Introduction
Benign paroxysmal positional vertigo (BPPV), first described by Bárány, is the most common cause of vertigo. The definite etiology of BPPV is still unclear. It was reported that advanced age, head trauma, inactivity, viral labyrinthitis, and ischemia of the anterior vestibular artery may dispose individuals to BPPV. The major pathophysiological process in BPPV involves displaced otoconia from the macula of the utricular otolith which drop in the semicircular canals. When the position of the head changes relative to the direction of gravity, the otolithic debris will move to a new position within the semicircular canals, causing a false sense of rotation. Typical BPPV manifests as recurring transient positional-related vertigo and torsional or horizontal nystagmus on provocative head motion. Nearly 94% of BPPV cases affects the posterior semicircular canals (PSCs), while the horizontal semicircular canal is the next most common. The diagnosis of BPPV depends on the patient’s history and the characteristics of nystagmus on positional testing. In many cases, BPPV settles spontaneously within a few weeks. It was revealed that more than 95% of cases can be cured by canalith repositioning therapy (CRT). Although BPPV generally responds well to CRT, there is a high recurrence rate after the initial resolution. It was reported that 10% to 18% patients relapse during a 1-year follow-up period. Brandt suggested that relapse rate can be as high as 50% in 10 years and in addition, most recurrences are observed during the first year after the CRM. Several studies have demonstrated that age, family history, diabetes and hypertension, comorbidities, delayed BPPV treatment using CRT, multiple canal involvement, endolymphatic hydrops, bone mineral density (BMD), and serum vitamin D levels, may be associated with the recurrence of BPPV. However, the results are conflicting. Thus, in this study, we try to identify the factors associated with recurrent BPPV.
Methods
We carried out a systematic review and meta-analysis to test the association between potential risk factors and recurrent BPPV along with a protocol developed in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA).
Literature Search
A systematic literature search of PubMed, the Cochrane Library, Web of Science, Embase, Chinese National Knowledge Infrastructure (CNKI) and Sino Med was conducted to identify relevant studies published from database inception until November 3, 2019. The search strategy is detailed in Supplementary 1. All considered studies were imported into a reference management software (Endnote X·8·0·0) and duplicate publications were deleted.
Study Selection
All search results were reviewed by the lead author (Li) who read study titles to remove any clearly nonrelevant articles based on the inclusion and exclusion criteria listed below. The remaining abstracts were read and judged as possibly relevant based on inclusion and exclusion criteria. All studies deemed to be possibly relevant were read in full and independently judged against inclusion and exclusion criteria of the individual phase by 2 reviewers (Li and Wang). Disputes were resolved by consensus and consultation when necessary with a third reviewer (Zheng). Hand searches of the references from all the included studies were conducted to identify studies missed in the bibliographic searches.
Inclusion and Exclusion Criteria
Studies were included if they satisfied the following PICO(S) (participant, intervention, comparator, outcome [study design]) criteria:
Participant: adults aged 18 years and older, and BPPV were diagnosed according to guideline.
Intervention: assessment of the risk factors of BPPV patients and recurrent BPPV patients, for example, gender, age, the affected side, smoking, alcohol, hyperlipidemia, diabetes mellitus, hypertension, migraine, cervical spondylosis, complication, osteopenia/osteoporosis, ischemic heart disease, and so on.
Comparator: comparison groups of recurrence and non-recurrence.
Outcome: Recurrence of BPPV was used as an outcome data on the risk factors for recurrence of BPPV reported as odds ratios (ORs) with 95% CI or equivalent.
Study design: observational studies including cohort, case–control, or cross-sectional studies.
Studies were excluded if the following criteria were met:
If they lacked a control group or if they were reviews, theoretical research, systematic reviews and/or meta-analysis, conference reports, expert comments, or economic analysis and case reports.
Chapters from a book
Duplication of results
Studies with too little information or unclear data description
Animal studies
Data Extraction
Two reviewers (Li and Wang) independently selected studies on the basis of inclusion and exclusion criteria. Disparities in selection were resolved through discussion and ultimately by a third reviewer (Zheng). Studies were initially reviewed on the basis of title and abstract, and those deemed relevant were reviewed in full text to establish the final set of studies ultimately included. From these studies, data were abstracted in duplicate (by Li and Wang) to verify the accuracy. The following data were independently extracted by 2 researchers: first author, year of publication, location of study, type of study design, population size (recurrence/non-recurrence), sample ages, follow-up time, risk factors, and other relevant information. We also contacted the authors about unclear or missing information when necessary.
Quality Assessment
Risk of bias assessment was analyzed for each article by 2 investigators (Li and Wang) using the Newcastle-Ottawa Scale (NOS), which is a scale for quality assessment of nonrandomized studies in meta-analyses, and disagreement was resolved by discussion. The NOS is based on an accumulative score in each of 3 categories: selection, comparability, and exposure or outcome. The NOS scores range between 0 and 9 stars. Studies with 6 to 9 stars were considered to be at low risk of bias (ROB), studies with 4 to 5 stars were considered to be at medium ROB, and studies with 1 to 3 stars were considered to be at high ROB. Detailed results of the NOS quality appraisal are summarized in Table 1.
Statistical Analysis
Studies evaluating the risk factors for recurrent BPPV were included in a meta-analysis if relevant outcomes were reported in at least 2 articles. Dichotomous data were summarized using OR and 95% CI. Continuous data were summarized using mean difference (MD) and 95% CI. Statistical significance was set at .05. Included studies were then tested for heterogeneity using the χ2 test with significance set at P < .10 and the amount of heterogeneity was quantified by the I2 statistic as follows: (1) low 25%, (2) moderate 50%, and (3) high 75%. Where statistical heterogeneity (I2) was >50%, a random effects model was used. Where statistical heterogeneity (I2) was <50%, a fixed-effects model was used. Where meta-analysis was not possible, a narrative summary of study results is provided. Publication bias was visually evaluated using a funnel plot and Harbord test. Meta-analyses were conducted where feasible for each risk factor using Stata SE (version 15.0) software.
