Key Messages
What is already known on this topic
In many chronic conditions, there is consistent evidence that physical activity (PA) results in health benefits, but this has not yet been investigated in the chronic pancreatitis patient group.
This study was carried out to compare PA levels of patients with chronic pancreatitis to those of matched controls and to compare the PA levels with the current WHO guidelines for chronic conditions.
What this study adds
This study offers early objective evidence that activity levels in patients with chronic pancreatitis are lower than those in healthy controls and do not meet current international recommendations for chronic disease.
How this study might affect research, practice, or policy
PA may improve physical function and reduce disease progression in chronic pancreatitis, but research on this topic is urgently required to both measure PA and investigate the possible benefits of exercise interventions.
Introduction
Chronic pancreatitis is a progressive inflammatory disorder of the pancreas []. It is defined by the destruction of the pancreatic secretory parenchyma and its replacement by fibrous tissue which compromises both the endocrine and exocrine systems [, ]. Both pharmacological and lifestyle interventions are recommended treatment interventions []. Sarcopenia is a common complication of chronic pancreatitis. For the diagnosis of sarcopenia, the European Working Group on Sarcopenia in Older People (EWGSOP) recommends using the presence of both low muscle mass and low muscle function (strength or performance) []. Sarcopenia is associated with adverse health-related outcomes [], increased likelihood of frailty [], and is a predictor of greater healthcare utilisation [].
The beneficial effects of exercise and physical activity (PA) have been demonstrated in many chronic inflammatory diseases, including cardiovascular disease, type 2 diabetes mellitus, metabolic syndrome, and cancer [–]. Recently updated guidelines by the World Health Organisation (WHO) recommended that those with chronic conditions should engage in a minimum of 150–300 min of moderate-intensity aerobic PA per week, or 75–150 min of vigorous-intensity aerobic PA per week, or an equivalent combination for substantial health benefits []. There are currently no specific PA recommendations for individuals with chronic pancreatitis.
The authors recently conducted a systematic review [] examining the evidence for PA as an intervention in the chronic pancreatitis patient population. The single paper retrieved reported that a 12-week yoga-based exercise intervention improved quality of life and stress indicators in individuals with chronic pancreatitis when compared to a non-intervention group []. This dearth of literature precluded a conclusion regarding the overall effectiveness of PA in individuals with chronic pancreatitis. However, extrapolating from studies in HIV [] and cancer [], PA may improve physical function, reduce disease progression, and reduce anxiety and depression in those with chronic pancreatitis. To date, the effects of PA interventions have not been determined objectively in this patient group [], and few (if any) studies have evaluated PA levels of individuals with chronic pancreatitis.
This study therefore sought to compare PA levels of patients with chronic pancreatitis to those of matched controls. As secondary aims, we sought to compare the PA levels with the WHO guidelines for chronic conditions.
Materials and Methods
Study Design
Investigation of levels of PA was conducted in parallel to a larger cross-sectional study investigating the dietary intake of individuals with chronic pancreatitis using a cross-sectional controlled design. Written informed consent was sought and received from all participants. This study was approved by the Joint St. James’s Hospital/Tallaght University Hospital Research Ethics Committee 2021 (Reference 2020-03 List 11).
Inclusion and Exclusion Criteria
Adult subjects (>18 years) with a confirmed diagnosis of chronic pancreatitis who were living in the community and managed an oral diet were eligible for participation. Those with a history of pancreatic cancer, total pancreatectomy, or cystic fibrosis were excluded. Pregnant or breastfeeding subjects, subjects with a prognosis of <6 months, subjects with another malabsorptive disorder including coeliac disease or inflammatory bowel disease (IBD), those requiring artificial nutrition support, individuals with active illness or who were current inpatients, subjects who were not independently mobile, subjects with a recent non-controlled cardiac event, or subjects who declined or were unable to consent were excluded.
Controls were eligible for inclusion if they did not have a history of pancreatic disease including pancreatitis, a history of pancreatic cancer, total pancreatectomy, or cystic fibrosis. They were excluded if they were pregnant or breastfeeding, had a prognosis of <6 months, had another malabsorptive disorder including coeliac disease or IBD, were on artificial nutrition support, were current inpatients, were not independently mobile, had a recent non-controlled cardiac event, or were those who declined or were unable to provide informed consent.
