Introduction
Atrial fibrillation (AF) and atrial flutter (AFL) are the most frequently encountered arrhythmias. AF-related symptoms and the risk of strokes are significantly improved with ventricular rate control and anticoagulation; controlling sinus rhythm can improve exercise capacity and quality of life in patients with AF., As demonstrated in the recent EAST-AFNET 4 trial, early comprehensive rhythm control [such as antiarrhythmic drugs (AADs)] as part of a structured holistic management pathway is associated with a lower risk of cardiovascular (CV) outcomes in patients with newly diagnosed AF.
AF/AFL is estimated to occur in 15–40% of patients with chronic kidney disease (CKD). Additionally, mild CKD and moderate-to-severe CKD have been found to be independent risk factors for all-cause mortality in patients with AF/AFL. However, the use of AADs in patients with CKD is challenging because of the increased proarrhythmic risks, especially in patients with CKD and concomitant structural heart disease. A further concern is that administration of drugs that rely on kidney elimination can result in accumulation and drug toxicity, especially as renal function deteriorates over time. Because CKD and AF are often coexisting and increase with an advancing age, it is important to establish the efficacy and safety for AAD treatment in patients with AF and CKD. A post hoc analysis of the BALKAN-AF survey found that AF patients with CKD received less rhythm control than rate control; when they did receive rhythm control, it was almost exclusively amiodarone, speaking to the need for increased awareness of the safety and efficacy of available alternative rhythm control therapies.
ATHENA (A Placebo-Controlled, Double-Blind, Parallel Arm Trial to Assess the Efficacy of Dronedarone 400 mg bid for the Prevention of Cardiovascular Hospitalization or Death from Any Cause in Patients with Atrial Fibrillation/Atrial Flutter; NCT00174785) was a randomized, double-blind, placebo-controlled trial to evaluate the use of dronedarone in patients with AF/AFL who had additional risk factors for death. The study found that dronedarone reduced the incidence of CV hospitalization or death in patients with paroxysmal or persistent AF/AFL.
The aim of this post hoc analysis of the ATHENA trial was to evaluate the impact of dronedarone on CV hospitalization or death from any cause and safety outcomes in patients enrolled in the trial across a range of renal function.
Methods
Study design
Details of the ATHENA trial have been described previously., Briefly, ATHENA was a randomized, placebo-controlled, multicentre, double-blind, parallel-group trial that assessed the efficacy of dronedarone for the prevention of CV hospitalization or death from any cause in patients ≥70 years of age with paroxysmal or persistent AF/AFL and additional CV risk factors (arterial hypertension, previous stroke, transient ischaemic attack or systemic embolism, diabetes mellitus, left atrial diameter ≥50 mm, or left ventricular ejection fraction ≤40%). Patients with an estimated glomerular filtration rate (eGFR) of ≥10 mL/min (Cockcroft–Gault) were included in the study. Detailed patient eligibility and exclusion criteria can be found in the Supplementary material online. The study protocol was approved by the institutional review board at each participating institution.
Randomization and follow-up
Eligible patients were randomly assigned to either oral dronedarone 400 mg twice daily or a matching placebo (1:1 ratio). Assessment of vital signs and electrocardiography (ECG) were performed on Days 7 and 14 and at 1, 3, 6, 9, 12, 15, 18, 21, 24, 27, and 30 months. Renal function was assessed by clearance of serum creatinine at each follow-up visit.
Study endpoints
The primary outcome of the ATHENA study, and this exploratory post hoc analysis was first CV hospitalization or death from any cause. Secondary endpoints were as follows: death from any cause, death from CV causes, first hospitalization due to CV events, and first documented recurrence of AF/AFL (assessed using standard ECG at follow-up visits). Safety outcomes including treatment-emergent adverse events (TEAEs), defined as an adverse event occurring between first dose of the study drug and 10 days after the last dose, and TEAE leading to discontinuation were also evaluated.