Results
Study Selection
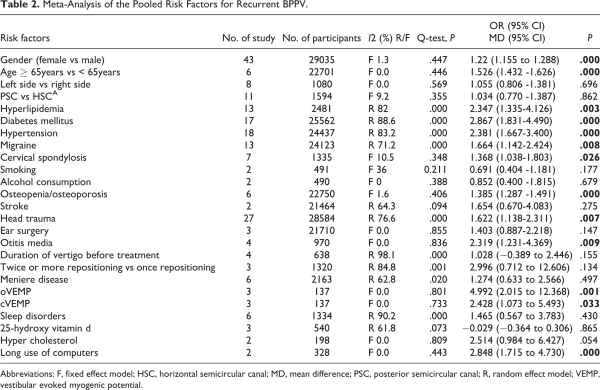
According to the search strategy, 4076 records were initially identified, in which 2036 articles were excluded because of duplication and 1736 articles were excluded after screening of abstract. After further evaluation of remaining 99 full-text studies, sixty eligible studies with 36 646 patients were finally included in this meta-analysis.-,,,,- A PRISMA flow diagram were presented to describe the detail selection process (Figure 1).
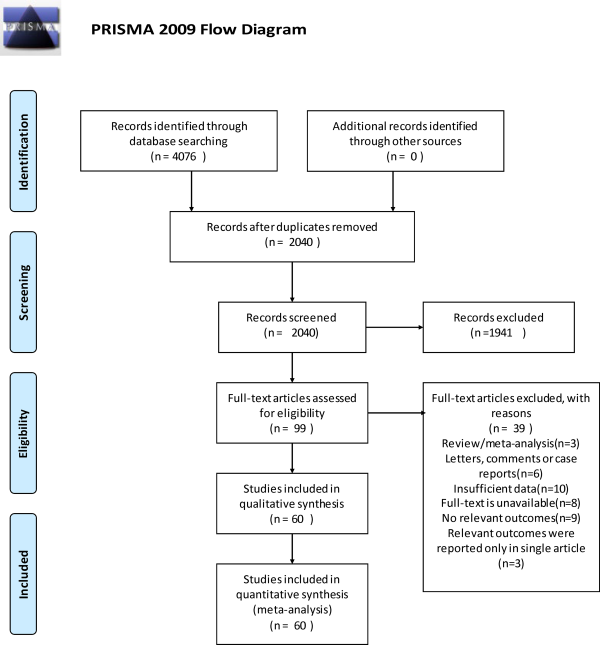
Figure 1
Flow diagram of the literature search.
Study Characteristics
Sixty-three studies examined 24 risk factors for recurrence of BPPV, and they included 36 646 participants. Twenty-three were retrospective cohort studies, and 37 were retrospective case–control studies. The number of patients in the included studies ranged from 42 to 21355 (Table 1). Studies were conducted in Israel (4), Korea (11), Japan (4), Germany (1), Egypt (2), India (1), Italy (2), United States (4), Uruguay (1), Croatia (1), Greece (1), Turkey (1), Serbia (1), and China (26; Table 1).
Quality Assessment
All 60 articles were observational studies. Based on the NOS, 50 studies were deemed to have high quality, while 10 studies were judged to be of moderate quality (Table 1). Supplementary Table S1 provides an overview of the results of the risk of bias assessments.
Findings
Thirty risk factors were identified. Thirteen of these were found to be risk factors for recurrence of BPPV: gender, age, hyperlipidemia, diabetes, hypertension, migraine, cervical spondylosis, osteopenia/osteoporosis, trauma, otitis media, ocular vestibular evoked myogenic potential (oVEMP), cervical vestibular evoked myogenic potential (cVEMP), and long use of computers. Side, type of the involved semicircular canals, smoking, alcohol consumption, stroke, ear surgery, duration of vertigo before treatment, the times of repositioning, Meniere disease, sleep disorders, hypercholesterolemia, and 25-hydroxy vitamin D did not correlate with BPPV recurrence (Table 2).
Gender
Forty-three studies provided data on gender, and they included 29 035 patients. Female are more likely to relapse than men (OR = 1.22, 95% CI: 1.155-1.288). We did not find significant heterogeneity among these studies (I2 = 1.3%, P = .000) in a fixed-effects model (Figure 2).
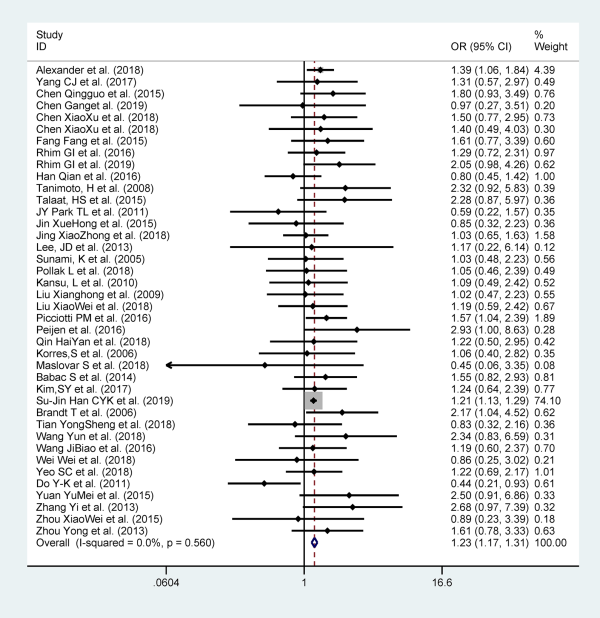
Figure 2
Forest plot of association between gender and recurrent BPPV. BPPV indicates benign paroxysmal positional vertigo.
Age
Six studies examined age as a risk factor for recurrence of BPPV, and they included 22 701 participants. Patients older than 65 years are more likely to relapse when compared to patients <65 years (OR: 1.526; 95% CI: 1.432-1.626, I2 = 0%, P = .000; Figure 3).
