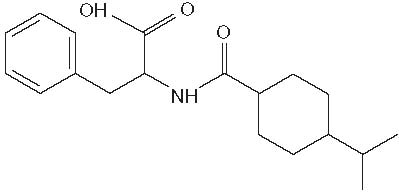
Senaglinide
A 4166, AY 4166, Fastic, SDZ DJN 608, Starlix®, Starsis®, YM 026, Nateglinide
Adis Comments
Senaglinide (Starlix®, Starsis®,A4166,AY4166,Nateglinide, SDZDJN 608, YM 026) is the lead compound of a series of amino acid derivatives developed by Ajinomoto. Ajinomoto has licensed the drug to Yamanouchi in Japan and to Novartis worldwide, except for China, Korea, Taiwan, the UK and Ireland, for which the rights were retained. Senaglinide has been recommended for approval in Japan, for use in the treatment of patients with type 2 diabetes mellitus. It will be jointly marketed by Yamanouchi (as Starsis®) and Nippon Hoechst Marion Roussel (as Fastic®). In addition, the drug is undergoing phase III development as an antidiabetic with Novartis in the US (as Starlix®), Canada and Europe, and is in phase II trials with Britannia in the UK.
Senaglinide is an oral hypoglycaemic agent with a unique mechanism of action. It is a more rapid and short term enhancer of insulin secretion than the sulphonylureas and may be effective in controlling postprandial hyperglycaemia in patients with type 2 diabetes mellitus.