Statistical analysis
The original analysis of the ATHENA study used the Cockcroft–Gault formula. In this exploratory subanalysis, renal function (eGFR) was assessed using the CKD-Epidemiology Collaboration (CKD-EPI) equation. Patients were then grouped by eGFR strata in the following subgroups: ≥60, ≥45 and <60, and <45 mL/min. For confirmation purposes, outcomes were also analysed in eGFR strata classified according to the Modification of Diet in Renal Disease (MDRD) Study Group criteria and the Cockcroft–Gault formula.
Log-rank testing and Cox regression were used to compare time to events between treatment groups. Modelling was performed of time-to-event according to treatment, baseline CKD (eGFR assessed as a continuous variable), and its interaction, using restricted cubic spline for CKD. The restricted cubic spline analysis enabled the model to fit the non-linearity of the CKD effect by the addition of k-2 covariates of degree 3, with k being the number of knots. A Cox model with interaction was used to analyse time-to-event adjusted for treatment, CKD (CKD itself + three cubic terms = four covariates), and the four treatment-by-CKD interaction terms using SAS Proc PHREG.
Results
Baseline characteristics
The analysis included data from 4588 participants of the 4628 recruited for the ATHENA trial (Table1). In total, 57% of patients had either no decrease or a mild decrease in eGFR (≥60 mL/min subgroup), 29% had a mild-to-moderate decrease in eGFR (≥45 and <60 mL/min subgroup), and the remaining 14% presented with moderate-to-severe decreases in eGFR (<45 mL/min subgroup). Median baseline eGFR for placebo and dronedarone were 74.1 and 73.2 mL/min, respectively, in the ≥60 mL/min subgroup, 53.4 and 53.5 mL/min, respectively, in the ≥45 and <60 mL/min subgroup, and 37.9 and 39.5 mL/min, respectively, in the <45 mL/min subgroup. The proportion of males in the subgroups decreased with worsening renal function. There was a trend towards increasing mean age and greater proportions with structural heart disease and coronary heart disease as renal function worsened. Mean CHA2DS2-VASc scores (congestive heart failure, hypertension, age ≥75 [doubled], diabetes, stroke [doubled], vascular disease, age 65 to 74, sex category [female]) also increased with decreasing renal function. In the ≥60 mL/min subgroup, ∼50% of participants had CHA2DS2-VASc scores >2, compared with ∼85% in the <45 mL/min subgroup.
Primary outcome: first cardiovascular hospitalization or death from any cause
In an analysis of all patients, rates of first CV hospitalization or death were 857/2621 (32.7%) patients in the ≥60 mL/min subgroup, 496/1332 (37.2%) in the ≥45 and <60 mL/min subgroup, and 279/635 (43.9%) in the <45 mL/min subgroup. The effect of treatment with dronedarone vs. placebo on first CV hospitalization or death from any cause vs. baseline eGFR assessed as a continuous variable is depicted in Figure1, showing a relatively consistent hazard ratio (HR) for the effect of dronedarone vs. placebo across a wide range of renal function, with no significant interaction between the study treatment group and CKD (P = 0.743). The number of patients experiencing first CV hospitalization or death from any cause was also analysed according to assigned renal function group (eGFR) (Figure2). Fewer patients experienced first CV hospitalization or death from any cause in the dronedarone vs. placebo groups, showing an HR ranging from 0.73 [95% confidence interval (CI) 0.64–0.84] to 0.82 (95% CI 0.65–1.03) across the renal function groups, with no interaction effect of treatment group and CKD subgroup (P = 0.727). In a subanalysis of patients with severe renal impairment, similar results were observed [HR 0.77 (0.43–1.39) for eGFR ≥10 and <30 mL/min; HR 0.83 (0.64–1.08) for eGFR ≥30 and <45 mL/min; Supplementary material online, Figure S1].