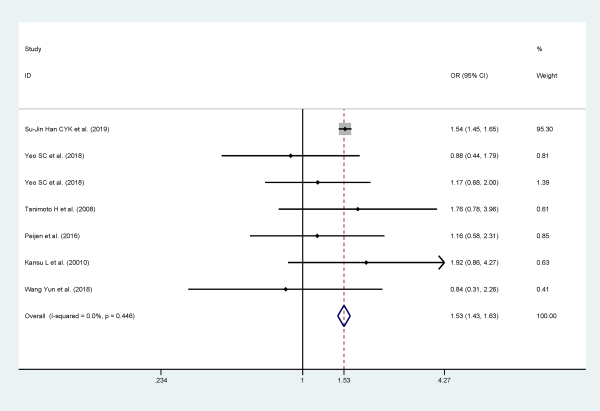
Figure 3
Forest plot of association between age (≥65 years or <65 years) and recurrent BPPV. BPPV indicates benign paroxysmal positional vertigo.
Hyperlipidemia
A high heterogeneity was detected (I2 = 82%, P = .003) to analyze data from 13 studies and a random effects model was applied. Overall results indicate that patients with hyperlipidemia are twice as likely to have recurrence BPPV when compared with patients without hyperlipidemia (OR 2.347, 95% CI: 1.335-4.126). As the heterogeneity was significant, we tried to seek heterogeneity sources through stratification analysis. The results showed that area and study design were not sources of heterogeneity. Stratified analysis based on follow-up time can significantly reduce heterogeneity (Figure 4).
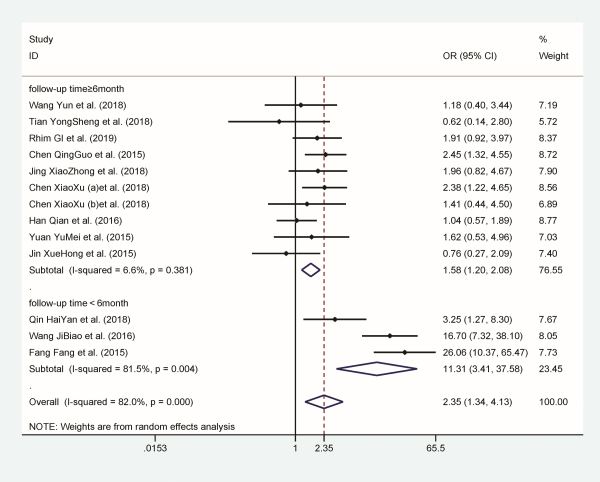
Figure 4
Forest plot of association between hyperlipidemia and recurrent BPPV. BPPV indicates benign paroxysmal positional vertigo.
Diabetes mellitus
Seventeen studies reported diabetes mellitus as a risk factor for recurrent BPPV, and they included 25 562 participants. Patients with diabetes mellitus are more likely to relapse when compared to patients without diabetes mellitus (OR 2.867; 95% CI: 1.831-4.490). The analysis was performed with a random effects model for the evidence of I2 = 88.6% and P = .000. Similarly, in order to investigate potential sources of heterogeneity, we performed subgroup analysis by contrasting the area, study design, and follow-up time of included studies. The results showed neither area nor study design could significantly reduce heterogeneity, except follow-up time (Figure 5).
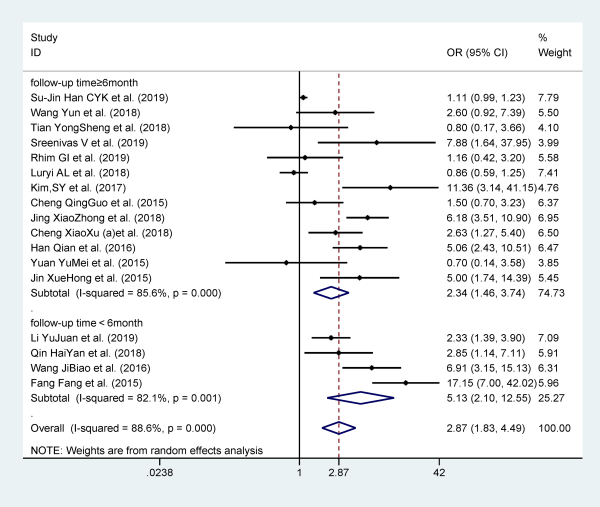
Figure 5
Forest plot of association between diabetes and recurrent BPPV. BPPV indicates benign paroxysmal positional vertigo.
Hypertension
Eighteen studies reported hypertension as a risk factor for recurrent BPPV, and they included 24 437 participants. Patients with hypertension are more likely to relapse when compared to patients without hypertension (OR: 2.381; 95% CI: 1.667-3.400). However, statistically significant heterogeneity was observed among these results (I2 = 83.2%, P = .000), which are shown in Figure 6. Stratified analysis based on follow-up time can significantly reduce heterogeneity.
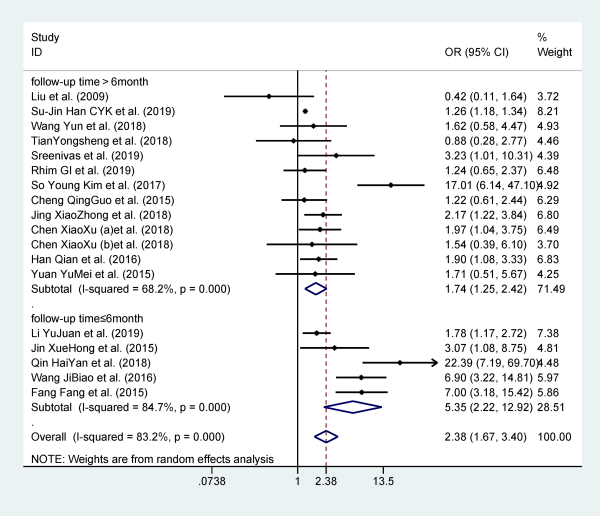
Figure 6
Forest plot of association between hypertension and recurrent BPPV. BPPV indicates benign paroxysmal positional vertigo.
Migraine
Thirteen studies reported migraine as a risk factor for recurrent BPPV, and they included 24 123 participants. Patients with migraine are more likely to relapse when compared to patients without migraine (OR 1.664; 95% CI: 1.142-2.424). The analysis was performed with a random effects model for the evidence of I2 = 71.2% and P = .008. Stratified analysis based on follow-up time can significantly reduce heterogeneity (Figure 7).
