1 BACKGROUND
Excess adiposity is becoming the most common worldwide health condition. The number of people living with overweight (body mass index [BMI] of 25 to < 30 kg/m2 in Western populations, 23 to < 25 kg/m2 in Asian populations, ) may increase to 4 billion or higher by 2035, affecting more than 50% of the global population. The prevalence of obesity (BMI ≥ 30 kg/m2 in Western populations and ≥ 25 kg/m2 in Asian populations, ) is expected to affect ~2 billion people by 2035. Younger individuals are not spared: in 2022, more than 390 million children and adolescents aged 5‐19 years globally were overweight or obese, and the prevalence of overweight and obesity in this age group is also expected to increase substantially over time.
Obesity is associated with multiple metabolic complications, including insulin resistance, diabetes, dyslipidaemia, hypertension, metabolic dysfunction‐associated steatotic liver disease (MASLD; previously non‐alcoholic fatty liver disease [or NAFLD])/metabolic dysfunction‐associated steatohepatitis (MASH; previously non‐alcoholic steatohepatitis [NASH]), cardiovascular complications and heart failure., , , , , , Management of patients with obesity relies on lifestyle interventions, pharmacological therapy and bariatric/metabolic surgery., , ,
Pharmacological therapy included orlistat, phentermine/topiramate (United States only), naltrexone/bupropion and the glucagon‐like peptide‐1 (GLP‐1) receptor agonist liraglutide at a dose of 3 mg once‐daily (QD),, until 2021, when the GLP‐1 receptor agonist semaglutide at a dose of 2.4 mg once‐weekly (QW) was approved, initially by the US Food and Drug Administration (FDA) and then by the European Medicines Agency (EMA), for chronic weight management in adults with a BMI of 30 kg/m2 or higher or 27 kg/m2 or higher with co‐morbidities. Both semaglutide 1.0 mg QW and liraglutide 1.2 mg QD were approved previously for glycaemic control of type 2 diabetes (T2D)., More recently, the dual GLP‐1‐GIP (glucose‐dependent insulinotropic polypeptide) receptor agonist, tirzepatide, first approved for T2D in 2022, has been approved for treatment of obesity by the FDA, the EMA and the UK regulatory agency., , The approval of high‐dose semaglutide and tirzepatide for obesity has renewed interest in the potential of incretin‐based medications for obesity management. The objective of this narrative review is to summarize the available data on approved and emerging incretin‐based agents for the treatment of obesity. For each of the currently approved agents, papers reporting data from the main clinical trials were sourced, and other information was obtained using targeted topic‐specific keywords in PubMed as well as via the authors, who recommended supporting references according to their areas of expertise.
2 THE ROLE OF INCRETINS AND OTHER HORMONES IN OBESITY
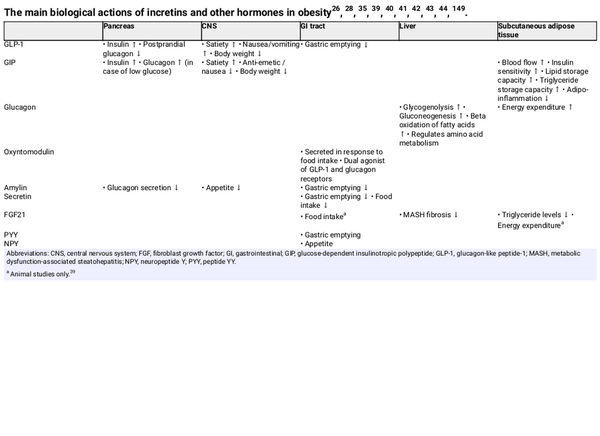
The incretins, GLP‐1 and GIP, are peptide hormones secreted by enteroendocrine cells in the intestine following food intake. They bind to receptors on pancreatic beta cells and potentiate insulin secretion in a glucose‐dependent manner, accounting for 60% or more of total secreted insulin in response to food ingestion. GLP‐1 and GIP receptors are present on many other tissues where they can exert several biological actions relevant to obesity, including enhanced satiety, regulation of gastric emptying (GLP‐1), lowering of adipose tissue inflammation (GIP) and increasing postprandial lipid clearance via stimulation of lipoprotein lipase activity (GIP) (Table 1).,
Mechanistic studies in animals have shown that GLP‐1 receptor agonists reduce food intake, increase fatty acid oxidation and induce weight loss after being transported into the brain. In addition to directly entering the brain, gut‐derived GLP‐1 can activate intestinal vagal afferents, leading to activation of GLP‐1–producing neurons that project into several areas of the brain responsible for regulation of food ingestion. Brain‐derived GLP‐1 synthesized in neurons can also project into GLP‐1 receptor‐expressing brain regions, including the hindbrain and hypothalamus. Glycaemic changes can modulate entry of GLP‐1 receptor agonists into the brain, although obesity and/or high fat feeding can blunt such an effect.
GIP is also present in the brain, and brain‐derived GIP has been shown to regulate body weight and food intake, an effect mediated by GIP receptor signalling in inhibitory GABAergic neurons., GIP analogues have been shown to augment weight loss, reduce food intake and prevent fat mass accumulation when co‐administered with GLP‐1 receptor agonists. GIP receptor agonism has been shown to attenuate GLP‐1 receptor agonist‐related nausea and vomiting. These features of GIP provide a rationale for dual GLP‐1/GIP receptor agonism.
Glucagon is a pancreatic peptide hormone whose receptors are mostly expressed in the liver where it exerts a hyperglycaemic effect by increasing glycogenolysis and gluconeogenesis (Table 1). Glucagon also regulates amino acid metabolism, stimulates beta oxidation of fatty acids and increases energy expenditure., Under discussion remains its ability to generate a satiety signal and reduce food intake, as suggested by studies in animals. Increased glucagon secretion has been observed in obesity, T2D and MASH. Glucagon has a peptide sequence related to that of GLP‐1 and GIP, making it possible to create agonists for more than one receptor, such as GLP‐1/GIP, GLP‐1/glucagon or GLP‐1/GIP/glucagon.
In addition to GLP‐1 and GIP, and glucagon, other potential therapeutic targets in obesity include the peptide hormones oxyntomodulin, amylin, secretin, peptide tyrosine tyrosine (PYY), neuropeptide Y (NPY) and fibroblast growth factor (FGF) 21 (Table 1)., , Oxyntomodulin is a dual agonist of the GLP‐1 and glucagon receptors that is secreted from the gut in response to food intake. Amylin is co‐secreted with insulin, and acts to suppress appetite, decrease glucagon secretion and delay gastric emptying., The addition of calcitonin to amylin in dual amylin/calcitonin agonists results in enhanced potency at the amylin receptor versus amylin analogues alone. Secretin inhibits gastric emptying and food intake. FGF21‐based therapies have been shown to lower circulating triglyceride levels and improve MASH‐related fibrosis, and animal studies have suggested roles in energy expenditure and regulation of food intake. PYY and NPY have roles in gastric emptying and appetite, respectively.,
3 INCRETIN‐BASED TREATMENTS FOR OBESITY
3.1 GLP‐1 receptor agonists
3.1.1 Liraglutide
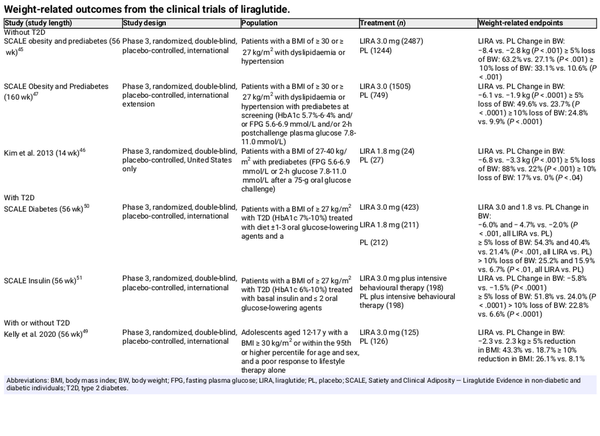
The impact of liraglutide on weight loss and metabolic characteristics in people living with obesity has been investigated in several clinical trials. In most trials, the dose used was higher than that approved in T2D (3.0 vs. 1.8 mg/d). In one study, adults with obesity had significantly decreased body weight after 56 weeks of liraglutide 3.0‐mg treatment (−8.4 kg, −8.0% vs. baseline) compared with placebo (Table 2). Liraglutide also improved glycaemic control and decreased the prevalence of prediabetes. Similar results were seen in studies of adults with overweight/obesity and prediabetes, with liraglutide 3.0‐ or 1.8‐mg recipients losing significantly more weight than placebo recipients (Table 2), along with significant improvements in glycaemic control, systolic blood pressure,, triglycerides and high sensitivity C‐reactive protein. Over a 3‐year treatment period, liraglutide 3.0 mg delayed the onset of diabetes, and 52 weeks of liraglutide 1.8‐mg treatment was associated with improvements in glucose tolerance, fasting plasma glucose (FPG), HbA1c and body weight, and a significant reduction in the prevalence of prediabetes in women with prior gestational diabetes. In adolescents with obesity with or without T2D, body weight reduction was superior with liraglutide 3.0 mg versus placebo (Table 2), although this was not associated with differences in glycaemic and cardiometabolic parameters. Weight loss (Table 2), as well as improvements in glycaemic control and a decreased need for glucose‐lowering therapies, has been documented in overweight/obese subjects with T2D treated with liraglutide 3.0 or 1.8 mg.,
Liraglutide 3.0‐mg treatment has been shown to maintain and even promote additional weight loss when started after low‐calorie‐diet–induced weight loss., Liraglutide can also be effective for lowering body weight after metabolic/bariatric surgery, as shown in the BARI‐OPTIMIZE (3.0 mg) and GRAVITAS (1.8 mg) studies.
In overweight patients with MASH, 48 weeks of treatment with liraglutide 1.8 mg resulted in a greater proportion of patients having resolution of definite MASH than placebo (39% vs. 9%; P = .019) and fewer patients having progression of fibrosis (9% vs. 36%; P = .03).
In people with T2D, liraglutide 1.8 mg has been proven to reduce the risk of death from cardiovascular causes, non‐fatal myocardial infarction or non‐fatal stroke (13.0% vs. 14.9%; P < .001 for non‐inferiority, P = .01 for superiority). No cardiovascular outcomes trials have been performed in non‐diabetic obese subjects, but a post hoc analysis of the Satiety and Clinical Adiposity—Liraglutide Evidence in non‐diabetic and diabetic individuals (SCALE) trials found that liraglutide 3.0 mg was not associated with excess cardiovascular risk in patients with overweight/obesity without T2D.