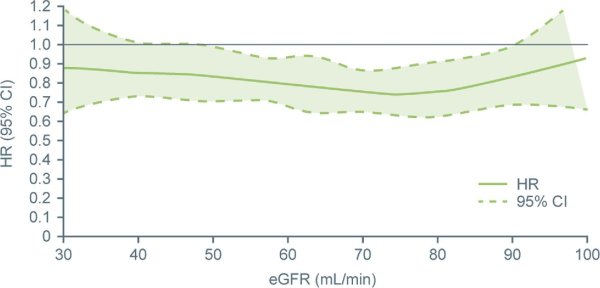
Figure 1
Hazard ratio (95% confidence interval) for first cardiovascular hospitalization or death from any cause related to treatment with dronedarone vs. placebo according to baseline estimated glomerular filtration rate. Hazard ratio and 95% confidence interval (dotted lines) shown. Test of interaction between treatment group and estimated glomerular filtration rate (Chronic Kidney Disease-Epidemiology Collaboration) as a continuous variable: P-value = 0.7434. CI, confidence interval; eGFR, estimated glomerular filtration rate; and HR, hazard ratio.
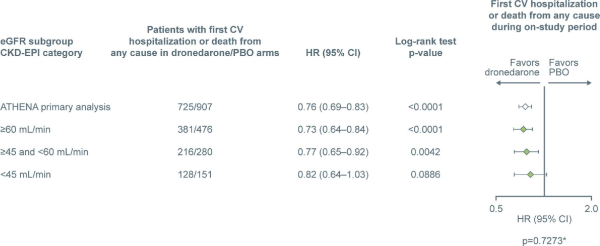
Figure 2
Number of patients experiencing first cardiovascular hospitalization or death from any cause. The ‘ATHENA primary analysis’ data are from Hohnloser et al.*Probability of interaction between the treatment group and the subgroup. Total patients in dronedarone and placebo subgroups—≥60 mL/min: 1320 and 1301; ≥45 and <60 mL/min: 649 and 683; <45 mL/min: 313 and 322. CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio; and PBO, placebo.
The number of patients who experienced a first CV hospitalization was lower with dronedarone compared with placebo, with an HR of 0.72 to 0.84 (P < 0.01 in the ≥60 mL/min and ≥45 and <60 mL/min groups; P > 0.05 in the <45 mL/min group) (Figure3A). There was no interaction effect of treatment group and CKD subgroup (P = 0.573). The number of patients who experienced death from any cause was numerically lower with dronedarone compared with placebo in the ≥60 and <45 mL/min subgroups, and similar in the ≥45 and <60 mL/min subgroup (Figure3B). However, log-rank test P-values were all >0.05, likely due to the smaller patient populations following stratification. There was no interaction effect of treatment group and CKD subgroup (P = 0.576).
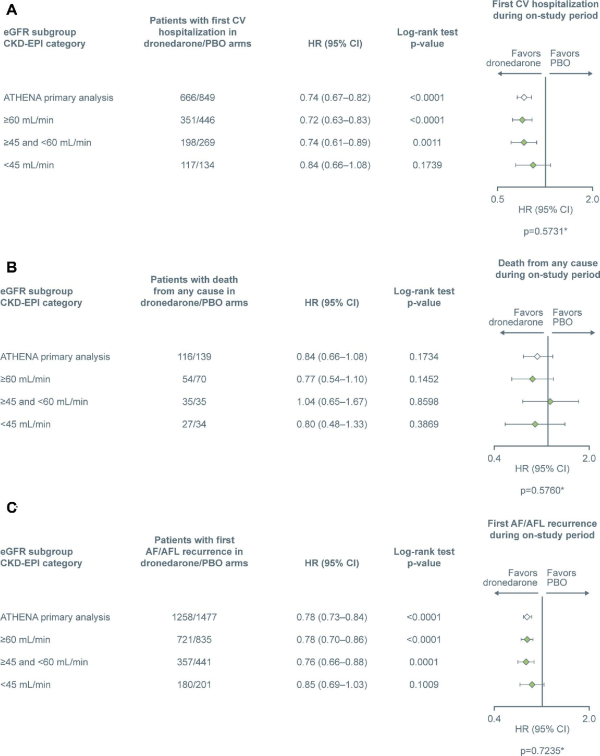
Figure 3
Number of patients with (A) first cardiovascular hospitalization, (B) death from any cause, and (C) first atrial fibrillation / atrial flutter recurrence. The ‘ATHENA primary analysis’ data are from Hohnloser et al. *Probability of interaction between the treatment group and the subgroup. Total patients in dronedarone and placebo subgroups—≥60 mL/min: 1320 and 1301; ≥45 and <60 mL/min: 649 and 683; <45 mL/min: 313 and 322. AF, atrial fibrillation; AFL, atrial flutter; CKD-EPI, Chronic Kidney Disease-Epidemiology Collaboration; CI, confidence interval; CV, cardiovascular; eGFR, estimated glomerular filtration rate; HR, hazard ratio; and PBO, placebo.
