, Anilkumar N, Cave AC, Marber M, Monaghan MJ, Shah AM. Involvement of Nox2 NADPH oxidase in adverse cardiac remodeling after myocardial infarction. Hypertension2008;51:319–325.
- 86. Yang J, Brown ME, Zhang H, Martinez M, Zhao Z, Bhutani S, Yin S, Trac D, Xi JJ, Davis ME. High-throughput screening identifies microRNAs that target Nox2 and improve function after acute myocardial infarction. Am J Physiol Hear Circ Physiol2017;312:H1002–H1012.
- 87. Chen X, Dong S, Zhang N, Chen L, Li MG, Yang PC, Song J. MicroRNA-98 plays a critical role in experimental myocarditis. Int J Cardiol2017;229:75–81.
- 88. Chen L, Hou X, Zhang M, Zheng Y, Zheng X, Yang Q, Li J, Gu N, Zhang M, Sun Y, Wu J, Yu B. MicroRNA-223-3p modulates dendritic cell function and ameliorates experimental autoimmune myocarditis by targeting the NLRP3 inflammasome. Mol Immunol2020;117:73–83.
- 89. Wang J, Han B. Dysregulated CD4+ T cells and microRNAs in myocarditis. Front. Immunol2020;11:539–554.
- 90. Yan L, Hu F, Yan X, Wei Y, Ma W, Wang Y, Lu S, Wang Z. Inhibition of microRNA-155 ameliorates experimental autoimmune myocarditis by modulating Th17/Treg immune response. J Mol Med2016;94:1063–1079.
- 91. Corsten MF, Papageorgiou A, Verhesen W, Carai P, Lindow M, Obad S, Summer G, Coort SLM, Hazebroek M, Leeuwen R, Van Gijbels MJJ, Wijnands E, Biessen EAL, Winther MPJ, De Stassen FRM, Carmeliet P, Kauppinen S, Schroen B, Heymans S. MicroRNA profiling identifies microRNA-155 as an adverse mediator of cardiac injury and dysfunction during acute viral myocarditis. Circ Res2012;111:415–425.
- 92. Zhang Y, Zhang M, Li X, Tang Z, Wang X, Zhong M, Suo Q, Zhang Y, Lv K. Silencing microRNA-155 attenuates cardiac injury and dysfunction in viral myocarditis via promotion of M2 phenotype polarization of macrophages. Sci Rep2016;6:13.
- 93. Ong S, Ligons DL, Barin JG, Wu L, Talor MV, Diny N, Fontes JA, Gebremariam E, Kass DA, Rose NR, Čiháková D. Natural killer cells limit cardiac inflammation and fibrosis by halting eosinophil infiltration. Am J Pathol2015;185:847–861.
- 94. Trotta R, Chen L, Ciarlariello D, Josyula S, Mao C, Costinean S, Yu L, Butchar JP, Tridandapani S, Croce CM, Caligiuri MA. miR-155 regulates IFN-γ production in natural killer cells. Blood2012;119:3478–3485.
- 95. Loebel M, Holzhauser L, Hartwig JA, Shukla PC, Savvatis K, Jenke A, Gast M, Escher F, Becker SC, Bauer S, Stroux A, Beling A, Kespohl M, Pinkert S, Fechner H, Kuehl U, Lassner D, Poller W, Schultheiss HP, Zeller T, Blankenberg S, Papageorgiou AP, Heymans S, Landmesser U, Scheibenbogen C, Skurk C. The forkhead transcription factor Foxo3 negatively regulates natural killer cell function and viral clearance in myocarditis. Eur Heart J2018;39:876–887.
- 96. Everett BM, Cornel JH, Lainscak M, Anker SD, Abbate A, Thuren T, Libby P, Glynn RJ, Ridker PM. Anti-inflammatory therapy with canakinumab for the prevention of hospitalization for heart failure. Circulation2019;139:1289–1299.
- 97. Adamo L, Rocha-Resende C, Prabhu SD, Mann DL. Reappraising the role of inflammation in heart failure. Nat Rev Cardiol2020;17:269–285.