3.1.2 Subcutaneous semaglutide
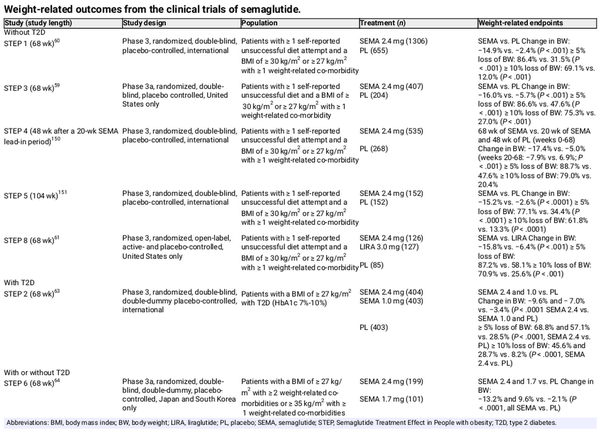
The effect of subcutaneous semaglutide on weight, metabolic and other outcomes has been extensively investigated in the Semaglutide Treatment Effect in People with obesity (STEP) programme. Again, in most studies, higher doses of subcutaneous semaglutide compared with the doses approved for use in T2D were studied (2.4 vs. 1.0 mg QW). Adults with overweight/obesity without T2D were included in STEP 1, STEP 3 and STEP 8. In STEP 1 and STEP 3, subcutaneous semaglutide 2.4 mg plus lifestyle interventions or intensive behavioural therapy, respectively, resulted in significantly greater weight loss than placebo over 68 weeks (Table 3)., In STEP 8, subcutaneous semaglutide 2.4‐mg treatment was associated with significantly greater weight loss than liraglutide over 68 weeks (Table 3), and significantly greater odds of achieving 10% or higher, 15% or higher, or 20% or higher weight loss. In STEP TEENS, adolescents aged 12‐17 years with overweight or obesity were enrolled and received subcutaneous semaglutide 2.4 mg or placebo for 68 weeks, as well as lifestyle intervention. Results were similar to those seen with adults, with semaglutide treatment inducing greater body weight reductions (Table 3) and improvement in cardiometabolic risk factors versus placebo.
STEP 2 investigated subcutaneous semaglutide 2.4 or 1.0 mg versus placebo in adults with T2D and obesity; the weight loss achieved with semaglutide 2.4 mg was superior to that achieved with placebo (estimated treatment difference [ETD] for semaglutide 2.4 mg vs. placebo: −6.2 percentage points, 95% confidence interval [CI] −7.3 to −5.2; P < .0001) (Table 3). Superior and clinically meaningful weight loss with subcutaneous semaglutide 2.4 mg versus placebo was also found in adult Asian patients with or without T2D enrolled in STEP 6 (ETD for semaglutide 2.4 mg vs. placebo: −11.1 percentage points [95% CI −12.9 to −9.2]; P < .0001) (Table 3). The loss of body weight was associated with reduction in abdominal visceral fat (semaglutide 2.4 mg vs. placebo: 40% vs. 6.9%; ETD −33.2% [95% CI −42.1 to −24.2]).
In patients experiencing weight regain or insufficient weight loss following metabolic surgery, subcutaneous semaglutide has been shown to induce weight loss., , In one study, 6 months of subcutaneous semaglutide 1.0 mg resulted in a body weight loss corresponding to 67.4% of the postsurgery weight regain, and in another, subcutaneous semaglutide (titrated from 0.25 to 1.0 mg QW according to tolerability) resulted in sustained weight loss over 12 months postsurgery. The weight loss seen with subcutaneous semaglutide 1.0 mg in a group of patients with postsurgery weight gain was superior to that seen with liraglutide.
Data on subcutaneous semaglutide for the treatment of MASH show modest results,, , with some studies showing MASH resolution with no worsening of fibrosis, but no difference between groups in terms of improvement during the fibrosis stage, and others showing no statistically significant improvement of fibrosis or achievement of MASH resolution.
Analyses of data from STEP 1 and STEP 4 showed that, in general, there were greater improvements in blood pressure, lipids and FPG with subcutaneous semaglutide 2.4 mg versus placebo, and patients receiving semaglutide had a net reduction in use of antihypertensive and lipid‐lowering medications versus placebo. Data from patients with prediabetes in STEP 1, STEP 3 and STEP 4 showed that, compared with placebo, subcutaneous semaglutide 2.4‐mg treatment was associated with a higher likelihood of normoglycaemia, and significant improvements in HbA1c, FPG and insulin resistance. Of note, the STEP 1 extension study and STEP 1 and STEP 4 analyses of the impact of subcutaneous semaglutide 2.4 mg on cardiometabolic risk factors showed a loss of cardiometabolic treatment benefits after semaglutide discontinuation.,
In the SELECT trial, a total of 17 604 patients with overweight or obesity and established cardiovascular disease were randomized to subcutaneous semaglutide 2.4 mg QW or placebo. Over a mean follow‐up of 39.8 months, a 20% relative risk reduction versus placebo (hazard ratio, 0.80; 95% CI 0.72 to 0.90; P < .001) in the primary cardiovascular endpoint (a composite of death from cardiovascular causes, non‐fatal myocardial infarction or non‐fatal stroke) occurred in the semaglutide group.
Adult patients with obesity and heart failure with preserved ejection fraction (HFpEF) were enrolled into STEP HFpEF., A prespecified analysis of the STEP HFpEF trial by obesity class and degree of body weight reduction found that subcutaneous semaglutide improved outcomes across obesity classes, with the magnitude of benefit directly related to the degree of weight loss.
3.1.3 Oral GLP‐1 receptor agonists
Oral semaglutide is a co‐formulation of semaglutide and the permeation enhancer sodium N‐(8‐[2‐hydroxybenzoyl]amino)caprylate (SNAC). SNAC increases gastric pH to protect semaglutide from enzymatic degradation, promotes monomerization of semaglutide and fluidizes the lipid membrane of the gastric epithelium, all of which enhances the absorption of semaglutide. Oral semaglutide has been investigated in people living with T2D in the PIONEER studies (reviewed by Singh and colleagues). Compared with placebo, oral semaglutide recipients had significantly greater improvements in HbA1c and body weight, and weight loss with oral semaglutide was greater than with dulaglutide and liraglutide in the active‐controlled PIONEER studies. In the OASIS 1 study, 68 weeks of oral semaglutide 50 mg QD in adults with overweight or obesity led to a mean body weight change from baseline of −15.1% versus −2.4% for placebo (P < .0001), and significantly more participants losing 5% or more of baseline body weight (85% vs. 26%; P < .0001); these weight changes with oral semaglutide were superior and clinically meaningful versus placebo.
Orforglipron is an oral, once‐daily non‐peptide GLP‐1 receptor agonist in phase 2 clinical development for obesity and type 2 diabetes., In contrast to oral semaglutide, which has complex administration requirements with respect to food, water and other medications, and limited bioavailability (0.4%‐1.0%), orforglipron has no food or water restrictions and a bioavailability of 20%‐40%. In patients with obesity or overweight without diabetes, 26 weeks of orforglipron treatment at doses of 12, 24, 36 or 45 mg resulted in a mean weight change from baseline of −8.6% to −12.6% versus −2.0% with placebo; after 36 weeks, the mean weight change was −9.4% to −14.7% with orforglipron versus −2.3% with placebo. At week 36, a body weight reduction of 10% or higher was seen in 46%‐75% of orforglipron recipients compared with 9% of placebo recipients. In patients with T2D, 26 weeks of orforglipron (3, 12, 24, 36 or 45 mg) resulted in a mean weight loss of up to −10.1 versus −2.2 and −3.9 kg in the placebo and dulaglutide groups, respectively. In addition, orforglipron treatment resulted in improvement on HbA1c in patients with T2D (mean change in HbA1c at week 26: up to −2.1% vs. −0.4% and −1.1% for placebo and dulaglutide, respectively), and improvements in cardiometabolic risk in obese/overweight individuals without T2D. The phase 3 ATTAIN‐1 study (NCT05869903), which is investigating orforglipron in adults with overweight or obesity, is currently ongoing.
With initial results now obtained with orforglipron, more non‐peptide GLP‐1 receptor agonists are expected to enter clinical development.
3.2 Dual co‐agonists
3.2.1 GIP/GLP‐1 receptor agonists
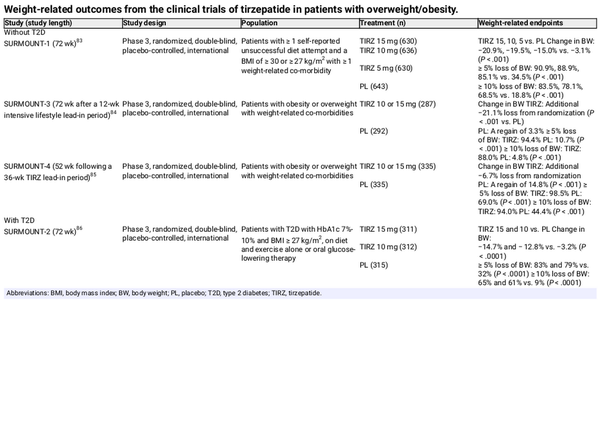
The efficacy and safety of tirzepatide is being assessed in the SURMOUNT programme, which was designed to specifically investigate tirzepatide at the same doses as those approved for T2D (up to 15 mg QW) in people with overweight/obesity (Table 4). In SURMOUNT‐1, 72 weeks of tirzepatide treatment (5, 10 or 15 mg) provided substantial and significantly greater weight reduction than placebo in patients with overweight/obesity without T2D (up to 21% vs. 3% for placebo; P < .001), with more than 90% and more than 80% of tirzepatide 15‐mg recipients achieving greater than 5% and greater than 10% weight loss from baseline, respectively. In SURMOUNT‐3, adults with obesity/overweight who achieved a 5.0% or higher weight reduction after a 12‐week intensive lifestyle intervention received tirzepatide 10 or 15 mg, or placebo for 72 weeks. After 72 weeks of treatment, tirzepatide recipients had additional weight loss that was substantially greater than that seen with placebo, and a greater proportion of participants losing 5.0% or higher, or 10.0% or higher body weight (Table 4). In SURMOUNT‐4, participants were randomized to 52 weeks of tirzepatide or placebo after a 36‐week, open‐label tirzepatide lead‐in period. Similar to SURMOUNT‐3, after 52 weeks, the tirzepatide group had substantially greater weight loss compared with the placebo group (Table 4).
