Significance
Mitotane is the first-line medical treatment in patients with adrenocortical carcinoma. It is well known that adrenal function can recover after its discontinuation. However, little is known about the frequency or predictors of adrenal recovery, although this is an important information for patients. Here, we demonstrate that adrenal function recovers in most patients, in median after 26 months with a wide range (3-67 months). Mitotane plasma levels (ie, maximum peak ≥ 27 mg/L) significantly correlate with a longer time to adrenal recovery. The other strong predictor was follow-up at a reference center, suggesting that education on glucocorticoid reduction and close monitoring might be the key to adrenal recovery. Mitotane plasma levels decline slowly after its discontinuation and require more than 1 year to become undetectable. This information might be crucial for planning future medical therapies in patients with adrenocortical carcinoma who experience progressive disease under mitotane treatment.
Introduction
Adrenocortical carcinoma (ACC) is a rare malignancy with an incidence of 1 to 2 cases per million persons per year. Even after complete resection, the rate of recurrence is high and many patients die from metastatic disease. To reduce the risk of recurrence, adjuvant mitotane treatment was introduced many decades ago, and more recent studies and guidelines have further suggested its value. Although the recent phase III ADIUVO trials demonstrated that adjuvant mitotane might not be indicated in patients with low-grade ACC (complete tumor resection, tumor stages I-III, and Ki67 ≤ 10%), adjuvant mitotane is still recommended in patients at high risk of recurrence representing most of ACC cases.,,
The mechanism of mitotane action on adrenal cortex cells is not fully understood, and involves endoplasmic reticulum stress,, mitochondrial damage, and down-regulation of different mitochondrial enzymes involved in the steroidogenesis, resulting in a reduction of steroid production. In addition, a strong induction of CYP3A4 by mitotane leads to a rapid metabolic clearance of many hormones (including glucocorticoids) and drugs, explaining a decrease in cortisol bioavailability. Although it is unclear to which extent mitotane is also toxic to normal cells of the adrenal cortex, the above described effects together result in adrenal insufficiency in virtually all treated patients., Therefore, replacement of glucocorticoids and sometimes mineralocorticoids is an essential part of the management of patients treated with mitotane., In addition, mitotane might affect the hypothalamic–pituitary–adrenal (HPA) axis at diverse levels, eg, by decreasing the adrenocorticotropic hormone (ACTH) levels and an inhibitory effect on the pituitary cells. Thus, patients under mitotane in addition to the primary adrenal insufficiency may experience a partial inhibition of the pituitary function.
Clinical experience tells that the HPA axis can recover after mitotane discontinuation, and clinicians are often faced with the challenge of trying to predict the likelihood of restoration of normal adrenal function. Until now, there are only 2 small studies that investigated adrenal recovery after mitotane treatment in a total of 47 patients with ACC. They reported restoration of the adrenal function in 62.5% and 78.3% of patients, respectively., However, no information about the frequency of repeat dynamic testing have been described and no predictive factors for adrenal recovery could be established., In addition to that, information about the HPA hormones change after the stop of mitotane is still missing.
Moreover, mitotane comes with multiple pharmacokinetic interactions with other drugs due to its capacity as one of the strongest inducers of CYP3A4. Its long half-life frequently prevents the successful administration of other anticancer drugs, above all tyrosine kinase inhibitors,, in case of disease progression. However, data on the exact half-life or the time until mitotane is completely eliminated after the drug is stopped are extremely scarce. Therefore, contemporary data on the mitotane plasma disappearance rate after termination are urgently needed to guide clinicians to plan future treatment of a given patients that interfere with mitotane even after discontinuation.
The aim of this study was to retrospectively investigate HPA axis recovery after adjuvant treatment with mitotane in a larger cohort of patients with ACC and to investigate possible factors influencing adrenal recovery that could be helpful for the clinicians to identify group of patients in whom a normal adrenal function is likely or not to be restored. Moreover, we investigated the elimination time of mitotane after its discontinuation as well as the change of ACTH and adrenal hormonal levels.
Subjects and methods
Study population
This retrospective cohort study was part of the European Network for the Study of Adrenal Tumors (ENSAT) registry study (www.ensat.org/registry) in 3 German reference centers for ACC (Würzburg, Berlin, and Munich). The study was conducted in accordance with the principles of the Declaration of Helsinki and approved by the local Ethics Committees of the Universities of Wuerzburg, Charité Berlin, and Munich. All patients provided written informed consent.
Patients with histologically proven ACC and adjuvant mitotane treatment for a minimum of 12 months were included if they discontinued mitotane at a time when no evidence of disease was present. Patients with lack of relevant information on primary diagnosis or follow-up or with disease recurrence within the first 5 years after mitotane discontinuation before adrenal recovery and who started new medical treatment (including mitotane) were excluded. Patients with concomitant systemic adjuvant or neo-adjuvant anti-tumor treatment as well as radiotherapy of the tumor bed during mitotane treatment were also eligible. We consecutively enrolled all patients who qualified for these selection criteria that were treated with adjuvant mitotane between 2000 and 2020 in one of the reference centers. Follow-up was closed in December 2022. Patients initially treated in our centers who had their follow-up visits after the discontinuation of mitotane in other non-reference centers (including general practitioner or other hospitals treating <25 patients with ACC per year) were also included.
Demographic, clinical, and histological parameters, including sex, body mass index (BMI), body surface area (BSA), age at mitotane start and discontinuation, evidence of hormonal excess, ENSAT tumor stage, as well as comprehensive data (see below) on mitotane and glucocorticoid replacement therapy, mineralocorticoid replacement with fludrocortisone, adrenal function, and gamma-glutamyl transferase (GGT), were retrieved from the ENSAT ACC registry and medical records.
Details on mitotane treatment and glucocorticoid replacement therapy
The following information on mitotane treatment was captured: duration of mitotane treatment, mitotane dose and plasma levels at each available measurement during treatment, as well as plasma levels after mitotane discontinuation. Mitotane blood concentration was centrally measured using the Lysosafe® service provided for free by HRA Pharma for all patients treated with mitotane in Europe (https://lysosafe.clinfile.com). Mitotane exposure was expressed as the area under the curve (AUC) of mitotane plasma levels. Cumulative dose of mitotane (g) was estimated as the sum of the reported daily dose of mitotane multiplied for the number of days on the respective dosage of mitotane during the entire period of treatment. Cumulative mitotane dose was also evaluated adjusted for BSA (g/m²). Mitotane average daily dose (g/d) was calculated as cumulative dose divided by the days of treatment.