- 98. Zeng Z, Wang K, Li Y, Xia N, Nie S, Lv B, Zhang M, Tu X, Li Q, Tang T, Cheng X. Down-regulation of microRNA-451a facilitates the activation and proliferation of CD4+ T cells by targeting Myc in patients with dilated cardiomyopathy. J Biol Chem2017;292:6004–6013.
- 99. Owczarz M, Budzinska M, Domaszewska-Szostek A, Borkowska J, Polosak J, Gewartowska M, Slusarczyk P, Puzianowska-Kuznicka M. miR-34a and miR-9 are overexpressed and SIRT genes are downregulated in peripheral blood mononuclear cells of aging humans. Exp Biol Med (Maywood)2017;242:1453–1461.
- 100. Rao DS, O'Connell RM, Chaudhuri AA, Garcia-Flores Y, Geiger TL, Baltimore D. MicroRNA-34a perturbs B lymphocyte development by repressing the forkhead box transcription factor Foxp1. Immunity2010;33:48–59.
- 101. Nogueira E, Hamour S, Sawant D, Henderson S, Mansfield N, Chavele KM, Pusey CD, Salama AD. Serum IL-17 and IL-23 levels and autoantigen-specific Th17 cells are elevated in patients with ANCA-associated vasculitis. Nephrol Dial Transplant2010;25:2209–2217.
- 102. Abdulahad WH, Stegeman CA, Limburg PC, Kallenberg CGM. Skewed distribution of Th17 lymphocytes in patients with Wegener’s granulomatosis in remission. Arthritis Rheum2008;58:2196–2205.
- 103. Abdulahad WH, Geld YM, Van Der Stegeman CA, Kallenberg CGM. Persistent expansion of CD4+ effector memory T cells in Wegener’s granulomatosis. Kidney Int2006;70:938–947.
- 104. Abdulahad WH, Stegeman CA, Geld YM, Van Der Doornbos-Van Der Meer B, Limburg PC, Kallenberg CGM. Functional defect of circulating regulatory CD4+ T cells in patients with Wegener’s granulomatosis in remission. Arthritis Rheum2007;56:2080–2091.
- 105. Morgan MD, Day CJ, Piper KP, Khan N, Harper L, Moss PA, Savage COS. Patients with Wegener’s granulomatosis demonstrate a relative deficiency and functional impairment of T-regulatory cells. Immunology2010;130:64–73.
- 106. Dekkema GJ, Bijma T, Jellema PG, A Van Den B, Kroesen BJ, Stegeman CA, Heeringa P, Abdulahad WH, Sanders JS. Increased miR-142-3p expression might explain reduced regulatory T cell function in granulomatosis with polyangiitis. Front Immunol2019;10:1–12.
- 107. Ni FF, Li CR, Li Q, Xia Y, Wang GB, Yang J. Regulatory T cell microRNA expression changes in children with acute Kawasaki disease. Clin Exp Immunol2014;178:384–393.
- 108. Rouas R, Fayyad-Kazan H, El Zein N, Lewalle P, Rothé F, Simion A, Akl H, Mourtada M, El Rifai M, Burny A, Romero P, Martiat P, Badran B. Human natural Treg microRNA signature: role of microRNA-31 and microRNA-21 in FOXP3 expression. Eur J Immunol2009;39:1608–1618.
- 109. Kalra H, Drummen GPC, Mathivanan S. Focus on extracellular vesicles: introducing the next small big thing. Int J Mol Sci2016;17:1–30.
- 110. Chang YJ, Li YS, Wu CC, Wang KC, Huang TC, Chen Z, Chien S. Extracellular microRNA-92a mediates endothelial cell-macrophage communication. ATVB2019;39:2492–2504.
- 111. Zhao J, Li X, Hu J, Chen F, Qiao S, Sun X, Gao L, Xie J, Xu B. Mesenchymal stromal cell-derived exosomes attenuate myocardial ischaemia-reperfusion injury through miR-182-regulated macrophage polarization. Cardiovasc Res2019;115:1205–1216.
- 112. Wu R, Gao W, Yao K, Ge J. Roles of exosomes derived from immune cells in cardiovascular diseases. Front Immunol2019;10:1–16.