We initially asked subjects to identify a matched, healthy control from within their social circle, but this method of recruitment was unsuccessful, recruiting only one subject. The healthy controls were then recruited by advertising on social media, advertising within the researchers’ base hospitals, and from the research team’s social circle. We recruited 15 age- and sex-matched controls.
Individuals were identified from the pancreatic database by a specialist pancreatic nurse, and their medical records were screened against the study’s inclusion criteria. A letter of invitation was sent, followed by a confirmation call. Due to the COVID-19 pandemic, all visits were scheduled and conducted by online video conference (Zoom v5.1.0, 55 Almaden Boulevard, 6th Floor, San Jose, CA, USA). The confirmation calls were conducted by the Surgical Registrar (lead researcher on a sister project) and the Clinical Specialist Physiotherapist (lead researcher for the present study) via Zoom. Where Zoom was not possible in a small number of cases, appointments were made by telephone.
During this call study, participants were provided with details of the methodology for the study including the PA measurement. Following a cooling-off period of 7 days and receipt of informed consent documents, they were then interviewed by the physiotherapist, given further information about the study, and provided with a PA monitor along with written instructions regarding application and usage.
Measurement of Activity
PA was measured over 1 week using the ActiGraph wGT3X-BT accelerometer (ActiGraph Corp., Pensacola, FL, USA). The device weighed approximately 27 g and was attached to an elasticated belt over the right hip. The ActiGraph accelerometer has been validated in both laboratory and field settings [, ]. Participants were asked to maintain their typical levels of PA during the assessment period, and they were instructed to remove the monitor for any water-based activities. An activity diary was provided to record hours of daily wear-time for cross-validation with the raw accelerometer output during waking hours. ActiLife software (Version 6.13.4) was used to download and process raw data. To identify PA at different intensities, vector magnitude count thresholds as described by Troiano et al. [] were used. PA intensity was classified as light, moderate, vigorous, and combined moderate-vigorous physical activity (MVPA). Low-intensity PA ranged from 100-2,019 counts per minute, moderate intensity ranged from 2,020 to 5,998 counts per minute and vigorous intensity was >5,999 counts per minute, with a further analysis of bouts of MVPA that were sustained for at least 10 min []. Wear-time was validated by application of the algorithms devised by Choi et al. []. Due to COVID-19 restrictions, clinical and demographic data including sex, date of birth, and weight (kg)/height (m) to determine body mass index (BMI, kg/m2) were self-reported.
Statistical Analysis
Statistical analysis was performed using the statistical package for the social sciences (SPSS, Version 25, IBM, Chicago, IL, USA). Continuous variables were tested for normality using the Kolmogorov-Smirnov test. Normally distributed data were analysed using 2-tailed Student’s t test, and non-normally distributed data were analysed using the Mann-Whitney U test. The level of significance was set a priori at p < 0.05.
Results
Recruitment and Participation
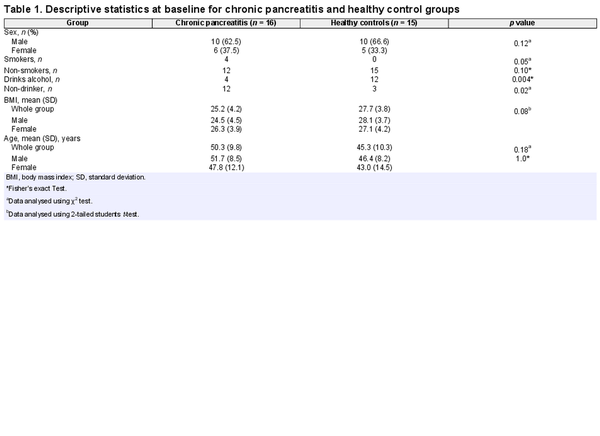
All patients from an active chronic pancreatitis database of n = 200 were screened for participation, and n = 57 were deemed suitable to be contacted by the research team according to the inclusion criteria. Following further telephone contact to schedule an appointment, n = 40 subjects agreed to participate. Of these, n = 17 ultimately participated in an online interview and then received ActiGraph accelerometers and activity diaries by post. Sixteen of the 17 completed the full ActiGraph PA assessment over the required 1-week period, along with n = 15 controls. There were no statistical differences between patients with chronic pancreatitis and control subjects regarding sex, age, or BMI. There were significantly more smokers in the chronic pancreatitis group and fewer current drinkers (Table 1).