The body weight‐lowering efficacy of tirzepatide has been confirmed in SURMOUNT‐2, which included people with obesity and T2D (Table 4). After 72 weeks of treatment with tirzepatide 10 or 15 mg, change in body weight from baseline and the proportion of participants losing 5%, 10%, or more was significantly greater with tirzepatide versus placebo. HbA1c levels were also significantly lower with tirzepatide than with placebo, and a greater proportion of participants receiving tirzepatide were able to decrease their glucose‐lowering medications. An impact on progression to T2D has also been reported for tirzepatide, with a post hoc analysis of SURMOUNT‐1 reporting a reduction of the 10‐year predicted risk of T2D development in people receiving tirzepatide of up to 69% at week 72, with significantly greater risk reductions with tirzepatide versus placebo, irrespective of the baseline glycaemic status.
Of note, the SURMOUNT studies also reported improvements in patient‐reported outcomes, with participants reporting improvements in physical functioning with tirzepatide versus placebo,, , , as well as improvements in emotional and mental health scores.
Underway is the SURMOUNT‐MMO study (NCT05556512), which is investigating the impact of tirzepatide on the reduction of morbidity and mortality in adults with obesity with an established or increased risk of cardiovascular disease.
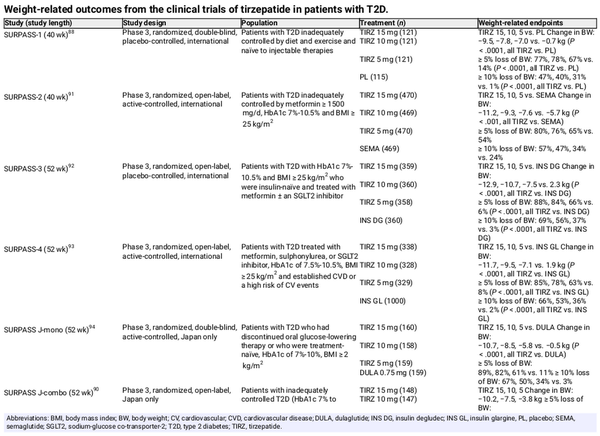
Table 5 summarizes the weight‐related endpoints across the SURPASS clinical trial programme in individuals with T2D. Tirzepatide treatment resulted in significant body weight reduction versus placebo in patients with T2D naïve to injectable glucose‐lowering therapy and those inadequately controlled on insulin glargine. In Japanese patients receiving tirzepatide as an add‐on to oral glucose‐lowering therapy, body weight reduction occurred regardless of the type of background oral therapy. In the active‐controlled SURPASS trials, tirzepatide was found to be superior to semaglutide 1.0 mg, insulin degludec, insulin glargine and dulaglutide for reduction in body weight.
Some real‐world data are available for tirzepatide versus semaglutide, in a United States‐based study utilizing electronic health records in individuals with or without T2D receiving tirzepatide or semaglutide as per the T2D labels (n = 41 223 total; semaglutide 0.5 mg: 32 030; tirzepatide 5 mg: 9193). Those receiving tirzepatide had greater weight loss from baseline than semaglutide recipients at 3, 6 and 12 months, and were significantly more probable to achieve weight loss of 5%, 10% and 15% of baseline.
Post hoc analyses of the SURPASS trials indicate that tirzepatide can decrease liver fat content and the volume of visceral adipose tissue and abdominal subcutaneous adipose tissue. Preliminary results from patients with MASH enrolled in the phase 2 SYNERGY‐NASH study show that a greater proportion of patients receiving tirzepatide than those receiving placebo achieved resolution of MASH without worsening of fibrosis after 52 weeks of treatment (74% vs. 13%). In patients with T2D, higher doses of tirzepatide (10 or 15 mg) significantly decreased MASH‐related biomarkers such as alanine and aspartate aminotransferase, keratin‐18, procollagen III and adiponectin.
Tirzepatide treatment has also been associated with lower blood pressure and improved lipid profile in SURMOUNT‐1, −2 and −3,, , and with proven cardiovascular safety, as indicated by results from SURPASS‐4 in subjects with T2D and increased cardiovascular risk, and a recent meta‐analysis., A cardiovascular outcomes trial in patients with T2D and increased cardiovascular risk, SURPASS‐CVOT (NCT04255433), is currently underway, investigating the effect of tirzepatide versus dulaglutide on major cardiovascular events.
Tirzepatide is probable to be followed soon by other dual GIP/GLP‐1 receptor agonists such as NNC0090‐2746. In patients with T2D, NNC0090‐2746 significantly improved glycaemic control and reduced body weight versus placebo.
3.2.2 Glucagon/GLP‐1 receptor agonists
The rationale for the first dual glucagon/GLP‐1 receptor agonist originated from the observation that chronic infusion of a water‐soluble glucagon improves body weight and glycaemic control in diet‐induced obese mice. Soon after, another GLP‐1/glucagon co‐agonist was developed based on the structure of oxyntomodulin, a molecule with natural activity at both target receptors. In healthy volunteers, oxyntomodulin suppressed appetite and reduced food intake; similarly, in a study of people with overweight/obesity, energy intake was decreased in those receiving oxyntomodulin, and there was corresponding weight loss and altered levels of adipose hormones consistent with adipose tissue loss. Currently, the following dual glucagon/GLP‐1 receptor agonists are under investigation.
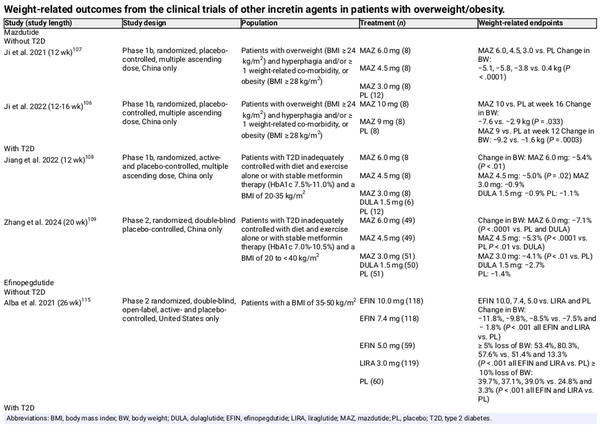
Mazdutide (IBI362, LY3305677) is a long‐acting analogue of oxyntomodulin that is currently in phase 2 development in China (Table 6)., , In Chinese people with overweight or obesity, mazdutide was associated with greater weight loss and reductions in BMI than placebo., In Chinese people with T2D, mazdutide was associated with clinically meaningful improvements in glycaemic variables (HbA1c, FPG, postprandial glucose, insulin resistance) and weight.,
Cotadutide (MEDI0382) is a glucagon analogue that has been modified to have dual glucagon and GLP‐1 receptor activity and was under development for MASH, T2D and obesity., However, the manufacturer has terminated the clinical programme for once‐daily cotadutide in favour of AZD9550, a once‐weekly injectable GLP‐1 receptor/glucagon agonist.
Efinopegdutide (MK‐6024, HM12525A, JNJ‐64565111) is a synthetic oxyntomodulin conjugated to the constant region of human immunoglobulin G4 that has been investigated in obesity with and without T2D and MASLD. In patients with obesity, efinopegdutide treatment resulted in a significant dose‐dependent reduction in body weight versus placebo, irrespective of diabetes status (Table 6)., Efinopegdutide treatment was also shown to result in a significant reduction of liver fat content versus semaglutide in patients with MASLD at 24 weeks (least‐squares mean relative reduction from baseline: 72.7% vs. 42.3%; P < .001).
Survodutide (BI 456906) is an acylated peptide containing a C18 fatty acid to extend its half‐life that acts via increasing energy expenditure and reducing food intake. In early‐stage trials of healthy volunteers and individuals with overweight/obesity, BI 456096 decreased body weight by ~6%‐14% after 16 weeks (placebo corrected)., Results from the phase 2 dose‐finding study of survodutide in people with overweight/obesity showed body weight loss ranging from −6.2% to −14.9% with survodutide versus −2.8% with placebo after 46 weeks of treatment. Of note, in this study, more than 90% of patients receiving survodutide experienced adverse events, and 24.6% stopped treatment during the study compared with 75% and 3.9% of placebo recipients, respectively. In people with MASH, preliminary data from a phase 2 trial showed a significantly greater proportion of participants receiving survodutide versus placebo had an improvement in MASH after 48 weeks (83.0% vs. 18.2%; P < .0001); liver fibrosis was also improved with survodutide versus placebo.
SAR425899 is another glucagon/GLP‐1 receptor agonist in early‐stage trials. In a trial of individuals with overweight/obesity, participants were randomized to a calorie‐reduced diet and either escalating doses of SAR425899 or placebo. Weight loss was seen with SAR425899 and placebo (−4.83 and −3.68 kg, respectively; P < .05), but recipients of SAR425899 lost more fat mass and fat free mass compareed to placebo (P < .05). SAR425899 treatment led to a significantly smaller reduction than placebo in body composition‐adjusted sleeping metabolic rate (P = .002) and greater increases in fat oxidation and ketogenesis (P < .05).
3.2.3 Other dual hormone agonists under investigation
Currently, dual calcitonin/amylin, GLP‐1/amylin, GLP‐1/secretin, GLP‐1/FGF21, GLP‐1/PYY and GLP‐1/NPY agonists are under investigation for use in obesity or T2D., , Of these, more data are available for the combination of cagrilintide, a long‐acting amylin analogue that has shown clinically significant weight loss that was significantly greater than placebo in clinical trials of people with overweight or obesity, and semaglutide. When co‐administered, GLP‐1 receptor/amylin agonism in individuals with T2D yielded a mean body weight change greater than the use of semaglutide 2.4 mg (P < .0001) or cagrilintide 2.4 mg (P < .0001) alone (cagrilintide/semaglutide: −15.6%; semaglutide: −5.1%; cagrilintide: −8·1%). The REDEFINE clinical programme currently underway is investigating the use of cagrilintide/semaglutide in individuals with overweight or obesity (REDEFINE 1; NCT05567796), individuals with overweight or obesity and T2D (REDEFINE 2; NCT05394519) and individuals with established cardiovascular disease (REDEFINE 3; NCT05669755).