- 113. Cortes-Troncoso J, Jang S-I, Perez P, Hidalgo J, Ikeuchi T, Greenwell-Wild T, Warner BM, Moutsopoulos NM, Alevizos I, Moutsopoulos NM, Alevizos I. T cell exosome-derived miR-142-3p impairs glandular cell function in Sjögren’s syndrome. JCI Insight2020;5:1–14.
- 114. Dewi IS, Celik S, Karlsson A, Hollander Z, Lam K, McManus JW, Tebbutt S, Ng R, Keown P, McMaster R, McManus B, Öhman J, Gidlöf O. Exosomal miR-142-3p is increased during cardiac allograft rejection and augments vascular permeability through down-regulation of endothelial RAB11FIP2 expression. Cardiovasc Res2017;113:440–452.
- 115. Guay C, Kruit JK, Rome S, Menoud V, Mulder NL, Jurdzinski A, Mancarella F, Sebastiani G, Donda A, Gonzalez BJ, Jandus C, Bouzakri K, Pinget M, Boitard C, Romero P, Dotta F, Regazzi R. Lymphocyte-derived exosomal microRNAs promote pancreatic β cell death and may contribute to type 1 diabetes development. Cell Metab2019;29:348–361.E6.
- 116. Cai L, Chao G, Li W, Zhu J, Li F, Qi B, Wei Y, Chen S, Zhou G, Lu X, Xu J, Wu X, Fan G, Li J, Liu S. Activated CD4+ T cells-derived exosomal miR-142-3p boosts post-ischemic ventricular remodeling by activating myofibroblast. Aging (Albany NY)2020;12:7380–7396.
- 117. Italiano JE, Mairuhu AT, Flaumenhaft R. Clinical relevance of microparticles from platelets and megakaryocytes. Curr Opin Hematol2010;17:578–584.
- 118. Chapnik E, Rivkin N, Mildner A, Beck G, Pasvolsky R, Metzl-Raz E, Birger Y, Amir G, Tirosh I, Porat Z, Israel L, Lellouche E, Michaeli S, Lellouche JP, Izraeli S, Jung S, Hornstein E. MiR-142 orchestrates a network of actin cytoskeleton regulators during megakaryopoiesis. Elife2014;3:1–22.
- 119. Bao H, Yao Q-P, Huang K, Chen X-H, Han Y, Jiang Z-L, Gao L-Z, Qi Y-X. Platelet-derived miR-142-3p induces apoptosis of endothelial cells in hypertension. Cell Mol Biol (Noisy-le-Grand)2017;63:3.
- 120. Bao H, Chen YX, Huang K, Zhuang F, Bao M, Han Y, Chen XH, Shi Q, Yao QP, Qi YX. Platelet-derived microparticles promote endothelial cell proliferation in hypertension via miR-142-3p. Faseb J2018;32:3912–3923.
- 121. Gomez I, Ward B, Souilhol C, Recarti C, Ariaans M, Johnston J, Burnett A, Mahmoud M, Luong LA, West L, Long M, Parry S, Woods R, Hulston C, Benedikter B, Niespolo C, Bazaz R, Francis S, Kiss-Toth E, Zandvoort M, van Schober A, Hellewell P, Evans PC, Ridger V. Neutrophil microvesicles drive atherosclerosis by delivering miR-155 to atheroprone endothelium. Nat Commun2020;11:18.
- 122. Tikhomirov R, Reilly-O’Donnell B, Catapano F, Faggian G, Gorelik J, Martelli F, Emanueli C. Exosomes: from potential culprits to new therapeutic promise in the setting of cardiac fibrosis. Cells2020;9:592.
- 123. Bouchareychas L, Duong P, Covarrubias S, Alsop E, Phu TA, Chung A, Gomes M, Wong D, Meechoovet B, Capili A, Yamamoto R, Nakauchi H, McManus MT, Carpenter S, Van K-JK, Raffai RL. Macrophage exosomes resolve atherosclerosis by regulating hematopoiesis and inflammation via microRNA cargo. Cell Rep2020;32:107881.