Light, Moderate, and Vigorous Activity
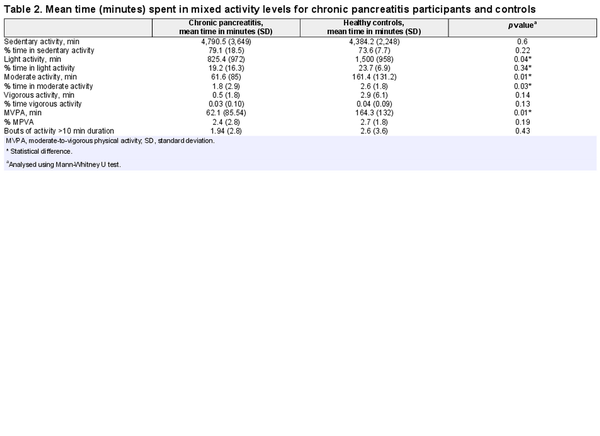
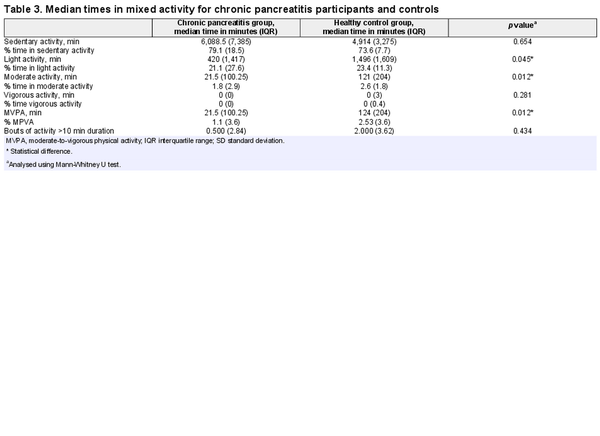
Participants with chronic pancreatitis spent a significantly lower amount of time in light, moderate, and moderate-vigorous activity than those in the control group. There was no significant difference when activity was assessed in bouts of 10-min activity. There was no significant difference between the groups for vigorous or sedentary activity (see Tables 2 and 3).
Sedentary Behaviour
There was no significant difference between patients and controls in the time spent on sedentary behaviour. The chronic pancreatitis group spent 79% of their time in sedentary behaviour (80 h during the week) compared with 73.6% (73 h) among control subjects.
Adherence to PA Recommendations
N = 6 control subjects (40%) met the WHO Guidelines [] for moderate activity in healthy adults, while n = 2 (12.5%) patients with chronic pancreatitis met this benchmark. The remainder of the chronic pancreatitis patients (10/16, 63%), and 4/15 (27%) of the control group undertook less than 60 min of moderate PA. Most of the pancreatitis and control subjects (88% and 67%, respectively) undertook no vigorous activity. There was no difference between the groups (Table 2).
Discussion
This novel exploratory study is the first of which we are aware to evaluate PA in the chronic pancreatitis population compared with a healthy control group. The results of our study indicate some disparity in activity levels between the two groups. While there was no difference in time spent in sedentary activity, patients with chronic pancreatitis spent less time engaged in light, moderate, and MVPA compared to matched controls. Vigorous activity participation was notably poor in both groups. In addition, the WHO recommendations for those with chronic conditions were not met by most patients with chronic pancreatitis.
Reduced muscle strength, fatigue, and reduced cognition have been reported in the chronic pancreatitis population [–]. A recent review on sarcopenia in chronic pancreatitis patients showed sarcopenia is highly prevalent in this group and is associated with reduced quality of life and increased hospitalisation, although the review data was limited to three full-text studies []. Failure of this group to meet the chronic disease recommendations for PA could conceivably contribute to sarcopenia, poor functional capacity, and poor clinical outcomes. The comprehensive HaPanEU guidelines [] for chronic pancreatitis made few references to PA, likely due to the dearth of studies on the topic. The two mentions of PA in the guidelines referred to importance of weight-bearing exercise to preserve bone density and the inclusion of PA in the treatment of type 3c diabetes along with other lifestyle changes.