Except for 1 patient treated with dexamethasone, all patients received hydrocortisone (HC) as glucocorticoid replacement therapy. To permit a comparison of the replacement therapy, HC equivalent (HCeq) dose was calculated for the patient who received dexamethasone as follows: 1 mg dexamethasone = 30 mg HC. HCeq dose before and after mitotane discontinuation was collected for each available time point. Cumulative HCeq dose (sum of the daily dose multiplied by the number of days on the respective HCeq dosage during the entire period of treatment, mg) during the time of mitotane therapy and the HCeq average dose (cumulative dose/days of treatment, mg/d) were evaluated. Both cumulative ad average HCeq were adjusted for BSA (mg/m² and mg/d m²).
Hormonal assessment
All available morning laboratory values for cortisol, ACTH, dehydroepiandrosterone sulfate (DHEAS), aldosterone, renin, and GGT were collected, as well as the results of all performed corticotropin stimulation test (also known as ACTH 1-24 stimulation test or short Synacthen test).,
Serum cortisol, both basal and after corticotropin stimulation test, and ACTH plasma levels were analyzed using standard Immunoassays (Immulite 2000 XPi, Siemens Healthcare GmbH, in Würzburg and Berlin, and CLIA, Liaison, DiaSorin, in Munich). For cortisol, ACTH, and corticotropin stimulation test, patients were instructed to interrupt their glucocorticoid supplementation for at least 18 h prior to the analysis. Corticotropin stimulation test was performed between 8 and 12 AM, and serum cortisol was measured before and 60 min after intravenous bolus injection of 250 μg corticotropin. The frequency of repeat testing was individually decided by clinicians on a case-by-case basis.
Regarding the other adrenal steroids, serum aldosterone and plasma renin concentrations were measured by Coat-a-Count® RIA (Siemens) and by Renin III Generation RIA (Cisbio), respectively, until September 2014, and from October 2014 by an automated chemiluminescence immunoassay (CLIA, iSYS, Immuno Diagnostic Systems). Dehydroepiandrosterone sulfate was measured by chemiluminescence immunoassay (Siemens Immulite 2000 XPi) up to 2017. Since 2017, all the above steroid measurements were performed in Würzburg by liquid chromatography tandem mass spectrometry (LC-MS/MS). In Berlin, aldosterone, renin, as well as DHEAS concentrations were measured by immunoassay (CLIA, Liaison, DiaSorin). In Munich, plasma aldosterone concentration was measured using radioimmunoassay by Coat-a-Count® RIA (Siemens) until 2014 and was replaced by the chemiluminescence immunoassay (CLIA, Liaison, DiaSorin). Chemiluminescence immunoassay was used also for the measurement of renin and DHEAS (CLIA, Liaison, DiaSorin).
Outcome assessment
Adrenal recovery and time to adrenal recovery were defined as the primary outcomes for the present analysis. The assessment of adrenal recovery was based on laboratory values and detailed clinical information provided in the medical records. Complete adrenal recovery was considered when serum cortisol concentration after corticotropin stimulation test reached a peak of at least 500 nmol/L (18 µg/dL). If no corticotropin stimulation test was performed, recovery of the HPA axis was assumed if the patient had stopped the glucocorticoid replacement therapy without any symptoms of adrenal insufficiency for at least 8 weeks and the morning serum cortisol was at least ≥140 nmol/L (5 µg/dL). Insufficient adrenal recovery was considered when serum cortisol levels after corticotropin stimulation test were <276 nmol/L (10 µg/dL), or morning serum cortisol concentration was <140 nmol/L (5 µg/dL) or if the patient was under glucocorticoid replacement with HCeq dose more than 10 mg/day. All cases with cortisol levels that do not fit in the criteria of either complete or insufficient adrenal recovery were considered as partial adrenal recovery. Partial recovery was considered if the patient did not achieve a complete HPA recovery and had serum cortisol levels after corticotropin stimulation test between 276 and 500 nmol/L (10-18 µg/dL) or morning serum cortisol was ≥140 nmol/L (5 µg/dL) and the patient was under HCeq dose ≤ 10 mg/day. The cutoff of 10 mg/day HCeq dose to differentiate insufficient or partial recovery is what we routinely use in our clinical practice.
Time to adrenal recovery was defined as the time elapsed from the first day of mitotane discontinuation to the date the definition of adrenal recovery was met. Patients who did not achieve HPA recovery were censored at the day of the last follow-up.
Statistical analysis
Data were analyzed using SPSS v.26 (IBM SPSS Statistics) and GraphPad Prism (version 9.0, La Jolla, CA, USA). Distribution of the data was evaluated by the Shapiro–Wilk test. Continuous variables were reported as median with lower and upper quartiles (Q1-Q3) if not otherwise specified whereas categorical variables as numbers and percentages. Two-sided t test or Mann–Whitney test and analysis of variance (ANOVA) or Kruskal–Wallis test were used to compare continuous variables, as appropriate, while chi-square (χ²) test was used to compare categorical variables. The AUC of mitotane plasma levels during the treatment and the AUC of mitotane dose were calculated using the linear trapezoid method considering all the available concentrations and doses during the treatment per each patient. Fitted-smoothing curves were used to explore the mitotane plasma levels after the discontinuation of the drug.