Animal studies of the dual calcitonin/amylin receptor agonist KBP‐089 combined with liraglutide have shown a complementary action on body weight, food intake and glucose tolerance.,
3.3 Triple co‐agonists
3.3.1 Glucagon/GIP/GLP‐1 receptor agonists
Retatrutide (LY3437943) is a fatty acid acylated peptide with agonist activity for the glucagon, GIP and GLP‐1 receptors. In phase 2 trials, retatrutide treatment resulted in substantial body weight reductions, regardless of T2D status. The percentage body weight reduction was dose dependent in both trials (obesity without T2D: −8.7% to −24.2% for 1‐12 mg doses vs. −2.1% for placebo; obesity with T2D: −3.2% to −16.9% for 0.5‐12 mg doses vs. 3.0% and 2.0% for dulaglutide)., In people with obesity, treatment with retatrutide for 48 weeks resulted in substantial weight loss, with 100% of patients who received the 8 and 12 mg doses having weight loss of 5% or higher, and more than 90% of patients receiving these doses having a weight loss of 10% or higher. In a substudy of this trial, the change in liver fat from baseline at week 24 ranged from −42.9% with retatrutide 1 mg to −82.4% with retatrutide 12 mg, versus +0.3% with placebo, and more than 85% of participants receiving the two highest retatrutide doses (8 and 12 mg) achieved resolution of steatosis (< 5% total liver fat content). These liver fat reductions were significantly related to reductions in body weight and abdominal adipose tissue and improvements in markers of insulin sensitivity and lipid metabolism. Other evidence suggests that adding glucagon agonism may provide additional liver‐related benefits that are independent of weight loss.
SAR441255 is another glucagon/GIP/GLP‐1 agonist that has been investigated in animal models and healthy subjects. In a phase 1 study of lean to overweight healthy subjects, SAR441255 improved glycaemic control during a mixed meal tolerance test.
Efocipegtrutide (HM15211) is a triple agonist being investigated in patients with MASH in a study currently in progress.
3.3.2 Other triple co‐agonists under investigation
GLP‐1/glucagon/leptin co‐agonism is also a potentially promising option to explore, with GLP‐1/glucagon administration able to restore the benefits of leptin on food intake, body weight and glycaemic control in a hypercaloric environment.
4 LIMITATIONS AND TOLERABILITY OF INCRETIN‐BASED TREATMENTS FOR OBESITY
Incretin‐based therapies come with some limitations, including high cost and potential loss of lean body mass., , The disadvantages of loss of muscle mass during weight loss are being recognized, and work is underway on ways to preserve muscle mass while losing fat, with animal studies suggesting antibody blockade of activin type II receptors may preserve lean mass and increase fat loss during treatment with GLP‐1 receptor agonists. Although the percentage of patients achieving weight loss of 5% or more in clinical trials is substantial, particularly for semaglutide and tirzepatide (Tables 3, 4, 5), no clinical trial reports 100% of patients achieving this weight loss goal, suggesting that a subset of patients do not respond to treatment with these agents. There is also the issue of weight regain once treatment is stopped,, suggesting that lifelong treatment with these medications may be required for many people, which may lead to safety issues for some. Although generally well tolerated, the gastrointestinal adverse events associated with incretin‐based therapies are well known., , For individuals undergoing endoscopy, the slowing of gastric motility in people treated with GLP‐1 receptor agonists can result in gastric residue, retention of solids and an increased risk of aspiration while sedated during the procedure.
Potential issues with self‐harm, pancreatitis, malignancies and gallbladder and biliary diseases with incretin therapies have also been investigated. In a study of self‐harm and depression in patients initiating incretin‐based therapies for T2D, no increased risk of depression or self‐harm was found, although patients with a prior history of depression, self‐harm or serious psychiatric conditions were excluded. Of note, the FDA is conducting an ongoing evaluation of reports of suicidal thoughts or actions in people receiving GLP‐1 receptor agonists; currently the preliminary evaluation does not suggest a causal link, but as an increased risk cannot be definitively ruled out, the evaluation is continuing.
In meta‐analyses, no increased risk of malignancies was found,, and an increased risk of pancreatitis was associated with dipeptidyl peptidase‐4 inhibitors, but not GLP‐1 receptor agonists; however, individuals with medullary thyroid cancers and pancreatitis were excluded from clinical trials. Other meta‐analyses have found an increased risk of gallbladder or biliary diseases and an increased risk of worsening diabetic retinopathy with GLP‐1 receptor agonists. The clinical trial of retatrutide reported cardiac arrythmia and parasthesia. Long‐term safety data for the newer incretin‐based therapies for obesity are needed.
5 SUMMARY AND FUTURE DIRECTIONS
Obesity and its associated complications are increasing in prevalence and have become a significant problem. While lifestyle modifications are generally the initial step, obesity is a chronic disease and most patients fulfil criteria for the use of pharmacotherapy; however, the recommended duration for and long‐term effect of pharmacotherapy for obesity is currently unknown.
There are few drugs approved for obesity, but the recent approval of the GLP‐1 receptor agonist semaglutide (high dose) and the dual GIP/GLP‐1 agonist tirzepatide have put renewed focus on incretin‐based therapies for the treatment of obesity. With the introduction of these incretin‐based therapies, weight‐lowering effects between 6% and 21% have been observed. The proportion of people achieving 10% or higher weight loss varies between 23% and 33% with liraglutide 3.0 mg, 46%‐79% with semaglutide 2.4 mg and 61%‐94% with tirzepatide 10 or 15 mg. Preliminary data for the triple co‐agonists seem to surpass these effects, and approach effects seen with bariatric/metabolic surgery. In addition to the weight‐lowering effects, beneficial effects on cardiovascular risk factors and outcomes have been seen, a significant advantage for agents used to treat obesity and T2D. Research is also broadening to include other patient populations, including adolescents, those experiencing weight regain following metabolic surgery, those with sleep apnoea and patients with MASLD/MASH, and to date favourable outcomes in these patients have been observed; in patients with MASLD/MASH, preliminary data suggest dual and triple co‐agonism may lead to greater benefits than single agonism. Prevention of diabetes in those with prediabetes has also been reported. Therefore, these newer pharmacological agents can be considered in some people (e.g. in those with a high cardiovascular risk profile, or where anaesthesia and extubation might become problematic) as an alternative to bariatric surgery. In people with severe obesity, surgery is hampered by an enlarged liver, and incretin‐based therapy could be a bridge to surgery in this scenario.
While the gastrointestinal effects associated with incretin therapies are well known and generally mild and tolerable, we recommend that therapy should be stopped in patients who develop intolerable gastrointestinal side effects, and in people who develop pancreatitis, because people with a history of pancreatitis were excluded from participation in clinical trials. In addition, people with medullary thyroid carcinoma, multiple endocrine neoplasia type 2A or depression were also excluded from participation in clinical trials, and while the risk of malignancy or suicidal ideation/attempts appears low, uncertainty remains. Furthermore, no data in pregnancy are available.
Many of the dual and triple co‐agonists are still in development, and further head‐to‐head data are needed to assess the efficacy of these emerging dual and triple agonists versus the established incretin therapies. Other current research gaps of interest include the prevention of excessive loss of lean mass in patients receiving these therapies, as well as the optimal maintenance regimen for patients who have reached their weight loss goal, including if the dose or dosing frequency can be reduced without the weight regain seen when treatment is fully withdrawn. Further data are needed in broader populations to those in clinical trials, including older people, for whom data are currently scarce. The cardiorenal benefits and effects on heart failure of incretin therapies requires further investigation, as does the use of these agents following the failure of bariatric surgery. However, despite these gaps, the available data to date are promising, and further results are eagerly awaited.
AUTHOR CONTRIBUTIONS
TF was involved in the conception of the manuscript, acquisition and interpretation of the data included in the manuscript and drafting and critical revision of the manuscript for important intellectual content. CDB was involved in the conception of the manuscript, interpretation of the data included in the manuscript and critical revision of the manuscript for important intellectual content. SDP was involved in analysis and interpretation of the data included in the manuscript and critical revision of the manuscript for important intellectual content. SA was involved in the conception and design of the manuscript, the acquisition, analysis and interpretation of the data included in the manuscript and drafting and critical revision of the manuscript for important intellectual content. JF was involved in the interpretation of the data included in the manuscript and critical revision of the manuscript for important intellectual content. AL was involved in the conception of the manuscript, the acquisition of the data included in the manuscript and critical revision of the manuscript for important intellectual content. BL was involved in the conception and design of the manuscript, the acquisition, analysis and interpretation of the data included in the manuscript and drafting and critical revision of the manuscript for important intellectual content. MM was involved in the conception of the manuscript and critical revision of the manuscript for important intellectual content. CM was involved in interpretation of the data included in the manuscript and critical revision of the manuscript for important intellectual content. TDM was involved in critical revision of the manuscript for important intellectual content. OS was involved in the conception and design of the manuscript, the interpretation of the data included in the manuscript and drafting the manuscript. All authors read and approved the final manuscript.
FUNDING INFORMATION
This review was funded by CRS Clinical Research Services GmbH, Germany.