Although regular PA has been shown to influence both risk of disease onset and disease progression and prevalence in many chronic disease populations [, ], few studies have measured or studied the effects of PA in subjects with chronic pancreatitis []. Among the few studies published on this topic, two reports highlighted risks associated with exercise in patients with chronic pancreatitis who had undergone total pancreatectomy with auto-islet transplantation. The risks related to the threat of hypoglycaemia due to failure to increase endogenous glucose production during moderate-intensity exercise in that specific group [, , ]. These studies demonstrated potential deleterious effects of exercise in a vulnerable patient group and therefore highlight the need for caution in those with a progressive, complicated chronic disease. A potentially positive effect on pancreatic cirrhosis and injury was detected in mice with (cerulein-induced) chronic pancreatitis. This study suggested that exercise may result in the secretion of the hormone irisin, which is known to mitigate liver cirrhosis, and might potentially alleviate pancreatic cirrhosis and injury [].
In other chronic conditions, there is ample, consistent evidence that engagement in PA results in health benefits. Cardiac problems, respiratory difficulties, type 2 diabetes, and obesity were lower in those engaged in vigorous activity in a recent large-scale European study []. PA also improves all-cause mortality for type 2 diabetes, HIV, hypertension, and cancer []. Another potential benefit relates to the positive effect of PA on cognition. Exercise and PA have been shown to improve cognition in older adults in some chronic conditions []. A 12-week aerobic exercise intervention improved short-term cognitive function, as well as sleep, depression, fatigue, cardiac fitness, and quality of life in patients with non-cirrhotic chronic hepatitis []. This may be relevant for those with chronic pancreatitis as chronic pain, one of the primary complications of this condition, has been associated with reduced cognition []. Regarding IBD, a recent review suggested putative benefits to the immune system, improvements in disease activity, better quality of life, and improved bone mineral density were possible with exercise intervention; however, the authors concluded that further investigation was required to establish specific exercise recommendations for this group [].
We found that PA within the chronic pancreatitis group fell well below WHO recommendations []. Notably, our control group also demonstrated low activity levels. The level for controls (40% achieving the WHO benchmark) was slightly less than the level of activity among “healthy adults” (46% achieved the WHO benchmark) in a recent Healthy Ireland 2019 report []. It is noteworthy that the Healthy Ireland report used a patient reported outcome questionnaire, but our study used an objective measurement of PA (accelerometery) which should reduce self-reporting bias. Previous work has noted limitations with PROMS [–]. Indeed, Boone et al. [] report overestimation as high as 165% in their study of 70 subjects which compared accelerometery with the self-reported New Zealand Physical Activity Questionnaire (NZPAQ-LF), the WHO guidelines [] also recommended that all adults should limit the amount of time spent being sedentary and recommends replacing sedentary time with any intensity of exercise, including light intensity. We found that sedentary activity did not differ between the chronic pancreatitis and control groups, both of which demonstrated significantly more time in sedentary behaviour than that reported for the general population in the Healthy Ireland Survey in 2019 [].
Limitations and Strengths
We acknowledge that recruitment in this study was limited with only 17 individuals with chronic pancreatitis recruited from 57 eligible patients (29.8%), with a recruitment rate of one every 2 weeks. The study period coincided with the COVID-19 pandemic, during which there was an unprecedented cessation/limitation of face-to-face clinic appointments in Ireland. Importantly, once subjects were recruited following the initial Zoom/telephone calls, almost all (16/17 patients) complied fully with the PA monitoring intervention and completed both the active wearing of the monitor and the movement diary completion as requested. Therefore, we showed that patients with chronic pancreatitis were amenable to measuring their levels of PA over a week-long period, setting the scene for future studies. Due to the lack of face-to-face contact, self-reported weight and heights were relied upon which potentially introduced a bias, for both patients and controls. Similarly, patients and control participants may have altered their activity levels because of taking part in the study (reactivity bias).
Conclusions
This exploratory study offers early objective evidence suggesting that activity levels in patients with chronic pancreatic disease are lower than healthy controls and are not meeting current international recommendations for chronic disease. Research in this area is lacking, both in the measurement of habitual activity, as well as in determining the effects of exercise interventions. Further investigation of this chronic illness population is strongly recommended.
Statement of Ethics
This study was approved by the Joint St. James’s Hospital/Tallaght University Hospital Research Ethics Committee 2021 (Reference 2020-03 List 11). Written informed consent was sought and received from all participants in this study.