Time to adrenal recovery was calculated using the Kaplan–Meier method, and comparisons between groups were done by log-rank statistics. We performed Cox proportional hazards regression univariate and multivariate analyses of factors that could potentially influence adrenal recovery after mitotane treatment: sex, BMI, age at mitotane discontinuation, follow-up at our 3 centers (defined as reference centers, where more than 25 patients with ACC per year are treated) or in non-reference centers, duration of mitotane treatment, mitotane exposure (evaluated as the AUC of mitotane plasma levels), AUC of mitotane dose, maximum peak of mitotane concentration, last mitotane concentration prior discontinuation, cumulative and average mitotane and HCeq replacement dose during the treatment, HCeq replacement dose at mitotane stop and after 6 months of mitotane discontinuation, fludrocortisone therapy, ACTH levels at mitotane stop and after 6 months of mitotane discontinuation, and GGT levels at mitotane discontinuation. For continuous variables, the median value was considered as cutoff for the analysis. In the Cox regressions, partial adrenal recovery and insufficient recovery were considered together as “no recovery.” Hazard ratio (HR) and 95% confidence interval (CI) were evaluated. Only variables with P < .10 at univariate analysis were included in the multivariate regression.
Changes in serum aldosterone and plasma renin, ACTH, and DHEAS concentrations were analyzed at the time of mitotane discontinuation and at the time of adrenal recovery or the last follow-up (if not recovery) using Wilcoxon matched-pairs signed rank test only in those patients with available paired measurement at the 2 time points.
All reported P values are 2-sided. P values <.05 were considered to indicate statistical significance.
Results
Patient characteristics
We identified 77 patients treated adjuvantly with mitotane who were disease-free at the time of treatment discontinuation. Of these, a final cohort of 56 patients was included in the study, comprising 38 patients (67.9%) followed up in reference centers and 18 (32.1%) followed up in non-reference centers (Figure 1).
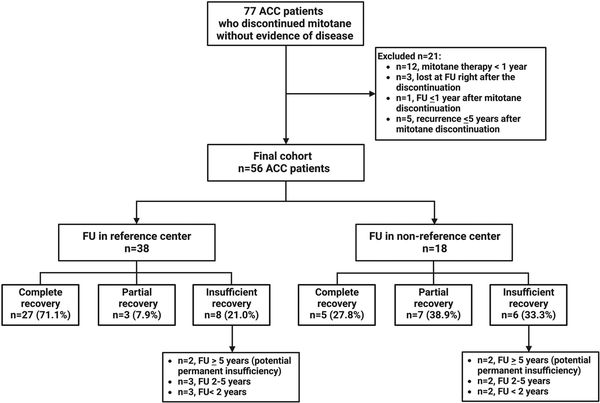
Figure 1
Final cohort of patients. Flowchart of the included patients. Abbreviations: ACC, adrenocortical carcinoma; FU, follow-up; n, number of patients. Reference centers for this study were defined as centers that treat more than 25 patients with ACC per year.
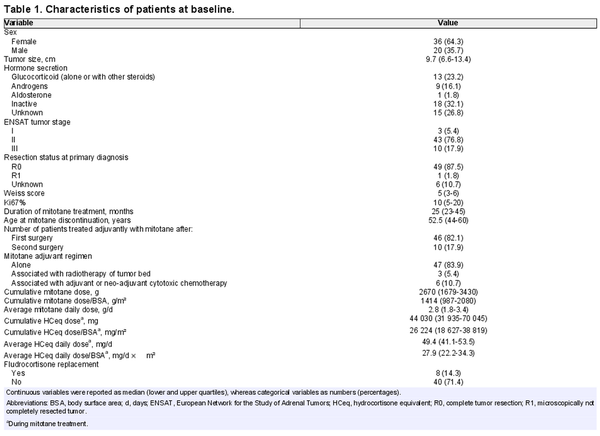
Baseline characteristics of patients were in the expected range for an ACC cohort undergoing adjuvant mitotane therapy (Table 1). Details on mitotane treatment and glucocorticoid replacement are also provided in Table 1.
Mitotane treatment and glucocorticoid replacement
The median duration of adjuvant mitotane treatment slightly exceeded 2 years (25 months, range 23.2-44.7), and the median cumulative mitotane dose was 2670 g (1679-3430), corresponding to an average daily dose of 2.8 g/d (1.8-3.4) (Table 1). Mitotane plasma levels during treatment were available in 44 patients (78.6%), and the median highest peak of mitotane plasma concentration was 21.0 mg/L (17.7-26.0). Last mitotane plasma concentration was measured 9 days (0-37) before treatment discontinuation reached a median of 13.6 mg/L (10.9-16.5).
Mitotane plasma concentrations were evaluated also after treatment discontinuation in a subgroup of 27 cases (48.2%) with available data and last mitotane plasma levels measured within 1 month before the discontinuation of the treatment. Mitotane plasma levels decreased slowly after discontinuation (Figure 2A), but with a very high variability between individual patients (Figure 2B). For the entire cohort, the median time to mitotane levels below 5, 2, and 1 mg/L (= detection limit) was 152 days (114-202), 280 days (192-370), and 395 days (227-546), respectively (Figure 2A).
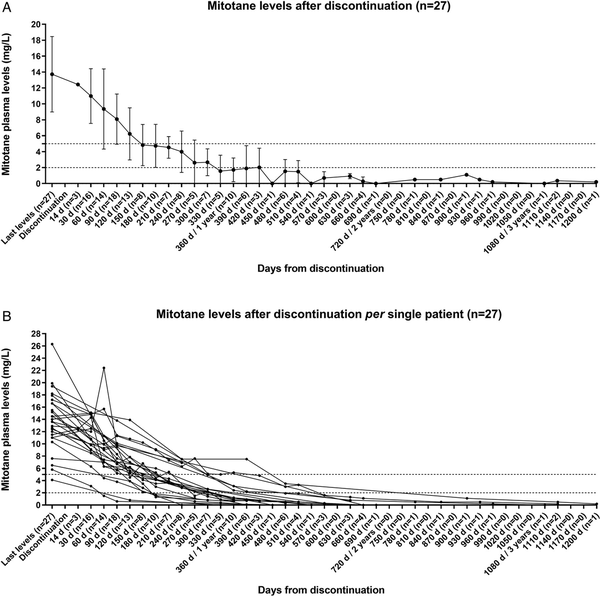
Figure 2
Mitotane plasma levels after its discontinuation. Decrease of the mitotane plasma levels (mg/L) after its discontinuation represented (A) as mean ± standard deviation for the cohort of patients with detailed follow-up information (n = 27) and (B) per single patient. Only patients with a last available mitotane plasma levels measured within 1 month before the discontinuation of the treatment were included. Time is reported in days from the mitotane discontinuation. The number of patients evaluated at each analyzed time point is reported in brackets.