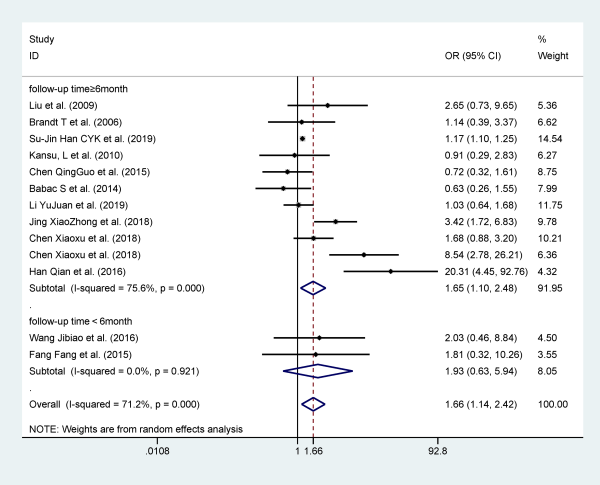
Figure 7
Forest plot of association between migraine and recurrent BPPV. BPPV indicates benign paroxysmal positional vertigo.
Cervical spondylosis
Seven studies reported cervical spondylosis as a risk factor for recurrent BPPV, including 1335 participants, and a fixed effects model was applied (I2 = 10.5%, P = 0.026). Patients with cervical spondylosis are more likely to relapse when compared to patients without cervical spondylosis (OR: 1.37; 95% CI: 1.038-1.803; Figure 8).
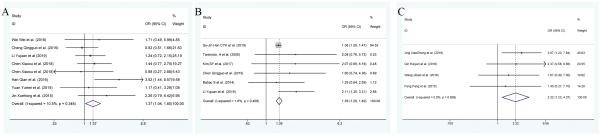
Figure 8
Forest plot of association between risk factors and recurrent BPPV. A, Cervical spondylosis. B, Osteoporosis. C, Otitis media. BPPV indicates benign paroxysmal positional vertigo.
Osteopenia/osteoporosis
Six studies provided data on osteoporosis, and they included 22 750 patients. Patients with osteoporosis are more likely to relapse when compared to patients without osteoporosis (OR 1.385; 95% CI 1.287-1.491). No significant heterogeneity was found across the results (I2 = 1.6%, P = .000), which are shown in Figure 8.
Head trauma
Twenty-seven studies reported head trauma as a risk factor for recurrent BPPV, and they included 28 584 participants. Patients with head trauma are more likely to relapse when compared to patients without head trauma (OR: 1.622; 95% CI: 1.138-2.311, I2 = 76.6%, P = .007, random effect model). Stratified analysis based on area can significantly reduce heterogeneity (Figure 9).
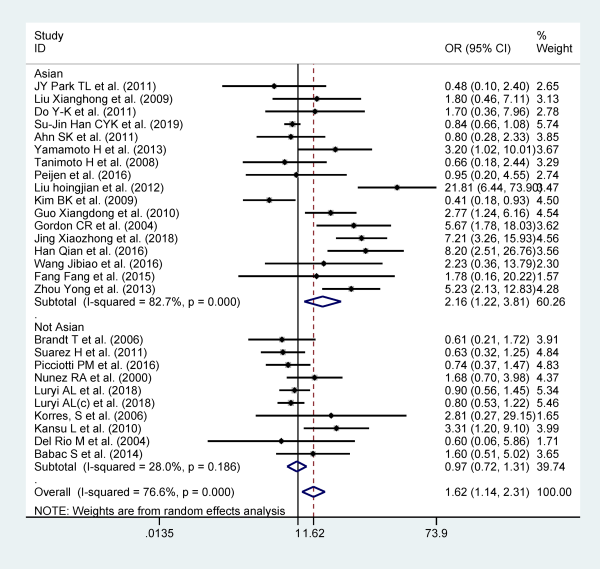
Figure 9
Forest plot of association between head trauma and recurrent BPPV. BPPV indicates benign paroxysmal positional vertigo.
Otitis media
Four studies reported otitis media as a risk factor for recurrent BPPV, and they included 970 participants. Patients with otitis media are more likely to relapse when compared to patients without otitis media (OR: 2.319; 95% CI: 1.231-4.369) with no evidence of heterogeneity in fixed effect mode (I2 = 0.0%; P = .836; Figure 8).
Vestibular evoked myogenic potential
Three studies reported abnormal VEMP as a risk factor for recurrent BPPV, and they included 137 participants. Patients with abnormal VEMP are more likely to relapse when compared to patients with normal VEMP (oVEMP: OR = 4.992; 95% CI: 2.015-12.368, I2 = 0.0%, P = .801; cVEMP: OR = 2.428; 95% CI: 1.073-5.493, I2 = 0.0%, P = .733), with no evidence of heterogeneity in fixed effect model (Figure 10).

Figure 10
Forest plot of association between risk factors and recurrent BPPV. A, oVEMP; (B) cVEMP; (C) long use of computers. BPPV indicates benign paroxysmal positional vertigo; VEMP, vestibular evoked myogenic potential.
Long use of computers
Two studies reported long use of computers as a risk factor for recurrent BPPV, and they included 328 participants. Patients who use computers for a long time are more likely to relapse when compared to patients who don’t (OR = 2.848; 95% CI: 1.715-4.730) with no evidence of heterogeneity in fixed effect mode (I2 = 0.0%, P = .443; Figure 10).
Meniere disease
Six studies reported Meniere’s disease as a risk factor for recurrent BPPV, and they included 2163 participants. There aren’t association between Meniere’s disease and recurrence of BPPV (OR = 1.274; 95% CI: 0.633-2.566). Moderate heterogeneity was found in a random effects model (I2 = 62.8% and P = .020; Figure 11).
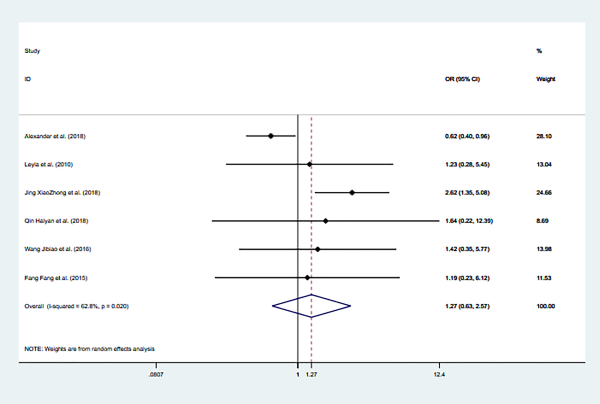
Figure 11
Forest plot of association between Meniere’s and recurrent BPPV.