CONFLICT OF INTEREST STATEMENT
TF is an employee of CRS Clinical Research Services GmbH, Germany, and has contributed to speaker panels for Amarin, AstraZeneca, Boehringer Ingelheim, Berlin Chemie, Cipla, Daiichi‐Sankyo, Eli Lilly and Company, Fortbildungskolleg, MSD, Novartis, Novo Nordisk, Sanofi and Santis; and contributed to advisory panels for AstraZeneca, Atrogi, Bayer, Cipla, Diabetes Akademie Bad Mergentheim, Eli Lilly and Company, Eysense, Fortbildungskolleg, Novo Nordisk, Pfizer, Sanofi, Remynd and Roche. CDB has contributed to advisory board panels for AstraZeneca, Abbott Diagnostics, Boehringer‐Ingelheim, Eli Lilly and Company, Indigo Diabetes, Insulet, Medtronic, Novo Nordisk and Sanofi. He is on the speaker panel for Abbott Diagnostics, Eli Lilly and Company, Insulet, Medtronic and Novo Nordisk. SDP consulted for Abbott, Amarin Corporation, Applied Therapeutics, AstraZeneca, Eli Lilly and Company, Eva Pharma, Menarini International, Novo Nordisk, Sanofi and Sun Pharmaceuticals, and received funding for these consulting services; received grant support from AstraZeneca and Boehringer Ingelheim; and received speaker fees from Abbott, AstraZeneca, Boehringer Ingelheim, Eli Lilly and Company, Laboratori Guidotti, Menarini International, MSD, Novartis Pharmaceuticals, Novo Nordisk and Sanofi. SA is an employee of CRS Clinical Research Services GmbH, Germany. JF reports research support from Akero, Altimmune, Boehringer Ingelheim, 89bio, Eli Lilly and Company, Janssen, Madrigal, Merck, Novartis, Novo Nordisk, Pfizer and Sanofi; advisory boards and consulting for Akero, Altimmune, Boehringer Ingelheim, Carmot Therapeutics, Echosens, 89bio, Eli Lilly and Company, Merck, Novo Nordisk, Pfizer and Sanofi; lectures and speaker bureau for Eli Lilly and Company, Novo Nordisk, Sanofi; and employee and stockholder: Biomea Fusion, Inc. BL received honoraria for advisory panels and lectures as well as study fees from AstraZeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Company, Novo Nordisk and Sanofi. AL reports research support from AstraZeneca and received honoraria as a consultant and speaker from Novo Nordisk, Eli Lilly and Company, Boehringer Ingelheim and AstraZeneca. BL reports research support from Amgen, Astra Zeneca, Bayer, Boehringer Ingelheim, Eli Lilly and Company, Madrigal, MSD, Novo Nordisk and Sanofi, and received honoraria as a consultant and speaker from Amgen, Astra Zeneca, Boehringer Ingelheim, Eli Lilly and Novo Nordisk. MM is an employee of Clinical Research Services GmbH, Germany. CM serves or has served on the advisory panel for Novo Nordisk, Sanofi, Merck Sharp and Dohme Ltd, Eli Lilly and Company, Novartis, AstraZeneca, Boehringer Ingelheim, Roche, Medtronic, ActoBio Therapeutics, Pfizer, Imcyse, Insulet, Zealand Pharma, Avotres, Mannkind, Sandoz and Vertex. Financial compensation for these activities has been received by KU Leuven; KU Leuven has received research support for CM from Medtronic, Imcyse, Novo Nordisk, Sanofi and ActoBio Therapeutics; CM serves or has served on the speakers bureau for Novo Nordisk, Sanofi, Eli Lilly and Company, Boehringer Ingelheim, AstraZeneca and Novartis. Financial compensation for these activities has been received by KU Leuven. CM is president of the European Association for the Study of Diabetes (EASD). All external support of EASD is to be found on www.easd.org. TDM receives funding from Novo Nordisk, holds stocks at Novo Nordisk and Eli Lilly, and received speaking fees within the last 3 years from Novo Nordisk, Eli Lilly, AstraZeneca and Merck. OS is founder and Chief Executive Officer of Sciarc GmbH, Germany, and has served on speaker panels and/or on advisory panels for Abbott, Bayer, Boehringer Ingelheim, Eli Lilly and Company, Glooko, LifeScan, Lilly, Mannkind, Sanofi and Woerwag.
ACKNOWLEDGEMENTS
Sheridan Henness and Francisco López de Saro (Rx Communications, Mould, UK) provided medical writing assistance with the preparation of this manuscript, funded by CRS Clinical Research Services GmbH, Germany.
REFERENCES
- 1. World Health Organization . Obesity and Overweight. 2024 Accessed June 5, 2024. https://www.who.int/news-room/fact-sheets/detail/obesity-and-overweight
- 2. Haam JH, Kim BT, Kim EM, et al. Diagnosis of obesity: 2022 update of clinical practice guidelines for obesity by the Korean Society for the Study of obesity. J Obes Metab Syndr. 2023;32(2):121–129.
- 3. Ogawa W, Hirota Y, Miyazaki S, et al. Definition, criteria, and core concepts of guidelines for the management of obesity disease in Japan. Endocr J. 2024;71(3):223–231.
- 4. World Obesity Federation . World Obesity Atlas. 2023 Accessed October 2, 2023. https://www.worldobesity.org/resources/resource‐library/world‐obesity‐atlas‐2023
- 5. Ogawa W, Miyazaki S. Diagnosis criteria for obesity and obesity disease. Health Eval Promot. 2015;42(2):301–306.
- 6. Pan WH, Yeh WT. How to define obesity? Evidence‐based multiple action points for public awareness, screening, and treatment: an extension of Asian‐Pacific recommendations. Asia Pac J Clin Nutr. 2008;17(3):370–374.
- 7. Ahmed B, Sultana R, Greene MW. Adipose tissue and insulin resistance in obese. Biomed Pharmacother. 2021;137:111315.
- 8. Lauterbach MA, Wunderlich FT. Macrophage function in obesity‐induced inflammation and insulin resistance. Pflugers Arch. 2017;469(3–4):385–396.
- 9. Zhang T, Chen J, Tang X, Luo Q, Xu D, Yu B. Interaction between adipocytes and high‐density lipoprotein:new insights into the mechanism of obesity‐induced dyslipidemia and atherosclerosis. Lipids Health Dis. 2019;18(1):223.
- 10. Fantin F, Giani A, Zoico E, Rossi AP, Mazzali G, Zamboni M. Weight loss and hypertension in obese subjects. Nutrients. 2019;11(7):1667.
- 11. Quek J, Chan KE, Wong ZY, et al. Global prevalence of non‐alcoholic fatty liver disease and non‐alcoholic steatohepatitis in the overweight and obese population: a systematic review and meta‐analysis. Lancet Gastroenterol Hepatol. 2023;8(1):20–30.
- 12. Powell‐Wiley TM, Poirier P, Burke LE, et al. Obesity and cardiovascular disease: a scientific statement from the American Heart Association. Circulation. 2021;143(21):e984–e1010.
- 13. Pandey A, Patel KV, Vaduganathan M, et al. Physical activity, fitness, and obesity in heart failure with preserved ejection fraction. JACC Heart Fail. 2018;6(12):975–982.
- 14. Durrer Schutz D, Busetto L, Dicker D, et al. European practical and patient‐centred guidelines for adult obesity management in primary care. Obes Facts. 2019;12(1):40–66.
- 15. Garvey WT, Mechanick JI, Brett EM, et al. American Association of Clinical Endocrinologists and American College of endocrinology comprehensive clinical practice guidelines for medical care of patients with obesity. Endocr Pract. 2016;22(Suppl 3):S1–S203.
- 16. Semlitsch T, Stigler FL, Jeitler K, Horvath K, Siebenhofer A. Management of overweight and obesity in primary care—a systematic overview of international evidence‐based guidelines. Obes Rev. 2019;20(9):1218–1230.
- 17. Yumuk V, Tsigos C, Fried M, et al. European guidelines for obesity management in adults. Obes Facts. 2015;8(6):402–424.
- 18. US FDA . FDA approves new drug treatment for chronic weight management, first since 2014. 2021 Accessed October 12, 2023. https://www.fda.gov/news-events/press-announcements/fda-approves-new-drug-treatment-chronic-weight-management-first-2014.
- 19. European Medicines Agency . Wegovy. 2022 Accessed October 12, 2023. https://www.ema.europa.eu/en/medicines/human/EPAR/wegovy.
- 20. Nordisk N. VICTOZA® (liraglutide) injection, for subcutaneous use. Prescribing information. 2017 Accessed October 3, 2023. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/022341s027lbl.pdf.
- 21. Nordisk N. OZEMPIC (semaglutide) injection, for subcutaneous use. Prescribing information. 2017 Accessed June 5, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/209637lbl.pdf.
- 22. Eli Lilly and Company . MOUNJARO® (tirzepatide) injection, for subcutaneous use. Prescribing information. 2023 Accessed October 13, 2023. https://pi.lilly.com/us/mounjaro-uspi.pdf?s=pi.
- 23. Reuters . Lilly weight‐loss drug gets US, UK approval to rival Wegovy. 2023 Accessed November 21, 2023.https://www.reuters.com/business/healthcare‐pharmaceuticals/us‐fda‐approves‐lillys‐weight‐loss‐drug‐2023‐11‐08/.
- 24. US FDA . ZEPBOUND™ (tirzepatide) injection, for subcutaneous use. Prescribing information. 2023 Accessed January 12, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2023/217806s000lbl.pdf.
- 25. Eli Lilly and Company . Mounjaro® (tirzepatide). Summary of product characteristics. 2022 Accessed February 28, 2024.https://www.ema.europa.eu/en/documents/product-information/mounjaro-epar-product-information_en.pdf.
- 26. Chia CW, Egan JM. Incretins in obesity and diabetes. Ann N Y Acad Sci. 2020;1461(1):104–126.
- 27. Muscelli E, Mari A, Natali A, et al. Impact of incretin hormones on beta‐cell function in subjects with normal or impaired glucose tolerance. Am J Physiol Endocrinol Metab. 2006;291(6):E1144–E1150.
- 28. Andreasen CR, Andersen A, Vilsboll T. The future of incretins in the treatment of obesity and non‐alcoholic fatty liver disease. Diabetologia. 2023;66(10):1846–1858.
- 29. Imbernon M, Saponaro C, Helms HCC, et al. Tanycytes control hypothalamic liraglutide uptake and its anti‐obesity actions. Cell Metab. 2022;34(7):1054–1063.e1057.
- 30. van Bloemendaal L, Ten Kulve JS, la Fleur SE, Ijzerman RG, Diamant M. Effects of glucagon‐like peptide 1 on appetite and body weight: focus on the CNS. J Endocrinol. 2014;221(1):T1–T16.
- 31. Baggio LL, Drucker DJ. Glucagon‐like peptide‐1 receptors in the brain: controlling food intake and body weight. J Clin Invest. 2014;124(10):4223–4226.
- 32. Bakker W, Imbernon M, Salinas CG, et al. Acute changes in systemic glycemia gate access and action of GLP‐1R agonist on brain structures controlling energy homeostasis. Cell Rep. 2022;41(8):111698.