Based on the last mitotane level before stopping treatment and using the lower and upper quartiles as cutoff, we divided the patients with at least 4 measurements of mitotane plasma level after its discontinuation (n = 22) in 3 subgroups: “low levels” (<11 mg/L, n = 5), “intermediate levels” (≥11 and <16.5 mg/L, n = 12), and “high levels” (≥16.5 mg/L, n = 5). As expected, we observed that mitotane plasma levels dropped faster below 5 mg/L (98 days [28-111]), below 2 mg/L (172 days [94-176]), and below detection limit (<1 mg/L) (199 days [125-217]) in patients with “low levels” before the discontinuation compared with those with intermediate and high levels (P < .03 in all cases) (Figure S1A-C). No significant differences were observed between patients with intermediate mitotane levels and those with high levels before discontinuation.
The average HCeq replacement daily dose during mitotane therapy was in median 49.4 mg/d (41.1-53.5), and the median cumulative HCeq dose reached 44 030 mg (31 935-70 045) (Table 1).
Mineralocorticoid replacement with fludrocortisone was administered in 8 patients (14.3%). At time of mitotane stop, data on antihypertensive medications were available in 47 patients, including 26 cases with drugs interfering with aldosterone and renin measurements. Considering patients without renin-interfering antihypertensive medication, at time of mitotane discontinuation, median plasma aldosterone and renin levels were 0.1 nmol/L (0.1-0.2) and 0.2 pmol/L (0.1-0.4), respectively, being the renin concentrations below the normal range (0.7-13.5 pmol/L).
Median plasma ACTH levels at the end of the treatment period reached 3.4 pmol/L (1.8-10.9) and were therefore in the lower part of the normal range (0-10.1 pmol/L). Dehydroepiandrosterone sulfate levels were below the lower normal range (0.5-4.1 µmol/L) in all patients at the time of mitotane discontinuation.
Adrenal recovery
The first laboratory analysis for the evaluation of the HPA axis after mitotane discontinuation was performed in median after 3 months. A median number of 2 corticotropin stimulation tests was available in 37 cases (66.1%).
In 42 out 56 patients (75%), complete and partial recovery of the adrenal function was observed (Figure 3A), including 32 cases (57.1%) in whom a complete recovery was documented after a median time of 792 days (95% CI 572-1011) (equivalent to 26 months [95% CI 19.6-32.4]) after mitotane discontinuation (Figure 3B). Among these, 22 cases (69%) achieved HPA recovery within the first 24 months after mitotane discontinuation (Figure S2). To note, a complete recovery after more than 67 months did not occur. Interestingly, among the 38 patients followed up in reference centers, the proportion of complete adrenal recovery was much higher in comparison with those followed up in non-reference centers (71% vs 27.8%, respectively, P = .002).
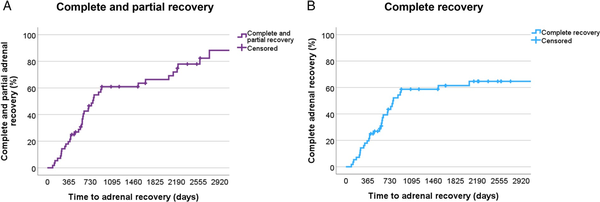
Figure 3
Adrenal recovery after mitotane discontinuation. Kaplan–Meier analysis of (A) time to complete and partial adrenal recovery and (B) time to complete adrenal recovery only.
Partial adrenal recovery was achieved by 10 additional patients (17.9%) after a median time of 84 months (95% CI 65.3-102.7), whereas 14 patients (25%) had insufficient recovery. Partial adrenal recovery and insufficient recovery were considered together as “no recovery.” In these patients, the median HCeq daily dose/BSA at the last follow-up was 12.5 mg/d m² (6.5-15.25). Of note, 13 of 24 patients (54.2%) who did not achieve complete adrenal recovery were not in follow-up in our centers. Furthermore, 5 patients who presented insufficient recovery had a follow-up time <2 years (median 15 months), which was clearly below the median time to adrenal recovery.
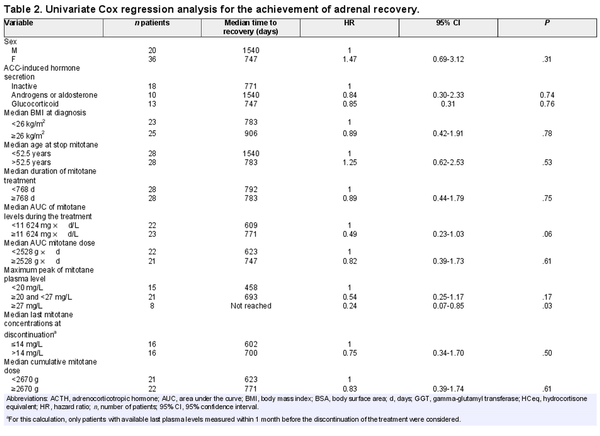
An explorative analysis for potential predictive factors influencing time to adrenal recovery has been performed. Table 2 depicts the detailed results for 24 factors including several clinical characteristics and variables on mitotane treatment as well as on replacement therapies (Table 2).
Most of the investigated potential predictor factors, including sex, age, BMI, hormone secretion, duration of mitotane treatment, median cumulative mitotane dose, cumulative and average HCeq dose during the mitotane treatment and after its stop, ACTH and GGT levels after mitotane stop, and fludrocortisone therapy, did not correlate with the duration to adrenal recovery. However, patients followed up in reference centers had a significantly shorter time to achieve adrenal recovery (median 623 days) in comparison with those followed up in non-reference centers (median not reached, HR = 4.51, 95% CI 1.71-11-89, P = .002; Figure 4A and Table 2). On the contrary, patients having higher mitotane exposure (AUC ≥ 11 624 mg d/L) had a longer median time to HPA recovery compared with those having lower mitotane exposure (771 vs 609 days, HR = 0.49, 95% CI 0.23-1.03, P = .06; Figure 4B and Table 2). Considering the highest peak of mitotane levels during the treatment, we observed a significant longer time to adrenal recovery for mitotane peak ≥ 27 mg/L (median time not reached vs. 693 days in those with mitotane peak 20-27 mg/L vs. 458 days in case of mitotane peak < 20 mg/L, HR = 0.24, 95% CI 0.07-0.85. P = .042; Figure 4C and Table 2). On the other hand, the last mitotane concentration before discontinuation did not have a significant impact on adrenal recovery (Figure 4D and Table 2). However, none of these parameters were significant at multivariate analysis (Table S1).