Conflict of Interest Statement
The authors have no competing or conflicting interests to declare.
Funding Sources
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Author Contributions
Brenda Monaghan acquired, reviewed, interpreted, and analysed the data; reviewed the literature; and drafted the manuscript. Qurat Ul Ain contributed to the study design and approved the final version. Kevin C. Conlon, Sinead N. Duggan, and John Gormley contributed to the study design and paper revision and approved the final version. Ann Monaghan and Sinead N. Duggan contributed to the study design and critically reviewed, interpreted, and approved the final version. John Gormley contributed to the study design, critically revised, and finally approved the article.
Data Availability Statement
All data generated or analysed during this study are included in this article. Any further enquiries can be directed to the corresponding author.
References
- 1. Yoh K, Nishikawa H, Enomoto H, Iwata Y, Ishii A, Yuri Y, et al Clinical influence of exercise therapy on sarcopenia in patients with chronic pancreatitis: a study protocol for a randomised controlled trial BMJ Open Gastroenterol 2018 5 1 e000190
- 2. Hall TC, Garcea G, Webb MA, Al-Leswas D, Metcalfe MS, et al The socio-economic impact of chronic pancreatitis: a systematic review J Eval Clin Pract 2014 20 3 203–7
- 3. Lohr JM, Dominguez-Munoz E, Rosendahl J, Besselink M, Mayerle J, Lerch MM, et al United European Gastroenterology evidence-based guidelines for the diagnosis and therapy of chronic pancreatitis (HaPanEU) United European Gastroenterol J 2017 5 2 153–99
- 4. Yoh K, Nishikawa H, Enomoto H, Iwata Y, Ishii A, Yuri Y, et al Clinical impact of physical exercise on sleep disorder as assessed by actigram in patients with chronic pancreatitis: a study protocol for a randomised controlled trial BMJ Open Gastroenterol 2018 5 1 e000193
- 5. Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Landi F, Cederholm T, et al Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older People Age Ageing 2010 Jul 39 4 412–23
- 6. Olesen SS, Buyukuslu A, Kohler M, Rasmussen HH, Drewes AM Sarcopenia associates with increased hospitalization rates and reduced survival in patients with chronic pancreatitis Pancreatology 2019 19 2 245–51
- 7. Roe L, Normand C, Wren MA, Browne J, O’Halloran AM The impact of frailty on healthcare utilisation in Ireland: evidence from the Irish longitudinal study on ageing BMC Geriatr 2017 17 1 203
- 8. Katzmarzyk PT, Friedenreich C, Shiroma EJ, Lee IM Physical inactivity and non-communicable disease burden in low-income, middle income and high-income countries Br J Sports Med 2022 56 2 101–6
- 9. Monaghan B, Monaghan A, Mockler D, Ul Ain Q, Duggan SN, Conlon KC, et al Physical activity for chronic pancreatitis: a systematic review HPB 2022 24 8 1217–22
- 10. Mijnarends DM, Koster A, Schols JM, Meijers JM, Halfens RJ, Gudnason V, et al Physical activity and incidence of sarcopenia: the population-based AGES-Reykjavik Study Age Ageing 2016 45 5 614–20
- 12. Sareen S, Kumari V, Gajebasia KS, Gajebasia NK Yoga: a tool for improving the quality of life in chronic pancreatitis World J Gastroenterol 2007 13 3 391–7
- 13. O'Brien KK, Tynan AM, Nixon SA, Glazier RH Effectiveness of aerobic exercise for adults living with HIV: systematic review and meta-analysis using the Cochrane Collaboration protocol BMC Infect Dis 2016 Apr 26 16 182
- 14. Stamatakis E, Lee IM, Bennie J, Freeston J, Hamer M, O’Donovan G, et al Does strength-promoting exercise confer unique health benefits? A pooled analysis of data on 11 population cohorts with all-cause, cancer, and cardiovascular mortality endpoints Am J Epidemiol 2018 May 1 187 5 1102–12