First recurrence of atrial fibrillation/atrial flutter
Median time (in days) from randomization until first documented recurrence of AF/AFL was longer with dronedarone vs. placebo in all three subgroups [≥60 mL/min, 533 (386–552) vs. 197 (178–312); ≥45 and <60 mL/min, 534 (378–593) vs. 187 (153–290); and <45 mL/min, 363 (95% CI 191–555) vs. 183 (89–305)]. There was also a difference in the number of patients experiencing first recurrence of AF/AFL between the dronedarone and placebo groups favouring dronedarone (HR 0.78 and 0.76, P < 0.01 for both, for the ≥60 mL/min and ≥45 and <60 mL/min subgroups, respectively). The <45 mL/min subgroup showed an HR of 0.85, P > 0.05 (Figure3C). There was no interaction effect of treatment group and CKD subgroup (P = 0.724). Patients with severe renal impairment (≥30 and <45 mL/min and ≥10 and <30 mL/min) showed HRs of 0.82 and 0.97, respectively (P > 0.05 for both groups).
Change in creatinine
Following dronedarone treatment, creatinine levels initially increased in all eGFR subgroups but appeared to plateau after the first measurement (performed at Week 1) (Figure4). In the more renally impaired subgroups, values returned close to baseline at later time points.
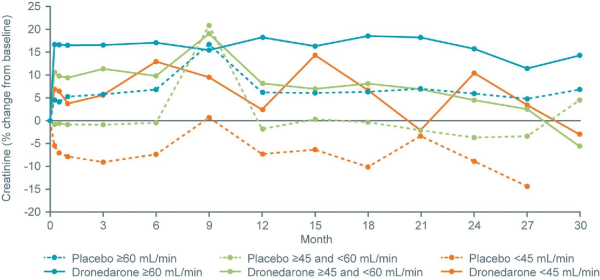
Figure 4
Percentage change from baseline in creatinine by estimated glomerular filtration rate category, with study time displayed on the x-axis.
Safety
There was a general trend towards more TEAEs, serious TEAEs, and TEAEs leading to discontinuation in patients with more impaired renal function (Table2). Differences between the dronedarone and placebo groups were small with regard to TEAEs and serious TEAEs. TEAEs leading to treatment discontinuation were more frequent in the dronedarone vs. placebo arm and were more prevalent in patients with poor renal function (ranging from 10.3% to 21.4% for dronedarone and from 7.8% to 9.6% for placebo). TEAEs leading to treatment discontinuation were primarily of gastrointestinal origin in both groups (Table2); these gastrointestinal TEAEs were mostly attributable to diarrhoea and nausea. Few patients in either treatment group reported QT prolongation as a serious TEAE leading to treatment discontinuation [placebo: one patient (0.04%); dronedarone: four patients (0.2%)].