Risk factors not associated with recurrent BPPV
The meta-analysis of side, type of the involved semicircular canals, smoking, alcohol consumption, stroke, ear surgery, duration of vertigo before treatment, the times of repositioning, hypercholesterolemia, sleep disorders, and 25-hydroxy vitamin D which did not correlate with recurrent BPPV were presented in supplement materials.
Sensitivity Analyses
Because of the heterogeneity between some studies, the results of meta-analysis may have changed significantly. We used sensitivity analysis to verify the reliability of the meta-analysis finding in this study. Statistical analysis of studies on hyperlipidemia, diabetes, hypertension, migraine, Meniere’s disease, and head trauma showed that the results of the meta-analysis did not change after each study was excluded. This indicated that the meta-analysis had good stability, and the results of the meta-analysis were reliable (Figure 12–16).
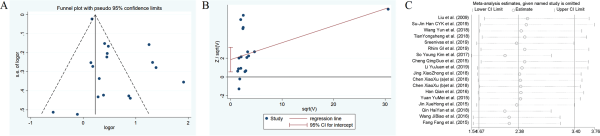
Figure 12
The funnel plots, Harbord test, sensitivity analysis of hypertension.
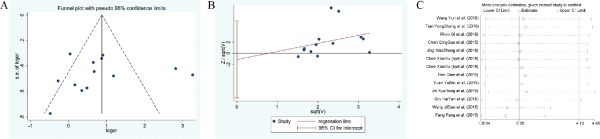
Figure 13
The funnel plots, Harbord test, sensitivity analysis of hyperlipidemia.
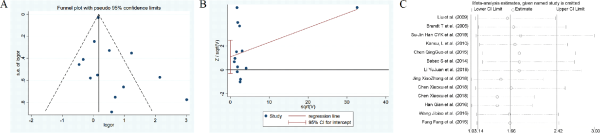
Figure 14
The funnel plots, Harbord test, sensitivity analysis of migraine.
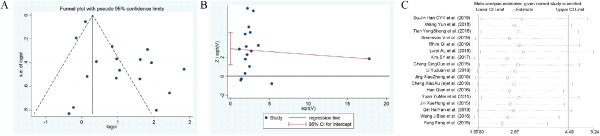
Figure 15
The funnel plots, Harbord test, sensitivity analysis of diabetes.
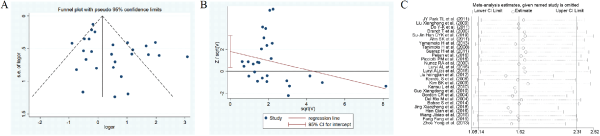
Figure 16
The funnel plots, Harbord test, sensitivity analysis of head trauma.
Subgroup Analyses
Studies on hyperlipidemia, diabetes, hypertension, migraine, and head trauma showed high heterogeneity. We searched for the source of heterogeneity of these studies through subgroup analysis and meta-regression. The included studies were divided into 2 groups according to the region, follow-up period (≤6 months or >6 months), and study design. We also analyzed the findings of this study by performing meta-regression. Follow-up time has a statistically significant contribution to the heterogeneity in the analysis of hyperlipidemia, diabetes, hypertension, migraine (P < .05), area, and heterogeneity in the analysis of head trauma (P < .05), indicating that follow-up time and area may be the source of heterogeneity in the study, but it cannot explain total heterogeneity
Assessment of Publication Bias
To determine the potential publication bias of the literature, we performed Harbord test. The publication bias was significant for hypertension, head trauma, diabetes (P = .009, P = .015, P = .002). We did not find any existence of publication bias in the results of hyperlipidemia, migraine, and Meniere’s disease (P = .724, P = .114, P = .290; Figure 11).
Discussion
This systematic review and meta-analysis provide comprehensive data on the risk factors contributing to recurrent BPPV. We find female gender, age (≥65years), hyperlipidemia, diabetes, hypertension, migraine, cervical spondylosis, osteopenia/osteoporosis, trauma, otitis media, abnormal VEMP, and long use of computers are risk factors for recurrence of BPPV, and there is no association between side, type of the involved semicircular canals, smoking, alcohol consumption, stroke, ear surgery, duration of vertigo before treatment, the times of repositioning, Meniere’s disease, sleep disorders, 25-hydroxy vitamin D,and the recurrence of BPPV.
Although paroxysmal positional vertigo is the most frequently observed pathology in otoneurological clinical practice, a definitive causative factor has not been identified yet. There are 2 main hypotheses to explain the development of BPPV. Schuknecht proposed the cupulolithiasis theory, which is based on the attachment of otolithic debris to the cupula in the crista ampullaris. Hall et al proposed the theory of canalithiasis, which is based on the presence of free-floating debris in the canal. Both these theories indicate that the presence of foreign particles in the semicircular canal may be the cause of vertigo. How these particles detach from utricle is still unclear. When position changes, particles move in the endolymph with the help of gravity and thus cause abnormal displacement of the cupula and aberrant neural stimulation, producing vertigo.
Some studies showed no correlation between gender, age, and recurrent BPPV.,,, However, Chau et al and von Brevern M et al reported that age and gender were related to recurrent BPPV., Our study showed that female and older patients (>65 years) have a higher level of relapses of BPPV (OR = 1.22, P = .000; OR = 1.526, P = .000). A menopause-related reduction in the secretion of female hormones may be involved in this phenomenon. A decrease in estrogen secretion may affects calcium/bone metabolism. Calcium metabolism also plays a chief role in the synthesis/absorption of otoconia made of calcium carbonate and so might be an etiological factor in the onset and recurrence of BPPV., Additionally, it is believed that during lifetime the number and volume of otoliths are progressively reduced and the interconnecting fibers between the otoliths may weaken from age-related reduction of calcium carbonate crystals in the process of demineralization. It was also supposed that age-related altered endolymphatic pH and calcium concentration may contribute to the pathogenesis of BPPV and worsen the symptoms.