- 15. Santos-Lozano A, Marin PJ, Torres-Luque G, Ruiz JR, Lucía A, Garatachea N Technical variability of the GT3X accelerometer Med Eng Phys 2012 34 6 787–90
- 16. Ozemek C, Kirschner MM, Wilkerson BS, Byun W, Kaminsky LA Intermonitor reliability of the GT3X+ accelerometer at hip, wrist, and ankle sites during activities of daily living Physiol Meas 2014 35 2 129–38
- 17. Troiano RP, Berrigan D, Dodd KW, Mâsse LC, Tilert T, McDowell M Physical Activity in the United States measured by accelerometer Med Sci Sports Exerc 2008 40 1 181–8
- 18. Freedson PS, Melanson E, Sirard J Calibration of the computer science and applications, Inc. accelerometer Med Sci Sports Exerc 1998 30 5 777–81
- 19. Choi LZ, Liu Z, Matthews CE, Buchowski MS Validation of accelerometer wear and non-wear time classification algorithm Med Sci Sports Exerc 2011 43 2 357–64
- 20. Cruz-Monserrate Z, Gumpper K, Pita V, Hart PA, Forsmark C, Whitcomb DC, et al Biomarkers of chronic pancreatitis: a systematic literature review Pancreatology 2021 Mar 21 2 323–33
- 21. Duggan SN, Smyth ND, O'Sullivan M, Feehan S, Ridgway PF, Conlon KC The prevalence of malnutrition and fat-soluble vitamin deficiencies in chronic pancreatitis Nutr Clin Pract 2014 Jun 29 3 348–54
- 22. Meyer F, Bannert K, Wiese M, Esau S, Sautter LF, Ehlers L, et al Evaluation of fatigue in patients with liver cirrhosis and chronic pancreatitis in a multicentre cross-sectional study Clinical Nutrition ESPEN 2020 40 515
- 24. Kuan LL, Dennison AR, Garcea G Prevalence and impact of sarcopenia in chronic pancreatitis: a review of the literature World J Surg 2021 45 2 590–7
- 25. Lin YK, Faiman C, Johnston PC, Hatipoglu BA, Stevens T, Bottino R, et al Spontaneous hypoglycemia after islet autotransplantation for chronic pancreatitis J Clin Endocrinol Metab 2016 Oct 101 10 3669–75
- 26. Ren Y, Zhang J, Wang M, Bi J, Wang T, Qui M, et al Identification of irisin as a therapeutic agent that inhibits oxidative stress and fibrosis in a murine model of chronic pancreatitis Biomed Pharmacother 2020 Jun 126 110101
- 27. Marques A, Peralta M, Sarmento H, Martins J, González Valeiro M Associations between vigorous physical activity and chronic diseases in older adults: a study in 13 European countries Eur J Public Health 2018 Oct 1 28 5 950–5
- 28. O’Gorman P, Strahan O, Ferguson D, Monaghan A, Kennedy M, Forde C, et al Improvement in cognitive impairment following a 12-week aerobic exercise intervention in individuals with non-cirrhotic chronic hepatitis C J Viral Hepat 2021 Apr 28 4 637–50
- 29. Engels M, Cross RK, Long MD Exercise in patients with inflammatory bowel diseases: current perspectives Clin Exp Gastroenterol 2017 Dec 22 11 1–11
- 31. Prince SA, Adamo KB, Hamel ME, Hardt J, Connor Gorber S, Tremblay M A comparison of direct versus self-report measures for assessing physical activity in adults: a systematic review Int J Behav Nutr Phys Act 2008 5 56
- 32. Boon RM, Hamlin MJ, Steel GD, Ross JJ Validation of the New Zealand Physical Activity Questionnaire (NZPAQ-LF) and the International Physical Activity Questionnaire (IPAQ-LF) with accelerometry Br J Sports Med 2010 Aug 44 10 741–6
- 33. Dyrstad SM, Hansen BH, Holme IM, Anderssen SA Comparison of self-reported versus accelerometer-measured physical activity Med Sci Sports Exerc 2014 46 1 99–106
- 11. WHO guidelines on physical activity and sedentary behaviour Geneva World Health Organization 2020. Licence: CC BY-NC-SA 3.0 IGO.
- 23. Jongsma ML, Postma SA, Souren P, Arns M, Gordon E, Vissers K, et al Neurodegenerative properties of chronic pain: cognitive decline in patients with chronic pancreatitis PLoS One 2011 6 8 e23363.
- 30. Healthy Ireland summary report 2019. Available from: https://www.gov.ie/en/publications/?q=healthy+ireland&sort_by=published_date&page=3.