- 33. Liskiewicz A, Khalil A, Liskiewicz D, et al. Glucose‐dependent insulinotropic polypeptide regulates body weight and food intake via GABAergic neurons in mice. Nat Metab. 2023;5(12):2075–2085.
- 34. Zhang Q, Delessa CT, Augustin R, et al. The glucose‐dependent insulinotropic polypeptide (GIP) regulates body weight and food intake via CNS‐GIPR signaling. Cell Metab. 2021;33(4):833–844 e835.
- 35. Del Prato S, Gallwitz B, Holst JJ, Meier JJ. The incretin/glucagon system as a target for pharmacotherapy of obesity. Obes Rev. 2022;23(2):e13372.
- 36. Hayes MR, Borner T, De Jonghe BC. The role of GIP in the regulation of GLP‐1 satiety and nausea. Diabetes. 2021;70(9):1956–1961.
- 37. Salem V, Izzi‐Engbeaya C, Coello C, et al. Glucagon increases energy expenditure independently of brown adipose tissue activation in humans. Diabetes Obes Metab. 2016;18(1):72–81.
- 38. Al‐Massadi O, Ferno J, Dieguez C, Nogueiras R, Quinones M. Glucagon control on food intake and energy balance. Int J Mol Sci. 2019;20(16):3905.
- 39. Clemmensen C, Finan B, Muller TD, DiMarchi RD, Tschop MH, Hofmann SM. Emerging hormonal‐based combination pharmacotherapies for the treatment of metabolic diseases. Nat Rev Endocrinol. 2019;15(2):90–104.
- 40. Pocai A. Unraveling oxyntomodulin, GLP1's enigmatic brother. J Endocrinol. 2012;215(3):335–346.
- 41. Andersen DB, Holst JJ. Peptides in the regulation of glucagon secretion. Peptides. 2022;148:170683.
- 42. Loomba R, Sanyal AJ, Kowdley KV, et al. Randomized, controlled trial of the FGF21 analogue pegozafermin in NASH. N Engl J Med. 2023;389(11):998–1008.
- 43. Milliken BT, Elfers C, Chepurny OG, et al. Design and evaluation of peptide dual‐agonists of GLP‐1 and NPY2 receptors for glucoregulation and weight loss with mitigated nausea and emesis. J Med Chem. 2021;64(2):1127–1138.
- 44. Steinert RE, Feinle‐Bisset C, Asarian L, Horowitz M, Beglinger C, Geary N. Ghrelin, CCK, GLP‐1, and PYY(3–36): secretory controls and physiological roles in eating and glycemia in health, obesity, and after RYGB. Physiol Rev. 2017;97(1):411–463.
- 45. Pi‐Sunyer X, Astrup A, Fujioka K, et al. A randomized, controlled trial of 3.0 mg of liraglutide in weight management. N Engl J Med. 2015;373(1):11–22.
- 46. Kim SH, Abbasi F, Lamendola C, et al. Benefits of liraglutide treatment in overweight and obese older individuals with prediabetes. Diabetes Care. 2013;36(10):3276–3282.
- 47. le Roux CW, Astrup A, Fujioka K, et al. 3 years of liraglutide versus placebo for type 2 diabetes risk reduction and weight management in individuals with prediabetes: a randomised, double‐blind trial. Lancet. 2017;389(10077):1399–1409.
- 48. Foghsgaard S, Vedtofte L, Andersen ES, et al. Liraglutide treatment for the prevention of glucose tolerance deterioration in women with prior gestational diabetes mellitus: a 52‐week randomized controlled clinical trial. Diabetes Obes Metab. 2023;26:201–214.
- 49. Kelly AS, Auerbach P, Barrientos‐Perez M, et al. A randomized, controlled trial of liraglutide for adolescents with obesity. N Engl J Med. 2020;382(22):2117–2128.
- 50. Davies MJ, Bergenstal R, Bode B, et al. Efficacy of liraglutide for weight loss among patients with type 2 diabetes: the SCALE diabetes randomized clinical trial. JAMA. 2015;314(7):687–699.
- 51. Garvey WT, Birkenfeld AL, Dicker D, et al. Efficacy and safety of liraglutide 3.0 mg in individuals with overweight or obesity and type 2 diabetes treated with basal insulin: the SCALE insulin randomized controlled trial. Diabetes Care. 2020;43(5):1085–1093.
- 52. Lundgren JR, Janus C, Jensen SBK, et al. Healthy weight loss maintenance with exercise, liraglutide, or both combined. N Engl J Med. 2021;384(18):1719–1730.
- 53. Wadden TA, Hollander P, Klein S, et al. Weight maintenance and additional weight loss with liraglutide after low‐calorie‐diet‐induced weight loss: the SCALE maintenance randomized study. Int J Obes (Lond). 2013;37(11):1443–1451.
- 54. Mok J, Adeleke MO, Brown A, et al. Safety and efficacy of liraglutide, 3.0 mg, once daily vs placebo in patients with poor weight loss following metabolic surgery: the BARI‐OPTIMISE randomized clinical trial. JAMA Surg. 2023;158(10):1003–1011.
- 55. Miras AD, Perez‐Pevida B, Aldhwayan M, et al. Adjunctive liraglutide treatment in patients with persistent or recurrent type 2 diabetes after metabolic surgery (GRAVITAS): a randomised, double‐blind, placebo‐controlled trial. Lancet Diabetes Endocrinol. 2019;7(7):549–559.
- 56. Armstrong MJ, Gaunt P, Aithal GP, et al. Liraglutide safety and efficacy in patients with non‐alcoholic steatohepatitis (LEAN): a multicentre, double‐blind, randomised, placebo‐controlled phase 2 study. Lancet. 2016;387(10019):679–690.
- 57. Marso SP, Daniels GH, Brown‐Frandsen K, et al. Liraglutide and cardiovascular outcomes in type 2 diabetes. N Engl J Med. 2016;375(4):311–322.
- 58. Davies MJ, Aronne LJ, Caterson ID, et al. Liraglutide and cardiovascular outcomes in adults with overweight or obesity: a post hoc analysis from SCALE randomized controlled trials. Diabetes Obes Metab. 2018;20(3):734–739.
- 59. Wadden TA, Bailey TS, Billings LK, et al. Effect of subcutaneous semaglutide vs placebo as an adjunct to intensive behavioral therapy on body weight in adults with overweight or obesity: the STEP 3 randomized clinical trial. JAMA. 2021;325(14):1403–1413.
- 60. Wilding JPH, Batterham RL, Calanna S, et al. Once‐weekly semaglutide in adults with overweight or obesity. N Engl J Med. 2021;384(11):989–1002.
- 61. Rubino DM, Greenway FL, Khalid U, et al. Effect of weekly subcutaneous semaglutide vs daily liraglutide on body weight in adults with overweight or obesity without diabetes: the STEP 8 randomized clinical trial. JAMA. 2022;327(2):138–150.
- 62. Weghuber D, Barrett T, Barrientos‐Perez M, et al. Once‐weekly semaglutide in adolescents with obesity. N Engl J Med. 2022;387(24):2245–2257.
- 63. Davies M, Faerch L, Jeppesen OK, et al. Semaglutide 2.4 mg once a week in adults with overweight or obesity, and type 2 diabetes (STEP 2): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3 trial. Lancet. 2021;397(10278):971–984.
- 64. Kadowaki T, Isendahl J, Khalid U, et al. Semaglutide once a week in adults with overweight or obesity, with or without type 2 diabetes in an east Asian population (STEP 6): a randomised, double‐blind, double‐dummy, placebo‐controlled, phase 3a trial. Lancet Diabetes Endocrinol. 2022;10(3):193–206.
- 65. Jensen AB, Renström F, Aczél S, et al. Efficacy of the glucagon‐like peptide‐1 receptor agonists liraglutide and semaglutide for the treatment of weight regain after bariatric surgery: a retrospective observational study. Obes Surg. 2023;33(4):1017–1025.
- 66. Lautenbach A, Kantowski T, Wagner J, Mann O, Stoll F, Aberle J. Sustained weight loss with semaglutide once weekly in patients without type 2 diabetes and post‐bariatric treatment failure. Clin Obes. 2023;13(5):e12593.
- 67. Murvelashvili N, Xie L, Schellinger JN, et al. Effectiveness of semaglutide versus liraglutide for treating post‐metabolic and bariatric surgery weight recurrence. Obesity (Silver Spring). 2023;31(5):1280–1289.
- 68. Loomba R, Abdelmalek MF, Armstrong MJ, et al. Semaglutide 2·4 mg once weekly in patients with non‐alcoholic steatohepatitis‐related cirrhosis: a randomised, placebo‐controlled phase 2 trial. Lancet Gastroenterol Hepatol. 2023;8(6):511–522.
- 69. Newsome PN, Buchholtz K, Cusi K, et al. A placebo‐controlled trial of subcutaneous semaglutide in nonalcoholic steatohepatitis. N Engl J Med. 2021;384(12):1113–1124.
- 70. Kosiborod MN, Bhatta M, Davies M, et al. Semaglutide improves cardiometabolic risk factors in adults with overweight or obesity: STEP 1 and 4 exploratory analyses. Diabetes Obes Metab. 2023;25(2):468–478.
- 71. Perreault L, Davies M, Frias JP, et al. Changes in glucose metabolism and glycemic status with once‐weekly subcutaneous semaglutide 2.4 mg among participants with prediabetes in the STEP program. Diabetes Care. 2022;45(10):2396–2405.
- 72. Wilding JPH, Batterham RL, Davies M, et al. Weight regain and cardiometabolic effects after withdrawal of semaglutide: the STEP 1 trial extension. Diabetes Obes Metab. 2022;24(8):1553–1564.
- 73. Lincoff AM, Brown‐Frandsen K, Colhoun HM, et al. Semaglutide and cardiovascular outcomes in obesity without diabetes. N Engl J Med. 2023;389(24):2221–2232.
- 74. Borlaug BA, Kitzman DW, Davies MJ, et al. Semaglutide in HFpEF across obesity class and by body weight reduction: a prespecified analysis of the STEP‐HFpEF trial. Nat Med. 2023;29(9):2358–2365.
- 75. Kosiborod MN, Abildstrom SZ, Borlaug BA, et al. Semaglutide in patients with heart failure with preserved ejection fraction and obesity. N Engl J Med. 2023;389(12):1069–1084.
