Oral and early-switch therapies can be used safely for community-acquired pneumonia
- Rhew, David C. MD
BACKGROUND
Hospital in-patients with community-acquired pneumonia usually receive antibiotics parenterally. Immediate administration of, or switching to, oral antimicrobials may save the cost of hospitalisation.
OBJECTIVE
To determine the efficacy, safety and cost of oral therapy for people with severe and nonsevere community-acquired pneumonia.
SETTING
Girona, Spain; October 1997 to December 1999.
METHOD
Randomised controlled trial.
PARTICIPANTS
Two hundred and thirty-five adults admitted to hospital diagnosed with pneumonia; temperature 37.5°C or higher and at least one clinical or two laboratory criteria; 70% male; average age 67 years. fifty-four percent had 'severe pneumonia' (i.e. at least one severity criterion: arterial/partial oxygen pressure <60 mmHg; arterial/partial oxygen pressure/inspiratory fraction of oxygen ratio <286; respiratory rate ≥30 min; heart rate ≥125−1 min; systolic blood pressure <90 mmHg; temperature ≥40°C; altered mental status; multilobar involvement; deterioration or no improvement after 72 h of treatment). Those discharged from an acute-care facility in the previous 8 days or with nosocomial or aspiration pneumonia; acquired immunodeficiency syndrome; extrapulmonary septic metastases; malabsorption or swallowing problems; pregnancy; lactation or criteria for admission to intensive care were excluded.
INTERVENTION
Of the severe pneumonia group, approximately half received antimicrobials parenterally for 2 days then switched to oral administration (the early-switch procedure). The other half had a full 10-day course of intravenous antibiotics. Half of the non-severe pneumonia group received oral therapy from the beginning. The rest were treated intravenously initially and switched to oral antibiotic administration after 72 hours without fever. All participants were monitored throughout hospitalisation, at 1 week and at 30 days after discharge.
OUTCOMES
Time to resolution of morbidity; adverse events; death.
MAIN RESULTS
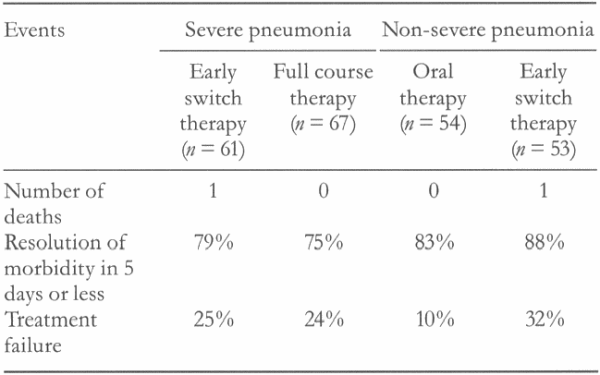
For non-severe cases, time to resolution of morbidity was 5 days or less in 83% of the oral therapy group and 88% of the parenteral therapy group. Treatment failure occurred in 10% of the oral therapy group and 32% of the parenteral group (p < 0.05). For those with severe pneumonia, time to resolution of morbidity was 5 days or less in 79% of the early-switch group and 75% of the full-course group (p > 0.05; Table 1). There were fewer adverse events in the oral therapy and early-switch groups because of a lower rate of infusion-related phlebitis. There were significant cost savings in the early-switch group.
AUTHORS' CONCLUSIONS
In-patients with non-severe community-acquired pneumonia can be effectively and safely treated with oral antimicrobials from the time of admission. Those with severe pneumonia can be treated with early-switch therapy.