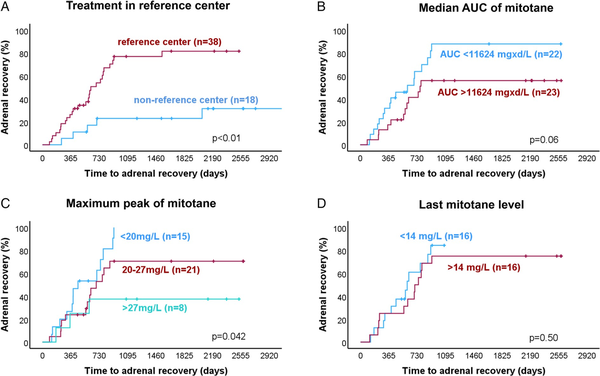
Figure 4
Predictive factors of time to adrenal recovery after mitotane discontinuation. Kaplan–Meier analysis of time to adrenal recovery (A) depending on treatment in reference center, (B) depending on the median area under the curve (AUC) of mitotane levels during the treatment period, (C) depending on the maximum peak of mitotane plasma level during the treatment period, and (D) depending on the last mitotane concentrations at discontinuation. Only patients with last mitotane plasma levels measured within 1 month before the mitotane discontinuation were included in the analysis of last mitotane concentrations. P values were calculated using the log-rank test.
Considering cases under fludrocortisone replacement (n = 8), 4 patients achieved adrenal recovery, among which 3 (75%) also stopped fludrocortisone, whereas in 1 patient fludrocortisone was still ongoing at the last follow-up after stopping glucocorticoid replacement therapy. None of the patients failing to achieve adrenal recovery stopped the fludrocortisone. All except 1 patient under fludrocortisone were treated with various antihypertensive drug classes interfering with the renin–angiotensin–aldosterone axis. Paired data on aldosterone and renin plasma levels at mitotane stop and time of recovery were available in only 9 and 8 patients, respectively, without antihypertensive drugs. No paired aldosterone and renin measurements were available for patients without antihypertensive medications and who did not achieved the HPA recovery. Median aldosterone levels significantly increased at time of recovery compared with the time of mitotane stopping (0.1 [0.1-0.2] vs 0.2 [0.1-0.4] nmol/L, respectively, P = .04) (Figure S3A). Renin concentrations did not change at the time of recovery and remained under the reference range in all except 1 patient (Figure S3B). Data on antihypertensive treatment change at the time of mitotane stop and after adrenal recovery or the last follow-up were available in 21 patients. Although a decrease of doses or number of antihypertensive treatments was slightly higher in patients who achieved the complete adrenal recovery compared with those who did not (42.9% vs 28.6%, respectively), no significant differences were observed between the 2 groups of patients in terms of changes in antihypertensive medications (χ² = 1.98, P = .37). We have also found 2 patients who achieved the HPA recovery who increased the doses or number of antihypertensive drugs.
The change in ACTH and DHEAS plasma levels between the time of stopping mitotane and time of recovery or the last follow-up (if not recovered) was also investigated in patients with available data at both time points. At the time of mitotane stop, 5 out 31 patients (16%) had ACTH levels > 2-fold the upper reference limit (median 60.3 pmol/L), indicating potential primary adrenal insufficiency (PAI) according to the Endocrine Society Guidelines, whereas most patients (84%) had median ACTH levels of 2.5 pmol/L, presumably suggesting a potential secondary adrenal insufficiency (SAI). However, all patients had been treated with HC, which might contribute to the low ACTH. Interestingly, a lower proportion of patients with potential PAI achieved complete adrenal recovery (40%) compared with 77% of those with potential SAI, but this difference was not significant (P = .13). More in details, in patients who recovered (n = 22), median ACTH levels significantly increased at the time of adrenal recovery (19.6 pmol/L [6.9-28.7]) compared with the time of mitotane discontinuation (2.5 pmol/L [1.5-8.0], P = .003; median time between the 2 measurement 16.5 months) (Figure S4A). A slightly but not significant increase in median ACTH levels was observed in patients without adrenal recovery (n = 9) at the last follow-up compared with the time of stopping mitotane (24.4 [2.5-177.3] vs 4.3 [2.5-59.5] pmol/L, respectively, P = .16; median time between the 2 measurement 36 months) (Figure S4B).
Dehydroepiandrosterone sulfate increased very slowly during follow-up and remained below the normal range in most patients (Figure S4C and D). However, in patients with adrenal recovery (n = 21), median DHEAS levels significantly increased in few patients (n = 7) at the time of recovery (0.03 µmol/L [0.03-3.34]) compared with the time of mitotane stopping (always undetected, P = .002; median time between the 2 measurements: 18 months) (Figure S4C). No patient without adrenal recovery (n = 10) showed normal values for DHEAS during the time of the follow-up (median time 48 months) (Figure S4D).
Discussion
Mitotane therapy causes adrenal insufficiency in virtually all treated patients. However, in clinical experience, adrenal function recovers in many patients, but the published evidence on HPA recovery after mitotane treatment is very limited and coming from 2 small previous studies including 23 and 24 patients, respectively., The primary aim of our study was to investigate the frequency of HPA recovery in a larger cohort of patients with ACC after discontinuation of adjuvant mitotane treatment. In 32 (57.1%) of our patients, a complete adrenal recovery was documented, which was achieved by the majority of patients (69% of those cases) within the first 2 years after mitotane discontinuation. However, among patients from reference centers, a higher proportion (71%) achieved normal adrenal function, underlining the importance of follow-up in specialized centers even after mitotane discontinuation. Our results are in line with 2 previous smaller studies, investigating 23 and 24 ACC patients, which reported complete adrenal recovery in 62.5% and 78.3% of cases, respectively. Of note, 16% of our cases (n = 9) had a partial recovery, with partial normalization of adrenal function but with ongoing glucocorticoid replacement therapy at the last follow-up. Moreover, among patients with insufficient adrenal recovery (n = 15), 33% of cases had a follow-up time <2 years, which was clearly below the median time to adrenal recovery. Patients with partial or insufficient HPA recovery but short follow-up could still have the possibility to achieve a complete adrenal recovery. In contrast, we identified 4 patients (7.1%) without any adrenal recovery after more than 5 years of mitotane discontinuation making a permanent adrenal insufficiency very likely.