TEAEs in a subanalysis of patients with severe renal impairment are presented in the Supplementary material online, Table S1. A higher proportion of patients receiving dronedarone reported TEAEs leading to treatment discontinuation compared with patients receiving placebo in all renal function groups; again, gastrointestinal TEAEs (primarily diarrhoea and nausea) leading to discontinuation were most commonly observed.
Modification of Diet in Renal Disease Study Group criteria and Cockcroft–Gault formula
As a sensitivity analysis, outcomes were also analysed in eGFR strata classified according to the MDRD Study Group criteria and the Cockcroft–Gault formula, used in the original ATHENA analysis. The findings were unchanged and these data are not presented herein.
Discussion
This exploratory post hoc analysis of the ATHENA trial aimed to determine the efficacy and safety of dronedarone in relation to renal function. Dronedarone was associated with a lower incidence of first CV hospitalization or death from any cause vs. placebo across a wide spectrum of renal function, consistent with the outcomes of the primary ATHENA trial. These findings are supported by modelling of treatment by baseline eGFR as a continuous variable, rather than choosing the specific cut-off points dividing patients into subgroups. The failure to achieve statistical significance separately in the <45 mL/min subgroup was most likely a reflection of the smaller population size resulting in reduced statistical power.
Secondary endpoints, including CV hospitalization and first recurrence of AF/AFL, showed improved or similar outcomes with dronedarone vs. placebo, with the ≥60 mL/min and ≥45 and <60 mL/min subgroups showing improvements that were statistically significant, but because of the post hoc nature of our analyses, further studies are needed to corroborate these findings. As expected, in ATHENA the risk for first CV hospitalization or death from any cause was higher in patients with renal impairment, in line with similar findings on the increased risk for all-cause and CV mortality with worsening renal impairment.
Dronedarone competes with creatinine for the renal tubular cation transport pathway, inhibiting tubular secretion of creatinine by ∼18% and subsequently increasing serum creatinine. Although this increase in serum creatinine was also described for dronedarone in the ANDROMEDA, EURIDIS-ADONIS, and PALLAS trials,, it does not represent a decrease in glomerular filtration rate (GFR). In this analysis, the increase in creatinine in the dronedarone groups was maintained until the end of the study in the ≥60 mL/min subgroup, but returned close to baseline in the subgroups with greater renal impairment.
Although the study was not powered to detect differences between the treatment arms in subgroups based on renal function, the results did not show any signs of unfavourable individual outcomes with dronedarone across a wide spectrum of renal function. No significant difference in deaths from any cause was observed between dronedarone and placebo in any eGFR subgroup. Serious TEAEs and deaths did not differ notably between dronedarone and placebo in each group, although TEAEs leading to discontinuation were numerically higher in patients with an eGFR of <45 mL/min receiving dronedarone vs. placebo. Another analysis of the ATHENA study has linked older age with a higher discontinuation rate; since older age was associated with worse renal function in the current analysis, this may explain the observed high rate of discontinuation in patients with severe renal impairment. CHA2DS2-VASc scores, a prognostic marker of increased risk for stroke or thromboembolic events and well recognized as a ‘frailty index’, were also higher in patients with more severe renal impairment, possibly reflecting a more fragile population of older age, with more comorbidities and medications with which possible drug–drug interactions and adverse effects are more common. While the higher medication burden in elderly vs. younger populations may increase the potential for adverse interaction, results from this analysis indicate that the rate of treatment discontinuation due to serious TEAEs involving bleeding/thrombotic events, QT prolongation, or heart failure was generally low, which may provide reassurance of the acceptable safety profile of dronedarone in this population.
Limitations
This was a post hoc analysis of a prospective randomized controlled trial, and patients were not stratified according to renal function in the main trial. Splitting the overall trial population into eGFR categories meant that the number of participants in each subgroup was reduced compared with the overall study population in the original trial. This resulted in a loss of power, particularly in the most renally impaired <45 mL/min subgroup [which constituted only 14% (n = 635) of included patients], although there was no interaction when studying eGFR as a continuous variable. The majority of patients had mild to moderate renal impairment, limiting the generalizability to patients with severe renal impairment. Finally, direct oral anticoagulants (DOACs) were not approved for atrial fibrillation at the time of the ATHENA study and so are not represented in the trial population; therefore, it is not possible to make any assessment of the safety/efficacy of dronedarone in conjunction with DOAC administration based on the current analysis.