- 76. Aroda VR, Blonde L, Pratley RE. A new era for oral peptides: SNAC and the development of oral semaglutide for the treatment of type 2 diabetes. Rev Endocr Metab Disord. 2022;23(5):979–994.
- 77. Singh G, Krauthamer M, Bjalme‐Evans M. Wegovy (semaglutide): a new weight loss drug for chronic weight management. J Investig Med. 2022;70(1):5–13.
- 78. Knop FK, Aroda VR, do Vale RD, et al. Oral semaglutide 50 mg taken once per day in adults with overweight or obesity (OASIS 1): a randomised, double‐blind, placebo‐controlled, phase 3 trial. Lancet. 2023;402(10403):705–719.
- 79. Wharton S, Blevins T, Connery L, et al. Daily oral GLP‐1 receptor agonist orforglipron for adults with obesity. N Engl J Med. 2023;389(10):877–888.
- 80. Frias JP, Hsia S, Eyde S, et al. Efficacy and safety of oral orforglipron in patients with type 2 diabetes: a multicentre, randomised, dose–response, phase 2 study. Lancet. 2023;402(10400):472–483.
- 81. Nordisk N. RYBELSUS (semaglutide) tablets, for oral use. Prescribing information. 2019 Accessed March 28, 2024. https://www.accessdata.fda.gov/drugsatfda_docs/label/2019/213051s000lbl.pdf.
- 82. Malik F, Li Z. Non‐peptide agonists and positive allosteric modulators of glucagon‐like peptide‐1 receptors: alternative approaches for treatment of type 2 diabetes. Br J Pharmacol. 2022;179(4):511–525.
- 83. Jastreboff AM, Aronne LJ, Ahmad NN, et al. Tirzepatide once weekly for the treatment of obesity. N Engl J Med. 2022;387(3):205–216.
- 84. Wadden TA, Chao AM, Machineni S, et al. Tirzepatide after intensive lifestyle intervention in adults with overweight or obesity: the SURMOUNT‐3 phase 3 trial. Nat Med. 2023;29(11):2909–2918.
- 85. Aronne LJ, Sattar N, Horn DB, et al. Continued treatment with tirzepatide for maintenance of weight reduction in adults with obesity: the SURMOUNT‐4 randomized clinical trial. JAMA. 2024;331(1):38–48.
- 86. Garvey WT, Frias JP, Jastreboff AM, et al. Tirzepatide once weekly for the treatment of obesity in people with type 2 diabetes (SURMOUNT‐2): a double‐blind, randomised, multicentre, placebo‐controlled, phase 3 trial. Lancet. 2023;402(10402):613–626.
- 87. Hankosky ER, Wang H, Neff LM, et al. Tirzepatide reduces the predicted risk of developing type 2 diabetes in people with obesity or overweight: post hoc analysis of the SURMOUNT‐1 trial. Diabetes Obes Metab. 2023;25(12):3748–3756.
- 88. Rosenstock J, Wysham C, Frias JP, et al. Efficacy and safety of a novel dual GIP and GLP‐1 receptor agonist tirzepatide in patients with type 2 diabetes (SURPASS‐1): a double‐blind, randomised, phase 3 trial. Lancet. 2021;398(10295):143–155.
- 89. Dahl D, Onishi Y, Norwood P, et al. Effect of subcutaneous tirzepatide vs placebo added to titrated insulin glargine on glycemic control in patients with type 2 diabetes: the SURPASS‐5 randomized clinical trial. JAMA. 2022;327(6):534–545.
- 90. Kadowaki T, Chin R, Ozeki A, Imaoka T, Ogawa Y. Safety and efficacy of tirzepatide as an add‐on to single oral antihyperglycaemic medication in patients with type 2 diabetes in Japan (SURPASS J‐combo): a multicentre, randomised, open‐label, parallel‐group, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(9):634–644.
- 91. Frias JP, Davies MJ, Rosenstock J, et al. Tirzepatide versus semaglutide once weekly in patients with type 2 diabetes. N Engl J Med. 2021;385(6):503–515.
- 92. Ludvik B, Giorgino F, Jodar E, et al. Once‐weekly tirzepatide versus once‐daily insulin degludec as add‐on to metformin with or without SGLT2 inhibitors in patients with type 2 diabetes (SURPASS‐3): a randomised, open‐label, parallel‐group, phase 3 trial. Lancet. 2021;398(10300):583–598.
- 93. Del Prato S, Kahn SE, Pavo I, et al. Tirzepatide versus insulin glargine in type 2 diabetes and increased cardiovascular risk (SURPASS‐4): a randomised, open‐label, parallel‐group, multicentre, phase 3 trial. Lancet. 2021;398(10313):1811–1824.
- 94. Inagaki N, Takeuchi M, Oura T, Imaoka T, Seino Y. Efficacy and safety of tirzepatide monotherapy compared with dulaglutide in Japanese patients with type 2 diabetes (SURPASS J‐mono): a double‐blind, multicentre, randomised, phase 3 trial. Lancet Diabetes Endocrinol. 2022;10(9):623–633.
- 95. Rodriguez PJ, Goodwin Cartwright BM, Gratzl S, et al. Comparative effectiveness of semaglutide and tirzepatide for weight loss in adults with overweight and obesity in the US: a real‐world evidence study. medRxiv. 2023. doi:10.1101/2023.11.21.23298775
- 96. Gastaldelli A, Cusi K, Fernandez Lando L, Bray R, Brouwers B, Rodriguez A. Effect of tirzepatide versus insulin degludec on liver fat content and abdominal adipose tissue in people with type 2 diabetes (SURPASS‐3 MRI): a substudy of the randomised, open‐label, parallel‐group, phase 3 SURPASS‐3 trial. Lancet Diabetes Endocrinol. 2022;10(6):393–406.
- 97. Eli Lilly and Company . Lilly reports strong fourth‐quarter 2023 financial results and provides 2024 guidance. 2024; Accessed June 4, 2024.https://investor.lilly.com/node/50281/pdf.
- 98. Hartman ML, Sanyal AJ, Loomba R, et al. Effects of novel dual GIP and GLP‐1 receptor agonist Tirzepatide on biomarkers of nonalcoholic Steatohepatitis in patients with type 2 diabetes. Diabetes Care. 2020;43(6):1352–1355.
- 99. Sattar N, McGuire DK, Pavo I, et al. Tirzepatide cardiovascular event risk assessment: a pre‐specified meta‐analysis. Nat Med. 2022;28(3):591–598.
- 100. Frias JP, Bastyr EJ 3rd, Vignati L, et al. The sustained effects of a dual GIP/GLP‐1 receptor agonist, NNC0090‐2746, in patients with type 2 diabetes. Cell Metab. 2017;26(2):343–352 e342.
- 101. Day JW, Ottaway N, Patterson JT, et al. A new glucagon and GLP‐1 co‐agonist eliminates obesity in rodents. Nat Chem Biol. 2009;5(10):749–757.
- 102. Müller TD, Finan B, Clemmensen C, DiMarchi RD, Tschop MH. The new biology and pharmacology of glucagon. Physiol Rev. 2017;97(2):721–766.
- 103. Pocai A, Carrington PE, Adams JR, et al. Glucagon‐like peptide 1/glucagon receptor dual agonism reverses obesity in mice. Diabetes. 2009;58(10):2258–2266.
- 104. Cohen MA, Ellis SM, Le Roux CW, et al. Oxyntomodulin suppresses appetite and reduces food intake in humans. J Clin Endocrinol Metab. 2003;88(10):4696–4701.
- 105. Wynne K, Park AJ, Small CJ, et al. Subcutaneous oxyntomodulin reduces body weight in overweight and obese subjects: a double‐blind, randomized, controlled trial. Diabetes. 2005;54(8):2390–2395.
- 106. Ji L, Gao L, Jiang H, et al. Safety and efficacy of a GLP‐1 and glucagon receptor dual agonist mazdutide (IBI362) 9 mg and 10 mg in Chinese adults with overweight or obesity: a randomised, placebo‐controlled, multiple‐ascending‐dose phase 1b trial. EClinicalMedicine. 2022;54:101691.
- 107. Ji L, Jiang H, An P, et al. IBI362 (LY3305677), a weekly‐dose GLP‐1 and glucagon receptor dual agonist, in Chinese adults with overweight or obesity: a randomised, placebo‐controlled, multiple ascending dose phase 1b study. EClinicalMedicine. 2021;39:101088.
- 108. Jiang H, Pang S, Zhang Y, et al. A phase 1b randomised controlled trial of a glucagon‐like peptide‐1 and glucagon receptor dual agonist IBI362 (LY3305677) in Chinese patients with type 2 diabetes. Nat Commun. 2022;13(1):3613.
- 109. Zhang B, Cheng Z, Chen J, et al. Efficacy and safety of mazdutide in Chinese patients with type 2 diabetes: a randomized, double‐blind, placebo‐controlled phase 2 trial. Diabetes Car. 2024;47(1):160–168.
- 110. Asano M, Sekikawa A, Sugeno M, Matsuoka O, Robertson D, Hansen L. Safety/tolerability, efficacy and pharmacokinetics of 600‐mug cotadutide in Japanese type 2 diabetes patients with a body mass index of 25 kg/m(2) or higher: a phase I, randomized, double‐blind, placebo‐controlled study. Diabetes Obes Meta. 2023;25(8):2290–2299.
- 111. Nahra R, Wang T, Gadde KM, et al. Effects of cotadutide on metabolic and hepatic parameters in adults with overweight or obesity and type 2 diabetes: a 54‐week randomized phase 2b study. Diabetes Care. 2021;44(6):1433–1442.
- 112. Parker VER, Robertson D, Wang T, et al. Efficacy, safety, and mechanistic insights of cotadutide, a dual receptor glucagon‐like peptide‐1 and glucagon agonist. J Clin Endocrinol Metab. 2020;105(3):803–820.
- 113. Technology P. AZ to terminate once‐daily cotadutide program to focus on weekly GLP‐1RA co‐agonist. 2023; Accessed November 21, 2023.https://www.pharmaceutical-technology.com/analyst-comment/az-terminate-cotadutide-coagonist/.
- 114. Romero‐Gomez M, Lawitz E, Shankar RR, et al. A phase IIa active‐comparator‐controlled study to evaluate the efficacy and safety of efinopegdutide in patients with non‐alcoholic fatty liver disease. J Hepatol. 2023;79(4):888–897.