Until now, no predictive factors of complete adrenal recovery have been investigated., Our analysis reveals potential predictive factors associated with the time to adrenal recovery. First, follow-up in a reference center seems to significantly increase the likelihood of adrenal recovery, which was 4.5 times higher in specialized centers. Although we cannot prove any causality, it is tempting to speculate that the required dose tapering of HC was probably done more stringent in these centers. Second, mitotane plasma levels also seem to play a role in the recovery of adrenal function. In fact, the highest peak of mitotane levels ≥ 27 mg/L during the treatment was associated with a significant longer time to adrenal recovery and a higher mitotane exposure showed a trend for a longer time to adrenal recovery. Again one could speculate that very high mitotane plasma level might be particularly toxic to adrenocortical cells or other cells in the HPA axis. However, before concluding clinical recommendations, these data that based only on 8 patients need confirmation in an independent series. Several other investigated parameters did not correlate with time to adrenal recovery, including sex, age at stop mitotane, BMI, hormone secretion, duration of mitotane treatment, median cumulative mitotane dose, cumulative and average HCeq dose during the mitotane treatment and levels after its stop, ACTH and GGT levels after mitotane stop, and fludrocortisone replacement therapy.
Due to induction of CYP3A4 and pharmacokinetic interaction with other drugs, mitotane could delay the administration of other therapies, above all tyrosine kinase inhibitors, in case of tumor progression., Until now, there was no reliable evidence on the elimination time of mitotane after its discontinuation. However, this information might be crucial for planning future therapy in a given patient. In our cohort, we observed that mitotane plasma levels sank slowly after discontinuation but with a huge variability between individual patients. Not surprisingly, the higher the mitotane level at discontinuation, the longer it takes until mitotane disappears. Even in patients with last concentrations < 11 mg/L, about 200 days are required until mitotane is undetectable, whereas this duration is more than 550 days in the group with last mitotane level > 16.5 mg/L.
Most of our patients (84%) presented low median ACTH levels (2.5 pmol/L) at the time of mitotane discontinuation, and their increase after treatment stop might point toward partial corticotropic insufficiency as a result of the inhibitory effect of mitotane on the HPA axis associated with a potential SAI., In our cohort, a slightly higher proportion of patients with potential SAI achieved complete adrenal recovery compared with those with potential PAI. However, the higher than usual glucocorticoid replacement doses during mitotane therapy with median HCeq dose of 50 mg/d (corresponding to a median of 28.6 mg HCeq dose/BSA) instead of 15-25 mg/d (corresponding to 5-8 mg HCeq dose/BSA) recommended for patients with adrenal insufficiency, due to a higher metabolic clearance of glucocorticoids induced by mitotane, could also be responsible for low ACTH levels. Therefore, we would assume that in some patients a mixed picture of corticotropic insufficiency and glucocorticoid overtreatment is present and a definitive conclusion on potential primary or secondary adrenal insufficiency in this setting is difficult to draw.
The effects of mitotane on the inhibition of the secretion of adrenal hormone levels are well known., However, until now, no data have been reported on potential restoration of other adrenal hormones. Therefore, a secondary outcome of our study was the evaluation of hormone changes after mitotane stop. Dehydroepiandrosterone sulfate levels increased very slowly after mitotane discontinuation only in few cases and remained below the normal range in most patients, even for more than 4 years after mitotane stopping. This observation is in line with previous studies reporting low or suppressed DHEAS levels for months to years after discontinuation of glucocorticoid treatment in patients with adrenal insufficiency or after normalization of cortisol levels in patients with endogenous hypercortisolism, even after endogenous glucocorticoid secretion has returned to normal., Moreover, it should be considered that in our cohort the median age is 52 years old. It is well demonstrated that DHEAS levels decline gradually with age., Considering the observations above, low DHEAS levels are not a reliable parameter to evaluate HPA axis suppression.
Regarding the changes in aldosterone and renin concentration after stopping mitotane, we were able to analyze only a small number of patients without antihypertensive drugs interfering with the renin–angiotensin–aldosterone axis (n = 9 and 8 for aldosterone and renin, respectively). However, we observed that aldosterone levels significantly increased at the time of adrenal recovery, whereas no change was observed for renin levels, which remained mostly below the normal range.
Our study has obvious limitations. First, the retrospective nature might have led to some selection bias and not all patients were treated in a standardized manner leading also to missing data on certain time points. Second, the total number of analyzed patients with just 56 is still low. However, due to the rarity of the disease and the small percentage of patient who are discontinuing mitotane without evidence of disease, the number is significant. A third limitation is the incomplete follow-up in some patients, particularly among those without a glucocorticoid discontinuation. Therefore, one could assume that the failure for adrenal recovery could be due to poor education on how to reduce glucocorticoid replacement after mitotane discontinuation. Furthermore, we have to acknowledge that the calculated average dosage of mitotane and HC is based on the documented dosage in the clinical records and might not be 100% accurate. However, we would expect that this approach did not lead to a systematic over- or underestimation of the dosage.
In conclusion, our results suggest that adrenal function will recover in the vast majority of patients, particularly in those who are adequately followed up. We provide also evidence that complete recovery was delayed in a subset of patients (31%) for more than 2 years after stopping mitotane. Therefore, tapering the glucocorticoid dosage and repeated corticotropin stimulation tests seems justified for up to 5 years before declaring a patient as permanent adrenal insufficient. In general, better patient education on glucocorticoid reduction is recommended, because this is probably associated with a higher rate of adrenal recovery after mitotane discontinuation. However, in few patients, yet unknown factors lead to permanent adrenal insufficiency. We have also demonstrated that that mitotane plasma levels decline slowly after discontinuation of mitotane, albeit with a wider inter-patient variability. The median time until mitotane is undetectable is slightly more than 1 year. This information might be crucial for planning future therapies with drugs that are metabolized by the cytochrome 3A4 in patients with ACC who have progressive disease under mitotane treatment.