The posterior SCC is the more frequently involved SCC in the pathogenesis of BPPV. The explanation for this is the anatomical location of the SCC at the most dependent position in the vestibular apparatus in a standing person. Von Brevern et al found BPPV predominantly affects the right labyrinth, and the probably reason is the habit—of most patients—of sleeping on the right side. Recent studies showed that side and semicircular canal attacked were not associated with recurrent BPPV.,, Our meta-analysis results also showed that the recurrence of BPPV wasn’t related to side and semicircular canal attacked (P > .05).
Increasing studies have proposed that systemic disease, including hyperlipidemia, hypertension, diabetes, cervical spondylosis, and stroke, could also increase the recurrence of BPPV.,,,,,, We conducted a meta-analysis of these indicators, and hyperlipidemia, hypertension, diabetes, and cervical spondylosis showed statistical significance (P < .05). This may be related to the known effect of hypertension and vascular disease on the inner ear, causing reduced blood flow to the labyrinth and contributing to dislodgement of otoliths and manifestation of BPPV. In addition to vascular damage, diabetes generates a balance disorder mediated by variations of blood glucose and plasma insulin levels with subsequent cupular deposits and free-floating debris in the semicircular canals. In a case–control histopathologic study of temporal bones in humans, the authors compared the prevalence of cupular and free-floating otoconia in the SCCs between the temporal bones of patients with type 1 diabetes and normal controls. They found that the presence of otoconia in the horizontal and posterior SCC was significantly higher in the patient group than in the control group. It has been reported that the occurrence of BPPV was higher in people with type 2 diabetes (46%) compared to those without the metabolic disease (37%). In a recent report, Jáuregui-Renaud et al studied the function of horizontal SCCs and utricle in patients with type 2 diabetes, who did not have any history of dizziness or other vestibular symptoms. The authors found that the patients had downsized responses to unilateral centrifugation compared to healthy volunteers, but their responses to horizontal SCC stimulation were similar and they had also a larger sway area and a lengthier sway path in the test of posturography. They assumed that utricular function may be defective even in the absence of horizontal SCC involvement or a history of falls. Similarly, in a postmortem study of temporal bones, containing 1031 semicircular canals, basophilic deposits (supposedly degenerated otoconia) were observed in 22% of the semicircular canal cupulae. Interestingly, none of those cases had a history of BPPV. It has been suggested that the size of the debris within the semicircular canal needs to reach a critical level before aberrant neural stimulation and BPPV symptoms develop., Circulation to the inner ear is from the vertebrobasilar system, primarily the anterior inferior cerebellar artery, which branches into the anterior vestibular artery. Its anatomical location within the inner ear causes the labyrinth particularly vulnerable to ischemia. The association between recurrent and stroke has been proposed by Su-Jin Han et al. Wada et al used carotid ultrasonography to evaluate the intima–media thickness of the common carotid artery in patients with BPPV and vestibular hypofunction. The study found that the percentage of abnormal intima–media thickness of common carotid arteries was significantly higher in patients with BPPV than those with other vestibular disorders. A study by Zhang et al also admitted increased abnormalities in the vertebrobasilar arteries of BPPV patients. The severity of vertigo was correlated with vertebral artery stenosis, occlusion, or tortuosity. These reports demonstrated that patients with BPPV had a higher prevalence of arteries injury. It is believed that BPPV can be a sequela to labyrinthine ischemia that probably facilitates detachment of otoconia from the otolith membrane. Additionally, diabetes mellitus or hyperuricemia can sometimes lead to metabolic acidosis in the blood and result in low pH in the endolymphatic fluids, which will facilitate the breakdown of calcium carbonate otoliths., Theoretically, age, hyperlipidemia, hypertension, diabetes, and stroke all serve as vascular risk factors and one could wonder that the common predisposing factor of BPPV might be ischemia. Yet we did not find an association between BPPV and other well established vascular risk factors such as stroke, smoking, and alcohol consumption (P > .05).
Back in 2003, Vibert et al proposed a connection between BPPV, osteoporosis, and osteopenia. Since then another independent group also noted that bone metabolism has a connection to BPPV. Moreover, there was a study demonstrating that the treatment of osteoporosis may have a protective effect against BPPV. Recent research brought to light the impact of the vitamin D levels on the BPPV with decreased levels being associated with its occurrence and more frequent recurrence.,, Recent studies demonstrated a possible seasonality to BPPV, with fewer cases occurring during the summer months., Theories behind this trend propose calcium homoeostasis to play a key role as serum Vitamin D levels surge with increasing daylight. However, Korpon et al suggests that the variable with the single strongest correlation between BPPV and seasonal variations is not sunlight or UV index, but rather barometric pressure. Yet, the correlative evidence may be weak because recent evidence suggests such associations to be purely coincidental. Yang et al evaluated the relationship between BMD and Vitamin D with the presence and recurrence of BPPV. The authors found that low BMD in women and low serum Vitamin D levels in men were significantly associated with the recurrence of BPPV, whereas age was an independent predictor of recurrence. In a rat model with impaired calcium turnover, due to the bilateral ovariectomy, the density of otoconia was decreased and their size was increased compared to the normal group. Our results indicate that osteoporosis/osteopenia was contributors to the recurrence of BPPV, while vitamin D was not.
Our analysis found that migraine is a risk factor for BPPV recurrence. Migraine and BPPV are among the most frequently encountered diseases in otoneurological clinics. The pathophysiologic mechanisms of migraine is still not clear, several studies have hypothesized that genetic and vascular factors and cortical spreading depression may be the underlying mechanism.- The mechanism for the vestibular symptoms and signs associated with migraine is poorly understood. Vasospasm of the labyrinthine arteries is a possible mechanism because vasospasm is a well-documented phenomenon with migraine. Repeated vasospasm may stress and injury the vestibular cells, inducing the dropping of otoconia from the macula. In addition, elimination of the inner-ear microvasculature as a result of vasospasm may generate cochlear symptoms, such as hearing disturbance and vestibular symptoms. Additionally, recurrent vasospasm is associated with the oxidative stress of endothelial cells, which is a possible pathogenetic mechanism common to both migraine and BPPV., The specific pathway for oxidative stress in migraine has not yet been acknowledged, but several studies have announced a reduction in superoxide dismutase activity in patients with migraine.- In a study of oxidative stress associated with BPPV, levels of pro-inflammatory mediators, such as interleukin 1β (IL-1β), IL-6, and tumor necrosis factor, were raised in the serum of patients experiencing a BPPV attack and declined after repositioning maneuvers such as the Epley maneuver. In addition, total antioxidant capacity and paraoxonase levels, which are antioxidant parameters, were decreased during a BPPV attack. Therefore, oxidative stress and inflammatory processes in the inner ear may be connected with the formation and migration of the otolith. Probably, patients with migraine have recurrent damage to the inner ear (due to vasospasm or some other mechanism) that predisposes them to recurrent bouts of BPPV.