Conclusions
Our findings demonstrate that dronedarone reduced first CV hospitalization or death from any cause in individuals with AF/AFL and additional risk factors across a wide range of renal function. Although dronedarone is an effective treatment in patients with structural heart disease and renal impairment compared with other AADs, which have limitations in their use or require dose reduction, further assessment of safety will be required in larger populations of patients with severe CKD.
Acknowledgements
Editorial assistance was provided by Alex van der Wateren (PhD), of Fishawack Communications Ltd, and was funded by Sanofi. We thank Wanda Stipek, PharmD, BCPS (Sanofi), for coordination of the development, facilitating author discussions, and critical review of this manuscript.
References
- 1. Singh BN, Singh SN, Reda DJ, Tang XC, Lopez B, Harris CL, Fletcher RD, Sharma SC, Atwood JE, Jacobson AK, Lewis HD Jr, Raisch DW, Ezekowitz MD. Sotalol Amiodarone Atrial Fibrillation Efficacy Trial I. Amiodarone versus sotalol for atrial fibrillation. N Engl J Med2005;352:1861–1872.
- 2.
- 3. Kirchhof P, Camm AJ, Goette A, Brandes A, Eckardt L, Elvan A, Fetsch T, van Gelder IC, Haase D, Haegeli LM, Hamann F, Heidbüchel H, Hindricks G, Kautzner J, Kuck K-H, Mont L, Ng GA, Rekosz J, Schoen N, Schotten U, Suling A, Taggeselle J, Themistoclakis S, Vettorazzi E, Vardas P, Wegscheider K, Willems S, Crijns HJGM, Breithardt G. Early rhythm-control therapy in patients with atrial fibrillation. N Engl J Med2020;383:1305–1316.
- 4. Turakhia MP, Blankestijn PJ, Carrero JJ, Clase CM, Deo R, Herzog CA, Kasner SE, Passman RS, Pecoits-Filho R, Reinecke H, Shroff GR, Zareba W, Cheung M, Wheeler DC, Winkelmayer WC, Wanner C, Conference Participants. Chronic kidney disease and arrhythmias: conclusions from a Kidney Disease: Improving Global Outcomes (KDIGO) Controversies Conference. Eur Heart J2018;39:2314–2325.
- 5. Goto S, Angchaisuksiri P, Bassand JP, Camm AJ, Dominguez H, Illingworth L, Gibbs H, Goldhaber SZ, Goto S, Jing ZC, Haas S, Kayani G, Koretsune Y, Lim TW, Oh S, Sawhney JPS, Turpie AGG, van Eickels M, Verheugt FWA, Kakkar AK, GARFIELD‐AF Investigators. Management and 1-year outcomes of patients with newly diagnosed atrial fibrillation and chronic kidney disease: results from the prospective GARFIELD-AF registry. J Am Heart Assoc2019;8:e010510.
- 6. Lea-Henry TN, Carland JE, Stocker SL, Sevastos J, Roberts DM. Clinical pharmacokinetics in kidney disease: fundamental principles. Clin J Am Soc Nephrol2018;13:1085–1095.
- 7. Jonsson KM, Wieloch M, Sterner G, Nyman U, Elmstahl S, Engstrom G, Svensson PJ. Glomerular filtration rate in patients with atrial fibrillation on warfarin treatment: a subgroup analysis from the AURICULA registry in Sweden. Thromb Res2011;128:341–345.