Acknowledgments
We are grateful to Michaela Haaf for coordinating the ENSAT Registry in Würzburg.
References
- 1. Bergenstal DM, Hertz R, Lipsett MB, Moy RH. Chemotherapy of adrenocortical cancer with o, p'-DDD. Ann Intern Med. 1960;53:672–682. https://doi.org/10.7326/0003-4819-53-4-672
- 2. Schteingart DE, Motazedi A, Noonan RA, Thompson NW. Treatment of adrenal carcinomas. Arch Surg. 1982;117(9):1142–1146. https://doi.org/10.1001/archsurg.1982.01380330010004
- 3. Fassnacht M, Dekkers OM, Else T, et al European Society of Endocrinology clinical practice guidelines on the management of adrenocortical carcinoma in adults, in collaboration with the European Network for the Study of Adrenal Tumors. Eur J Endocrinol. 2018;179(4):G1–G46. https://doi.org/10.1530/EJE-18-0608
- 4. Fassnacht M, Assie G, Baudin E, et al Adrenocortical carcinomas and malignant phaeochromocytomas: ESMO-EURACAN clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2020;31(11):1476–1490. https://doi.org/10.1016/j.annonc.2020.08.2099
- 5. Tang Y, Liu Z, Zou Z, Liang J, Lu Y, Zhu Y. Benefits of adjuvant mitotane after resection of adrenocortical carcinoma: a systematic review and meta-analysis. Biomed Res Int. 2018;2018:9362108. https://doi.org/10.1155/2018/9362108
- 6. Berruti A, Grisanti S, Pulzer A, et al Long-term outcomes of adjuvant mitotane therapy in patients with radically resected adrenocortical carcinoma. J Clin Endocrinol Metab. 2017;102(4):1358–1365. https://doi.org/10.1210/jc.2016-2894
- 7. Calabrese A, Basile V, Puglisi S, et al Adjuvant mitotane therapy is beneficial in non-metastatic adrenocortical carcinoma at high risk of recurrence. Eur J Endocrinol. 2019;180(6):387–396. https://doi.org/10.1530/EJE-18-0923
- 8. Puglisi S, Calabrese A, Ferrau F, et al New findings on presentation and outcome of patients with adrenocortical cancer: results from a national cohort study. J Clin Endocrinol Metab. 2023;108:2517–2525. https://doi.org/10.1210/clinem/dgad199
- 9. Else T, Kim AC, Sabolch A, et al Adrenocortical carcinoma. Endocr Rev. 2014;35(2):282–326. https://doi.org/10.1210/er.2013-1029
- 10. Terzolo M, Fassnacht M, Perotti P, et al Adjuvant mitotane versus surveillance in low-grade, localised adrenocortical carcinoma (ADIUVO): an international, multicentre, open-label, randomised, phase 3 trial and observational study. Lancet Diabetes Endocrinol. 2023;11:720–730. https://doi.org/10.1016/S2213-8587(23)00193-6
- 11. Terzolo M, Fassnacht M. Endocrine TUMOURS: our experience with the management of patients with non-metastatic adrenocortical carcinoma. Eur J Endocrinol. 2022;187(3):R27–R40. https://doi.org/10.1530/EJE-22-0260
- 12. Altieri B, Lalli E, Faggiano A. Mitotane treatment in adrenocortical carcinoma: mechanisms of action and predictive markers of response to therapy. Minerva Endocrinol (Torino). 2022;47(2):203–214. https://doi.org/10.23736/S2724-6507.21.03601-0
- 13. Haider MS, Ahmad T, Groll J, Scherf-Clavel O, Kroiss M, Luxenhofer R. The challenging pharmacokinetics of mitotane: an old drug in need of new packaging. Eur J Drug Metab Pharmacokinet. 2021;46(5):575–593. https://doi.org/10.1007/s13318-021-00700-5
- 14. Sbiera S, Leich E, Liebisch G, et al Mitotane inhibits sterol-O-acyl transferase 1 triggering lipid-mediated endoplasmic reticulum stress and apoptosis in adrenocortical carcinoma cells. Endocrinology. 2015;156(11):3895–3908. https://doi.org/10.1210/en.2015-1367
- 15. Weigand I, Altieri B, Lacombe AMF, et al Expression of SOAT1 in adrenocortical carcinoma and response to mitotane monotherapy: an ENSAT multicenter study. J Clin Endocrinol Metab. 2020;105(8):2642–2653. https://doi.org/10.1210/clinem/dgaa293
- 16. Hescot S, Slama A, Lombes A, et al Mitotane alters mitochondrial respiratory chain activity by inducing cytochrome c oxidase defect in human adrenocortical cells. Endocr Relat Cancer. 2013;20(3):371–381. https://doi.org/10.1530/ERC-12-0368
- 17. Poli G, Guasti D, Rapizzi E, et al Morphofunctional effects of mitotane on mitochondria in human adrenocortical cancer cells. Endocr Relat Cancer. 2013;20(4):537–550. https://doi.org/10.1530/ERC-13-0150
- 18. Seidel E, Walenda G, Messerschmidt C, et al Generation and characterization of a mitotane-resistant adrenocortical cell line. Endocr Connect. 2020;9(2):122–134. https://doi.org/10.1530/EC-19-0510
- 19. Chortis V, Taylor AE, Schneider P, et al Mitotane therapy in adrenocortical cancer induces CYP3A4 and inhibits 5alpha-reductase, explaining the need for personalized glucocorticoid and androgen replacement. J Clin Endocrinol Metab. 2013;98(1):161–171. https://doi.org/10.1210/jc.2012-2851
- 20. Kroiss M, Quinkler M, Lutz WK, Allolio B, Fassnacht M. Drug interactions with mitotane by induction of CYP3A4 metabolism in the clinical management of adrenocortical carcinoma. Clin Endocrinol (Oxf). 2011;75(5):585–591. https://doi.org/10.1111/j.1365-2265.2011.04214.x
- 21. Ghataore L, Chakraborti I, Aylwin SJ, et al Effects of mitotane treatment on human steroid metabolism: implications for patient management. Endocr Connect. 2012;1(1):37–47. https://doi.org/10.1530/EC-12-0028