- 124. Tkach M, Théry C. Communication by extracellular vesicles: where we are and where we need to go. Cell2016;164:1226–1232.
- 125. Chevillet JR, Kang Q, Ruf IK, Briggs HA, Vojtech LN, Hughes SM, Cheng HH, Arroyo JD, Meredith EK, Gallichotte EN, Pogosova-Agadjanyan EL, Morrissey C, Stirewalt DL, Hladik F, Yu EY, Higano CS, Tewari M. Quantitative and stoichiometric analysis of the microRNA content of exosomes. Proc Natl Acad Sci USA2014;111:14888–14893.
- 126. Kesidou D, Martins C, da PA, Windt LJ, de BM, Beqqali A, Baker AH. Extracellular vesicle miRNAs in the promotion of cardiac neovascularisation. Front Physiol2020;11:1–25.
- 127. Elias S, Rudensky AY. Therapeutic use of regulatory T cells for graft-versus-host disease. Br J Haematol2019;187:25–38.
- 128. Zhang X, Olsen N, Zheng SG. The progress and prospect of regulatory T cells in autoimmune diseases. J Autoimmun2020;111:102461.
- 129. Romano M, Fanelli G, Albany CJ, Giganti G, Lombardi G. Past, present, and future of regulatory T cell therapy in transplantation and autoimmunity. Front Immunol2019;10:1–14.
- 130. Bansal SS, Ismahil MA, Goel M, Zhou G, Rokosh G, Hamid T, Prabhu SD. Dysfunctional and proinflammatory regulatory T-lymphocytes are essential for adverse cardiac remodeling in ischemic cardiomyopathy. Circulation2019;139:206–221.
- 131. Bansal SS, Ismahil MA, Goel M, Patel B, Hamid T, Rokosh G, Prabhu SD. Activated T lymphocytes are essential drivers of pathological remodeling in ischemic heart failure. Circ Hear Fail2017;10:139–148.
- 132. Emmerson A, Trevelin SC, Mongue-Din H, Becker PD, Ortiz C, Smyth LA, Peng Q, Elgueta R, Sawyer G, Ivetic A, Lechler RI, Lombardi G, Shah AM. Nox2 in regulatory T cells promotes angiotensin II–induced cardiovascular remodeling. J Clin Invest2018;128:3088–3101.
- 133. Foks AC, Lichtman AH, Kuiper J. Treating atherosclerosis with regulatory T cells. Arterioscler Thromb Vasc Biol2015;35:280–287.
- 134. Ou Han-xiao, Guo Bing-bing, Liu Qi, Yang Zhen, Feng Wen-jie, Mo Zhong-cheng Regulatory T cells as a new therapeutic target for atherosclerosis. Acta Pharmacol Sin2018;39:1249–1258.
- 135. Albany CJ, Trevelin SC, Giganti G, Lombardi G, Scottà C. Getting to the heart of the matter: the role of regulatory T-cells (TREGS) in cardiovascular disease (CVD) and atherosclerosis. Front Immunol2019;10:1–11.
- 136. Liu X, Robinson SN, Setoyama T, Tung SS, D'Abundo L, Shah MY, Yang H, Yvon E, Shah N, Yang H, Konopleva M, Garcia-Manero G, McNiece I, Rezvani K, Calin GA, Shpall EJ, Parmar S. FOXP3 is a direct target of miR15a/16 in umbilical cord blood regulatory T cells. Bone Marrow Transplant2014;49:793–799.
- 137. Yang HY, Barbi J, Wu CY, Zheng Y, Vignali PDA, Wu X, Tao JH, Park BV, Bandara S, Novack L, Ni X, Yang X, Chang KY, Wu RC, Zhang J, Yang CW, Pardoll DM, Li H, Pan F. MicroRNA-17 modulates regulatory T cell function by targeting co-regulators of the Foxp3 transcription factor. Immunity2016;45:83–93.