The relationship between Meniere’s disease and BPPV is complex. The incidence of coexistence of BPPV and Meniere’s disease ranges from 0.5% to 44%., Predominance of ipsilateral existence of BPPV and Meniere’s disease have been reported in the majority of studies, which indicates a probably causal relationship between these 2 disorders. There is radiological evidence that detached saccular otoconia may cause obstruction of the reuniting duct and result in endolymphatic hydrops. The association between BPPV and Meniere’s disease has been confirmed by histopathologic temporal bone studies reporting significantly higher incidence of cupular or free-floating deposits in SCCs in patients with Meniere’s disease than in controls. Additionally, repeated episodes of hydrops may eventually generate sacculoutricular degeneration and detachment of the otoconia. Patients with unilateral hearing loss generally would like to sleep lying on the ear with hearing loss to keep the better hearing ear in the open environment. Shim et al and Sato et al have announced that sleeping habit is closely related with the effected side in BPPV. Otoconial debris dislodged from the utricle may fall into the lateral or PSCs of the undermost ear during sleep. This may account for more the common lateral canal involvement and ipsilateral occurrence of Meniere’s disease and BPPV. Dornhoffer and Colvin suggested that Meniere’s disease was associated with recurrent BPPV. Tanimoto et al reviewed risk factors in recurrent BPPV. They found that 75% of them have endolymphatic hydrops and all were in the same ear with BPPV. However, in a larger series, Luryi et al reported that there was no association between Meniere’s disease and recurrence of BPPV, which was keeping in line with our analysis result (P > .05). Gross et al noted that BPPV in Meniere’s disease was poorly response to repositioning maneuvers. This phenomenon could be explained by several potential mechanisms. Anatomical changes of labyrinth due to hydrops probably are a main reason for intractability of BPPV in Meniere’s disease. Partial obstruction of the posterior SCC by a dilated saccule could also result in adherence of otoliths to the membranous labyrinth. Partial obstruction allows otoliths to move within canals and keeps them from returning to vestibules. Stricture of the membranous labyrinth resulting from loss of its resilience after repeated distension due to endolymphatic hydrops may be another cause because the membrane may collapse inward and lead to severe narrowing of the SCC.
Approximately 13% of patients with traumatic brain injury report positional vertigo, and half of these patients have BPPV. When canalolithiasis occurs briefly after an impulsive head trauma (rapid head deceleration), a causative relationship may be suggested. When compared with idiopathic BPPV (i-BPPV), traumatic BPPV (t-BPPV) was associated with several poor prognostic features, including a higher rate of bilateral disease and a higher rate of recurrence., A subsequent whiplash injury might be responsible of the otoconia detachment, and microscopic hemorrhages, or “tissue shearing,” results in biochemical changes that boost the formation of otoconial clots. Following a successful maneuver, these microscopic changes may reactivate the production of new clots, explaining the recurrence of BPPV. While, in a large series, there was no important differences in outcomes were revealed, with the t-BPPV group having rates of resolution and recurrence similar to those in the i-BPPV group and requiring a similar number of treatment visits. Our result find that head trauma is a risk factor for recurrent BPPV and there is a high heterogeneity. This may be the result of lacking definite criteria for traumatic BPPV. According to Riga et al, for a causative association to be valid, BPPV should occur on the same side as the causative disease and the clinical symptoms should appear either concurrently or soon after the manifestation of the primary disease. Studies that specifically mention BPPV as one of the possible causes of posttraumatic dizziness or vertigo are lacking diagnostic clarity.
Dysfunction of the otoliths has been a suspected pathogenesis of BPPV. There have been some reports of VEMP abnormalities in patients with BPPV.- In explaining the pathophysiology of BPPV, the concept of a degenerative process that affects the macula of the utricle causing detachment of otoliths has gained popular support. Nonetheless, there were only a few reports of association between recurrence of BPPV and VEMP test. In our analysis, there was only 3 paper providing these data, and we find abnormal VEMP is a risk factor for the recurrence of BPPV, which is in accord with the findings by Lee et al. In BPPV, the degenerative process that affects the macula of the utricle and causes detachment of the otoliths might also affect the macula of the saccule.
Studies have found that otoliths can easily fall off the otoconial membrane and cause BPPV when exposed to inflammation, infection, or low pH. Ca2+ concentration in the endolymph may be increased due to recurrent inflammation, which will destroying the normal equilibrium between otolith formation and dissolution, and thus the likelihood of BPPV increases significantly. Otitis media and long use of computers are also risk factors for relapse in our analysis, but only 4 and 2 studies were included, and the quality of the studies was not high. Therefore, the results should be interpreted with caution. More high-quality studies are needed in the future to confirm these findings.
Strengths and Limitations
Our systematic review has several strengths. This is the first systematic review to comprehensively investigate the risk factors for recurrent BPPV. We used a strict search strategy to screen 6 databases with no language restrictions, including PubMed, Embase, Web of Science, Cochrane Library, CNKI, and Sino Med. Based on the NOS, 51 of 63 included studies scored ≥6 stars, which suggested high-quality studies. We included a range of publications involving different ethnic populations from across the world to ensure the applicability of our findings and to investigate a wide range of risk factors for recurrent BPPV. Furthermore, we also determined the heterogeneity between the studies included in subgroup analysis and found that most factors showed low levels of heterogeneity. Our sensitivity analysis showed that the sequential omission of a single study did not significantly influence the observed results and the magnitude of effects.
