Oral oseltamivir is an effective treatment of acute influenza in adultsEfficacy and safety of the oral neuraminidase inhibitor oseltamivir in treating acute influenza. A randomized controlled trial.
- Jefferson, Tom
- Treanor, JJ
- Hayden, FG
- Vrooman, PS
- Barbarash, R
- Bettis, R
- Riff, D
- Singh, S
- Kinnersley, N
- Ward, P
- Mills, RG
BACKGROUND
Oseltamivir has been shown to prevent influenza in experimentally infected animals and humans, and reduces viral replication rates in vitro.
OBJECTIVE
To assess effects of oseltamivir in natural influenza infection.
SETTING
Sixty primary care clinics and university centres in the United States during influenza season, January to March 1998.
METHOD
Randomised, placebo-controlled, double-blind study.
LITERATURE REVIEW
No explicit strategy; 38 references.
PARTICIPANTS
Six hundred and twenty-nine adults under 65 years, with respiratory and systemic symptoms, and pyrexia (≥38°C) lasting 36 hours or less.
INTERVENTION
Placebo (n = 209); low-dose oral oseltamivir (75 mg twice daily, n = 211); high-dose oral oseltamivir (150 mg twice daily, n = 209).
MAIN OUTCOMES
Time to resolution of illness (day on which symptoms resolved for at least 24 hours); symptom severity index in score-hours (product of daily severity score and duration of illness).
MAIN RESULTS
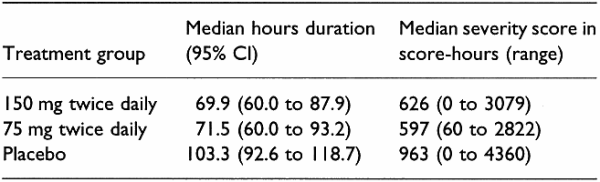
Three hundred and seventy-four people (60%) were found to have influenza. In this group, low-dose and high-dose oseltamivir significantly reduced duration and severity of illness compared with placebo (see Table 1; P value for duration compared with placebo < 0.001 for low-dose oseltamivir, 0.006 for high dose; P value for severity compared with placebo < 0.001 for low- and high-dose oseltamivir). Among all 629 participants, oseltamivir significantly reduced illness severity and duration compared with placebo, although differences were smaller (median severity 686 score-hours with low-dose oseltamivir versus 887 with placebo, P < 0.001; median duration with low-dose oseltamivir 76 hours versus 97 with placebo, P < 0.004). Nausea and vomiting were more frequent with oseltamivir than placebo (nausea occurred in 18% with oseltamivir versus 7.4% with placebo; vomiting in 14.1% with oseltamivir versus 3.4% with placebo).
AUTHORS' CONCLUSIONS
Oseltamivir is effective for relieving symptoms in adults with acute influenza.