- 138. Fayyad-Kazan H, Rouas R, Fayyad-Kazan M, Badran R, Zein N, El Lewalle P, Najar M, Hamade E, Jebbawi F, Merimi M, Romero P, Burny A, Badran B, Martiat P. MicroRNA profile of circulating CD4-positive regulatory T cells in human adults and impact of differentially expressed microRNAs on expression of two genes essential to their function. J Biol Chem2012;287:9910–9922.
- 139. Alrafas HR, Busbee PB, Nagarkatti M. Resveratrol downregulates miR-31 to promote T regulatory cells during prevention of TNBS-induced colitis. Mol Nutr Food Res2020;64:1–11.
- 140. Pan W, Zhu S, Dai D, Liu Z, Li D, Li B, Gagliani N, Zheng Y, Tang Y, Weirauch MT, Chen X, Zhu W, Wang Y, Chen B, Qian Y, Chen Y, Fang J, Herbst R, Richman L, Jallal B, Harley JB, Flavell RA, Yao Y, Shen N. MiR-125a targets effector programs to stabilize Treg-mediated immune homeostasis. Nat Commun2015;6:1–12.
- 141. Huang B, Zhao J, Lei Z, Shen S, Li D, Shen GX, Zhang GM, Feng ZH. miR-142-3p restricts cAMP production in CD4+CD25-T cells and CD4+CD25+ TREG cells by targeting AC9 mRNA. EMBO Rep2009;10:180–185.
- 142. Gao J, Gu J, Pan X, Gan X, Ju Z, Zhang S, Xia Y, Lu L, Wang X. Blockade of miR-142-3p promotes anti-apoptotic and suppressive function by inducing KDM6A-mediated H3K27me3 demethylation in induced regulatory T cells. Cell Death Dis2019;10:332.
- 143. Wang X, Cao J, Yu Y, Ma B, Gao C, Lu J, Lin Y, Li P, Qi F. Role of microRNA 146a in regulating regulatory T cell function to ameliorate acute cardiac rejection in mice. Transplant Proc2019;51:901–912.
- 144. Lu L-F, Boldin MP, Chaudhry A, Lin L-L, Taganov KD, Hanada T, Yoshimura A, Baltimore D, Rudensky AY. Function of miR-146a in controlling treg cell-mediated regulation of Th1 responses. Cell2010;142:914–929.
- 145. Lu Y, Hippen KL, Lemire AL, Gu J, Wang W, Ni X, Ranganathan P, Levine BL, Riley JL, June CH, Turka LA, Munn DH, Garzon R, Lu L, Blazar BR. MiR-146b antagomir-treated human TREGS acquire increased GVHD inhibitory potency. Blood2016;128:1424–1435.
- 146. Łyszkiewicz M, Winter SJ, Witzlau K, Föhse L, Brownlie R, Puchałka J, Verheyden NA, Kunze-Schumacher H, Imelmann E, Blume J, Raha S, Sekiya T, Yoshimura A, Frueh JT, Ullrich E, Huehn J, Weiss S, Gutierrez MG, Prinz I, Zamoyska R, Ziętara N, Krueger A. mir-181A/B-1 controls thymic selection of treg cells and tunes their suppressive capacity. PLoS Biol2019;17:e2006716.
- 147. Ghorbani S, Talebi F, Chan WF, Masoumi F, Vojgani M, Power C, Noorbakhsh F. MicroRNA-181 variants regulate T cell phenotype in the context of autoimmune neuroinflammation. Front Immunol2017;8:1–14.
- 148. Wang L, Yang X, Li W, Song X, Kang S. MiR-202-5p/MATN2 are associated with regulatory T-cells differentiation and function in allergic rhinitis. Hum Cell Springer Japan2019;32:411–417.
- 149. Mu Y, Zhang J, Liu Y, Ma J, Jiang D, Zhang X, Yi X, Cheng K, Shen S, Yang Y, Zhuang R, Zhang Y. CD226 deficiency on regulatory T cells aggravates renal fibrosis via up-regulation of Th2 cytokines through miR-340. J Leukoc Biol2020;107:573–587.