- 115. Alba M, Yee J, Frustaci ME, Samtani MN, Fleck P. Efficacy and safety of glucagon‐like peptide‐1/glucagon receptor co‐agonist JNJ‐64565111 in individuals with obesity without type 2 diabetes mellitus: a randomized dose‐ranging study. Clin Obes. 2021;11(2):e12432.
- 116. Di Prospero NA, Yee J, Frustaci ME, Samtani MN, Alba M, Fleck P. Efficacy and safety of glucagon‐like peptide‐1/glucagon receptor co‐agonist JNJ‐64565111 in individuals with type 2 diabetes mellitus and obesity: a randomized dose‐ranging study. Clin Obes. 2021;11(2):e12433.
- 117. Zimmermann T, Thomas L, Baader‐Pagler T, et al. BI 456906: discovery and preclinical pharmacology of a novel GCGR/GLP‐1R dual agonist with robust anti‐obesity efficacy. Mol Metab. 2022;66:101633.
- 118. Jungnik A, Arrubla Martinez J, Plum‐Morschel L, et al. Phase I studies of the safety, tolerability, pharmacokinetics and pharmacodynamics of the dual glucagon receptor/glucagon‐like peptide‐1 receptor agonist BI 456906. Diabetes Obes Metab. 2023;25(4):1011–1023.
- 119. Yazawa R, Ishida M, Balavarca Y, Hennige AM. A randomized phase I study of the safety, tolerability, pharmacokinetics and pharmacodynamics of BI 456906, a dual glucagon receptor/glucagon‐like peptide‐1 receptor agonist, in healthy Japanese men with overweight/obesity. Diabetes Obes Metab. 2023;25(7):1973–1984.
- 120. Le Roux C, Steen O, Lucas KJ, Startseva E, Unseld A, Hennige AM. 51‐OR: a phase 2, randomized, double‐blind, placebo‐controlled, dose‐finding study of BI 456906 in people with overweight/obesity. Diabete. 2023;72(Supplement):51‐OR.
- 121. Survodutide Phase II trial shows 83% of adults treated achieved groundbreaking results in liver disease due to MASH, with significant improvements in fibrosis [media release] [press release]. 26 February 2024.
- 122. Corbin KD, Carnero EA, Allerton TD, et al. Glucagon‐like peptide‐1/glucagon receptor agonism associates with reduced metabolic adaptation and higher fat oxidation: a randomized trial. Obesity (Silver Spring). 2023;31(2):350–362.
- 123. Lau DCW, Erichsen L, Francisco AM, et al. Once‐weekly cagrilintide for weight management in people with overweight and obesity: a multicentre, randomised, double‐blind, placebo‐controlled and active‐controlled, dose‐finding phase 2 trial. Lancet. 2021;398(10317):2160–2172.
- 124. Frias JP, Deenadayalan S, Erichsen L, et al. Efficacy and safety of co‐administered once‐weekly cagrilintide 2·4 mg with once‐weekly semaglutide 2·4 mg in type 2 diabetes: a multicentre, randomised, double‐blind, active‐controlled, phase 2 trial. Lancet. 2023;402(10403):720–730.
- 125. Gydesen S, Andreassen KV, Hjuler ST, Hellgren LI, Karsdal MA, Henriksen K. Optimization of tolerability and efficacy of the novel dual amylin and calcitonin receptor agonist KBP‐089 through dose escalation and combination with a GLP‐1 analog. Am J Physiol Endocrinol Metab. 2017;313(5):E598–E607.
- 126. Larsen AT, Gydesen S, Sonne N, Karsdal MA, Henriksen K. The dual amylin and calcitonin receptor agonist KBP‐089 and the GLP‐1 receptor agonist liraglutide act complimentarily on body weight reduction and metabolic profile. BMC Endocr Disord. 2021;21(1):10.
- 127. Coskun T, Urva S, Roell WC, et al. LY3437943, a novel triple glucagon, GIP, and GLP‐1 receptor agonist for glycemic control and weight loss: from discovery to clinical proof of concept. Cell Metab. 2022;34(9):1234–1247. e1239.
- 128. Jastreboff AM, Kaplan LM, Frias JP, et al. Triple‐hormone‐receptor agonist retatrutide for obesity – a phase 2 trial. N Engl J Med. 2023;389(6):514–526.
- 129. Rosenstock J, Frias J, Jastreboff AM, et al. Retatrutide, a GIP, GLP‐1 and glucagon receptor agonist, for people with type 2 diabetes: a randomised, double‐blind, placebo and active‐controlled, parallel‐group, phase 2 trial conducted in the USA. Lancet. 2023;402(10401):529–544.
- 130. Sanyal AJ, Kaplan LM, Frias JP, et al. Triple hormone receptor agonist retatrutide for metabolic dysfunction‐associated steatotic liver disease: a randomized phase 2a trial. Nat Med. 2024;30:1–12.
- 131. Brouwers B, Rao G, Tang Y, Rodríguez Á, Glass LC, Hartman ML. Incretin‐based investigational therapies for the treatment of MASLD/MASH. Diabetes Res Clin Pract. 2024;211:111675.
- 132. Bossart M, Wagner M, Elvert R, et al. Effects on weight loss and glycemic control with SAR441255, a potent unimolecular peptide GLP‐1/GIP/GCG receptor triagonist. Cell Metab. 2022;34(1):59–74. e10.
- 133. Abdelmalek MF, Suzuki A, Sanchez W, et al. A phase 2, adaptive randomized, double‐blind, placebo‐controlled, multicenter, 52‐week study of HM15211 in patients with biopsy‐confirmed non‐alcoholic steatohepatitis – study design and rationale of HM‐TRIA‐201 study. Contemp Clin Trials. 2023;130:107176.
- 134. Karagiannis T, Bekiari E, Tsapas A. Socioeconomic aspects of incretin‐based therapy. Diabetologia. 2023;66(10):1859–1868.
- 135. McCrimmon RJ, Catarig AM, Frias JP, et al. Effects of once‐weekly semaglutide vs once‐daily canagliflozin on body composition in type 2 diabetes: a substudy of the SUSTAIN 8 randomised controlled clinical trial. Diabetologia. 2020;63(3):473–485.
- 136. Heise T, DeVries JH, Urva S, et al. Tirzepatide reduces appetite, energy intake, and fat mass in people with type 2 diabetes. Diabetes Care. 2023;46(5):998–1004.
- 137. Kremer D, Sizoo D, Bakker SJL, van Beek AP. Obesity management strategies should cut fat, not muscle. Int J Obes (Lond). 2024;48:1039–1040. doi:10.1038/s41366‐024‐01502‐w
- 138. Nunn E, Jaiswal N, Gavin M, et al. Antibody blockade of activin type II receptors preserves skeletal muscle mass and enhances fat loss during GLP‐1 receptor agonism. Mol Metab. 2024;80:101880.
- 139. Deng Y, Park A, Zhu L, Xie W, Pan CQ. Effect of semaglutide and liraglutide in individuals with obesity or overweight without diabetes: a systematic review. Ther Adv Chronic Dis. 2022;13:20406223221108064.
- 140. Karagiannis T, Avgerinos I, Liakos A, et al. Management of type 2 diabetes with the dual GIP/GLP‐1 receptor agonist tirzepatide: a systematic review and meta‐analysis. Diabetologia. 2022;65(8):1251–1261.
- 141. Zhang P, Liu Y, Ren Y, Bai J, Zhang G, Cui Y. The efficacy and safety of liraglutide in the obese, non‐diabetic individuals: a systematic review and meta‐analysis. Afr Health Sci. 2019;19(3):2591–2599.
- 142. Hashash JG, Thompson CC, Wang AY. AGA rapid clinical practice update on the management of patients taking GLP‐1 receptor agonists prior to endoscopy: communication. Clin Gastroenterol Hepatol. 2024;22(4):705–707.
- 143. Gamble JM, Chibrikov E, Midodzi WK, Twells LK, Majumdar SR. Examining the risk of depression or self‐harm associated with incretin‐based therapies used to manage hyperglycaemia in patients with type 2 diabetes: a cohort study using the UK clinical practice research datalink. BMJ Open. 2018;8(10):e023830.
- 144. US FDA . FDA Drug Safety Communication: Update on FDA's ongoing evaluation of reports of suicidal thoughts or actions in patients taking a certain type of medicines approved for type 2 diabetes and obesity 2023. Accessed March 4, 2024.https://www.fda.gov/drugs/drug‐safety‐and‐availability/update‐fdas‐ongoing‐evaluation‐reports‐suicidal‐thoughts‐or‐actions‐patients‐taking‐certain‐type.
- 145. Abd El Aziz M, Cahyadi O, Meier JJ, Schmidt WE, Nauck MA. Incretin‐based glucose‐lowering medications and the risk of acute pancreatitis and malignancies: a meta‐analysis based on cardiovascular outcomes trials. Diabetes Obes Metab. 2020;22(4):699–704.
- 146. Nagendra L, Bg H, Sharma M, Dutta D. Semaglutide and cancer: a systematic review and meta‐analysis. Diabetes Metab Syndr. 2023;17(9):102834.
- 147. He L, Wang J, Ping F, et al. Association of glucagon‐like peptide‐1 receptor agonist use with risk of gallbladder and biliary diseases: a systematic review and meta‐analysis of randomized clinical trials. JAMA Intern Med. 2022;182(5):513–519.
- 148. Yoshida Y, Joshi P, Barri S, et al. Progression of retinopathy with glucagon‐like peptide‐1 receptor agonists with cardiovascular benefits in type 2 diabetes ‐ a systematic review and meta‐analysis. J Diabetes Complications. 2022;36(8):108255.
- 149. Melson E, Ashraf U, Papamargaritis D, Davies MJ. What is the pipeline for future medications for obesity? Int J Obes. 2024;48:1–19. doi:10.1038/s41366‐024‐01473‐y
- 150. Rubino D, Abrahamsson N, Davies M, et al. Effect of continued weekly subcutaneous semaglutide vs placebo on weight loss maintenance in adults with overweight or obesity: the STEP 4 randomized clinical trial. JAMA. 2021;325(14):1414–1425.
- 151. Garvey WT, Batterham RL, Bhatta M, et al. Two‐year effects of semaglutide in adults with overweight or obesity: the STEP 5 trial. Nat Med. 2022;28(10):2083–2091.