- 8. Koziel M, Simovic S, Pavlovic N, Nedeljkovic M, Kocijancic A, Paparisto V, Music L, Trendafilova E, Dan AR, Manola S, Kusljugic Z, Dan GA, Lip GYH, Potpara TS, Balkan-AF Investigators. Treatment implications of renal disease in patients with atrial fibrillation: the BALKAN-AF survey. J Arrhythm2020;36:863–873.
- 9. Hohnloser SH, Crijns HJ, van Eickels M, Gaudin C, Page RL, Torp-Pedersen C, Connolly SJ, ATHENA Investigators. Effect of dronedarone on cardiovascular events in atrial fibrillation. N Engl J Med2009;360:668–678.
- 10. Hohnloser SH, Connolly SJ, Crijns HJ, Page RL, Seiz W, Torp-Petersen C. Rationale and design of ATHENA: A placebo-controlled, double-blind, parallel arm Trial to assess the efficacy of dronedarone 400 mg bid for the prevention of cardiovascular Hospitalization or death from any cause in patiENts with Atrial fibrillation/atrial flutter. J Cardiovasc Electrophysiol2008;19:69–73.
- 11. Levey AS, Inker LA, Coresh J. GFR estimation: from physiology to public health. Am J Kidney Dis2014;63:820–834.
- 12. Tonelli M, Wiebe N, Culleton B, House A, Rabbat C, Fok M, McAlister F, Garg AX. Chronic kidney disease and mortality risk: a systematic review. J Am Soc Nephrol2006;17:2034–2047.
- 13. Tschuppert Y, Buclin T, Rothuizen LE, Decosterd LA, Galleyrand J, Gaud C, Biollaz J. Effect of dronedarone on renal function in healthy subjects. Br J Clin Pharmacol2007;64:785–791.
- 14. Connolly SJ, Camm AJ, Halperin JL, Joyner C, Alings M, Amerena J, Atar D, Avezum A, Blomstrom P, Borggrefe M, Budaj A, Chen SA, Ching CK, Commerford P, Dans A, Davy JM, Delacretaz E, Di Pasquale G, Diaz R, Dorian P, Flaker G, Golitsyn S, Gonzalez-Hermosillo A, Granger CB, Heidbuchel H, Kautzner J, Kim JS, Lanas F, Lewis BS, Merino JL, Morillo C, Murin J, Narasimhan C, Paolasso E, Parkhomenko A, Peters NS, Sim KH, Stiles MK, Tanomsup S, Toivonen L, Tomcsanyi J, Torp-Pedersen C, Tse HF, Vardas P, Vinereanu D, Xavier D, Zhu J, Zhu JR, Baret-Cormel L, Weinling E, Staiger C, Yusuf S, Chrolavicius S, Afzal R, Hohnloser SH, PALLAS Investigators. Dronedarone in high-risk permanent atrial fibrillation. N Engl J Med2011;365:2268–2276.
- 15. Kober L, Torp-Pedersen C, McMurray JJ, Gotzsche O, Levy S, Crijns H, Amlie J, Carlsen J, Dronedarone Study Group. Increased mortality after dronedarone therapy for severe heart failure. N Engl J Med2008;358:2678–2687.
- 16. Singh BN, Connolly SJ, Crijns HJ, Roy D, Kowey PR, Capucci A, Radzik D, Aliot EM, Hohnloser SH, Euridis ADONIS Investigators. Dronedarone for maintenance of sinus rhythm in atrial fibrillation or flutter. N Engl J Med2007;357:987–999.
- 17.
- 18. Philippart R, Brunet-Bernard A, Clementy N, Bourguignon T, Mirza A, Babuty D, Angoulvant D, Lip GY, Fauchier L. Prognostic value of CHA2DS2-VASc score in patients with ‘non-valvular atrial fibrillation’ and valvular heart disease: the Loire Valley Atrial Fibrillation Project. Eur Heart J2015;36:1822–1830.
- 19. Chen A, Stecker E, AW B. Direct Oral Anticoagulant Use: A Practical Guide to Common Clinical Challenges. J Am Heart Assoc2020;9:e017559.