- 150. Al-Ghezi ZZ, Singh N, Mehrpouya-Bahrami P, Busbee PB, Nagarkatti M, Nagarkatti PS. AhR activation by TCDD (2,3,7,8-tetrachlorodibenzo-p-dioxin) attenuates pertussis toxin-induced inflammatory responses by differential regulation of TREGS and Th17 cells through specific targeting by microRNA. Front Microbiol2019;10:1–14.
- 151. Hanna J, Hossain GS, Kocerha J. The potential for microRNA therapeutics and clinical research. Front Genet2019;10:1–6.
- 152. Bonneau E, Neveu B, Kostantin E, Tsongalis GJ, De Guire V. How close are miRNAs from clinical practice? A perspective on the diagnostic and therapeutic market. Electron J Int Fed Clin Chem Lab Med2019;30:114–127.
- 153. Nair N, Kumar S, Gongora E, Gupta S. Circulating miRNA as novel markers for diastolic dysfunction. Mol Cell Biochem2013;376:33–40.
- 154. Jiao M, You HZ, Yang XY, Yuan H, Li YL, Liu WX, Jin M, Du J. Circulating microRNA signature for the diagnosis of childhood dilated cardiomyopathy. Sci Rep2018;8:1–9.
- 155. Ellis KL, Cameron VA, Troughton RW, Frampton CM, Ellmers LJ, Richards AM. Circulating microRNAs as candidate markers to distinguish heart failure in breathless patients. Eur J Heart Fail2013;15:1138–1147.
- 156. Lee SWL, Paoletti C, Campisi M, Osaki T, Adriani G, Kamm RD, Mattu C, Chiono V. MicroRNA delivery through nanoparticles. J Control Release2019;313:80–95.
- 157. Bracamonte-Baran W, Chen G, Hou X, Talor MV, Choi HS, Davogustto G, Taegtmeyer H, Sung J, Hackam DJ, Nauen D, Čiháková D. Non-cytotoxic cardiac innate lymphoid cells are a resident and quiescent type 2-commited population. Front Immunol2019;10:1–18.
- 158. Deng Y, Wu S, Yang Y, Meng M, Chen X, Chen S, Li L, Gao Y, Cai Y, Imani S, Chen B, Li S, Deng Y, Li X. Unique phenotypes of heart resident type 2 innate lymphoid cells. Front Immunol2020;11:1–13.
- 159. Choi HS, Won T, Hou X, Chen G, Bracamonte-Baran W, Talor MV, Jurčová I, Szárszoi O, Čurnova L, Stříž I, Hooper JE, Melenovský V, Čiháková D. Innate lymphoid cells play a pathogenic role in pericarditis. Cell Rep2020;30:2989–3003.E6.
- 160. Newland SA, Mohanta S, Clément M, Taleb S, Walker JA, Nus M, Sage AP, Yin C, Hu D, Kitt LL, Finigan AJ, Rodewald HR, Binder CJ, McKenzie ANJ, Habenicht AJ, Mallat Z. Type-2 innate lymphoid cells control the development of atherosclerosis in mice. Nat Commun2017;8:11.
- 161. Berrien-Elliott MM, Sun Y, Neal C, Ireland A, Trissal MC, Sullivan RP, Wagner JA, Leong JW, Wong P, Mah-Som AY, Wong TN, Schappe T, Keppel CR, Cortez VS, Stamatiades EG, Li MO, Colonna M, Link DC, French AR, Cooper MA, Wang W, Le Boldin MP, Reddy P, Fehniger TA. MicroRNA-142 is critical for the homeostasis and function of type 1 innate lymphoid cells. Immunity2019;51:479–490.E6.
- 162. Pelosi A, Alicata C, Tumino N, Ingegnere T, Loiacono F, Mingari MC, Moretta L, Vacca P. An anti-inflammatory microRNA signature distinguishes group 3 innate lymphoid cells from natural killer cells in human decidua. Front Immunol2020;11:1–15.
- 163. Ramos-Ram P, Johansson K, Malmh C, Madeleine R. MicroRNA-155 is a critical regulator of type 2 innate lymphoid cells and IL-33 signaling in experimental models of allergic airway inflammation. J Allergy Clin Immunol2017;139:1007–1016.E9.