- 22. Daffara F, De Francia S, Reimondo G, et al Prospective evaluation of mitotane toxicity in adrenocortical cancer patients treated adjuvantly. Endocr Relat Cancer. 2008;15(4):1043–1053. https://doi.org/10.1677/ERC-08-0103
- 23. Reimondo G, Puglisi S, Zaggia B, et al Effects of mitotane on the hypothalamic-pituitary-adrenal axis in patients with adrenocortical carcinoma. Eur J Endocrinol. 2017;177(4):361–367. https://doi.org/10.1530/EJE-17-0452
- 24. Gentilin E, Tagliati F, Terzolo M, et al Mitotane reduces human and mouse ACTH-secreting pituitary cell viability and function. J Endocrinol. 2013;218(3):275–285. https://doi.org/10.1530/JOE-13-0210
- 25. Poirier J, Gagnon N, Terzolo M, et al Recovery of adrenal insufficiency is frequent after adjuvant mitotane therapy in patients with adrenocortical carcinoma. Cancers (Basel). 2020;12(3):639. https://doi.org/10.3390/cancers12030639
- 26. Vikner ME, Krogh J, Daugaard G, Andreassen M. Metabolic and hormonal side effects of mitotane treatment for adrenocortical carcinoma: a retrospective study in 50 Danish patients. Clin Endocrinol (Oxf). 2021;94(2):141–149. https://doi.org/10.1111/cen.14345
- 27. van Erp NP, Guchelaar HJ, Ploeger BA, Romijn JA, Hartigh J, Gelderblom H. Mitotane has a strong and a durable inducing effect on CYP3A4 activity. Eur J Endocrinol. 2011;164(4):621–626. https://doi.org/10.1530/EJE-10-0956
- 28. Kroiss M, Quinkler M, Johanssen S, et al Sunitinib in refractory adrenocortical carcinoma: a phase II, single-arm, open-label trial. J Clin Endocrinol Metab. 2012;97(10):3495–3503. https://doi.org/10.1210/jc.2012-1419
- 29. Kroiss M, Megerle F, Kurlbaum M, et al Objective response and prolonged disease control of advanced adrenocortical carcinoma with cabozantinib. J Clin Endocrinol Metab. 2020;105(5):1461–1468. https://doi.org/10.1210/clinem/dgz318
- 30. Altieri B, Ronchi CL, Kroiss M, Fassnacht M. Next-generation therapies for adrenocortical carcinoma. Best Pract Res Clin Endocrinol Metab. 2020;34(3):101434. https://doi.org/10.1016/j.beem.2020.101434
- 31. Moolenaar AJ, van Seters AP. O, p'-DDD values in plasma and tissue during and after chemotherapy of adrenocortical carcinoma. Acta Endocrinol. 1975;199:226.
- 32. Fassnacht M, Johanssen S, Quinkler M, et al Limited prognostic value of the 2004 international union against cancer staging classification for adrenocortical carcinoma: proposal for a revised TNM classification. Cancer. 2009;115(2):243–250. https://doi.org/10.1002/cncr.24030
- 33. US Food and Drug Administration CfDEaR. Guidance for industry: bioavailability and bioequivalence studies for orally administered drug products—general considerations. https://www.gmp-compliance.org/files/guidemgr/ucm154838.pdf.
- 34. Filipsson H, Monson JP, Koltowska-Haggstrom M, Mattsson A, Johannsson G. The impact of glucocorticoid replacement regimens on metabolic outcome and comorbidity in hypopituitary patients. J Clin Endocrinol Metab. 2006;91(10):3954–3961. https://doi.org/10.1210/jc.2006-0524
- 35. Bornstein SR, Allolio B, Arlt W, et al Diagnosis and treatment of primary adrenal insufficiency: an Endocrine Society clinical practice guideline. J Clin Endocrinol Metab. 2016;101(2):364–389. https://doi.org/10.1210/jc.2015-1710
- 36. Pofi R, Feliciano C, Sbardella E, et al The short synacthen (corticotropin) test can be used to predict recovery of hypothalamo-pituitary-adrenal axis function. J Clin Endocrinol Metab. 2018;103(8):3050–3059. https://doi.org/10.1210/jc.2018-00529
- 37. Hurtado MD, Cortes T, Natt N, Young WF Jr., Bancos I. Extensive clinical experience: hypothalamic-pituitary-adrenal axis recovery after adrenalectomy for corticotropin-independent cortisol excess. Clin Endocrinol (Oxf). 2018;89(6):721–733. https://doi.org/10.1111/cen.13803
- 38. Colling C, Nachtigall L, Biller BMK, Miller KK. The biochemical diagnosis of adrenal insufficiency with modern cortisol assays: reappraisal in the setting of opioid exposure and hospitalization. Clin Endocrinol (Oxf). 2022;96(1):21–29. https://doi.org/10.1111/cen.14587
- 39. Al-Aridi R, Abdelmannan D, Arafah BM. Biochemical diagnosis of adrenal insufficiency: the added value of dehydroepiandrosterone sulfate measurements. Endocr Pract. 2011;17(2):261–270. https://doi.org/10.4158/EP10262.RA
- 40. He X, Findling JW, Auchus RJ. Glucocorticoid withdrawal syndrome following treatment of endogenous Cushing syndrome. Pituitary. 2022;25(3):393–403. https://doi.org/10.1007/s11102-022-01218-y
- 41. Aribas E, Roeters van Lennep JE, De Rijke YB, et al Sex steroids and sex steroid-binding globulin levels amongst middle-aged and elderly men and women from general population. Eur J Clin Invest. 2022;52(12):e13866. https://doi.org/10.1111/eci.13866
- 42. Tang J, Chen LR, Chen KH. The utilization of dehydroepiandrosterone as a sexual hormone precursor in premenopausal and postmenopausal women: an overview. Pharmaceuticals (Basel). 2021;15(1):46. https://doi.org/10.3390/ph15010046