There are some important limitations to this systematic review that need to be considered. First, some risk factors (hyperlipidemia, diabetes, hypertension, migraine, head trauma, MD) have high degree of heterogeneity between the studies. These may be explained by differences in the criteria used to define recurrence, head trauma, and follow-up time. For example, the studies that specifically mention BPPV as one of the possible causes of posttraumatic dizziness or vertigo are lacking diagnostic clarity. Therefore, we should treat these results with some caution. Second, according to the figures (Figures 2 and 3), a paper was weighted (%) very highly and thus might have caused a bias. So we reanalyze the results after excluding this article. The results of reanalysis revealed that gender remains a risk factor for recurrent BPPV, while age is not. It implied that the biased weights of this paper might distort the results. Third, some publications involved in our meta-analysis were of low quality. Considering the limited number of studies for some factors (for example stroke), the accuracy and validity of the relationship between these factors and recurrent BPPV may also be questionable. Besides, because of the intrinsic differences in the design of included studies, such as study design, duration of follow-up, and so on, potential bias could not be completely ruled out. Therefore, the results should be interpreted carefully. Last but not least, some studies had fewer cases, and the negative results may not have been published. In terms of the assessment of publication bias, there were apparent publication bias with respect to hypertension, head trauma, and diabetes when using the Harbord test. All of these factors may have led to bias. Although our study had good stability, it cannot be entirely excluded potential bias. More high-quality studies are needed in the future to confirm some of these findings.
Conclusions
This systematic review and meta-analysis evaluated the risk factors for recurrent BPPV. Female, age, migraine, head trauma, otitis media, abnormal VEMP, long use of computers, hyperlipidemia, diabetes, hypertension, osteopenia/osteoporosis, and cervical spondylosis were associated with recurrent BPPV. Identification of these risk factors provides some insight into the falls risk evaluation and contributes to help clinicians counsel patients regarding the importance of follow-up after diagnosis of BPPV. Because of the limited quality and quantity of the included studies, rigorous studies with adequate sample sizes are needed to verify the conclusion.
Acknowledgments
The authors thank all the people for their work in the literature collecting, manuscript compiling, and their help with this work. The authors would like to express our gratitude to the reviewers and editors.
Authors’ Note Li, Wang, and Cao designed the study, reviewed the literature, conducted the statistical analysis, drafted the manuscript, and discussed the manuscript. Li, Liu, Zheng, Han, and Jing generated summary tables and edited pictures. Li, Ma, and Xia significantly contributed to the study design. Li, Yu contributed to the embellishment of language and revision of the manuscript.
Declaration of Conflicting Interests The author(s) declared the following potential conflicts of interest with respect to the research, authorship, and/or publication of this article: The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Shichang Li
https://orcid.org/0000-0003-1084-1252
Lisheng Yu
https://orcid.org/0000-0002-5482-6954
Supplemental Material Supplemental material for this article is available online.
References
- 1. Bárány E. Diagnose yon krankheitserscheinungen im bereiche des otolithenapparates. Acta Oto-Laryngologica. 1920;2(3):434–437. doi:10.3109/00016482009123103
- 2. Lemajic-Komazec S, Komazec Z. Initial evaluation of vertigo. Medicinski pregled. 2006;59(11-12):585–590.doi:10.2298/mpns0612585 l
- 3. Steenerson RL, Cronin GW, Marbach PM. Effectiveness of treatment techniques in 923 cases of benign paroxysmal positional vertigo. Laryngoscope. 2005;115(2):226–231. doi:10.1097/01.mlg.0000154723.55044.b5
- 4. Kim JS, Zee DS. Clinical practice. Benign paroxysmal positional vertigo. N Engl J Med. 2014;370(12):1138–1147. doi:10.1056/NEJMcp1309481
- 5. Honrubia V, Baloh RW, Harris MR, Jacobson KM. Paroxysmal positional vertigo syndrome. Am J Otol. 1999;20(4):465–470.
- 6. Riggio F, Dispenza F, Gallina S, Kulamarva G, Gargano R, Speciale R. Management of benign paroxysmal positional vertigo of lateral semicircular canal by Gufoni’s manoeuvre. Am J Otolaryngol. 2009;30(2):106–111. doi:10.1016/j.amjoto.2008.03.001
- 7. Dorigueto RS, Mazzetti KR, Gabilan YPL, Ganança FF. Benign paroxysmal positional vertigo recurrence and persistence. Brazil J Otorhinol. 2009;75(4):565–572. doi:10.1016/S1808-8694(15)30497-3
- 8. Casani AP, Cerchiai N, Navari E. Paroxysmal positional vertigo despite complete vestibular impairment: the role of instrumental assessment. Acta Otorhinolaryngol Ital. 2018;38(6):563–568. doi:10.14639/0392-100x-1549
- 9. Sakaida M, Takeuchi K, Ishinaga H, Adachi M, Majima Y. Long-term outcome of benign paroxysmal positional vertigo. Neurology. 2003;60(9):1532–1534. doi:10.1212/01.wnl.0000061477.03862.4d
- 10. Brandt T, Huppert D, Hecht J, Karch C, Strupp M. Benign paroxysmal positioning vertigo: a long-term follow-up (6-17 years) of 125 patients. Acta Oto-Laryngologica. 2006;126(2):160–163. doi:10.1080/00016480500280140
- 11. Do YK, Kim J, Park CY, Chung MH, Moon IS, Yang HS. The effect of early canalith repositioning on benign paroxysmal positional vertigo on recurrence. Clin Exp Otorhinol. 2011;4(3):113–117. doi:10.3342/ceo.2011.4.3.113
- 12. Liu X, Li G. [Clinical study of benign paroxysmal positional vertigo recurrence]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2009;23(7):304–306.
- 13. Kim SY, Han SH, Kim YH, Park MH. Clinical features of recurrence and osteoporotic changes in benign paroxysmal positional vertigo. Auris Nasus Larynx. 2017;44(2):156–161. doi:10.1016/j.anl.2016.06.006
- 14. De Stefano A, Dispenza F, Suarez H, et al. A multicenter observational study on the role of comorbidities in the recurrent episodes of benign paroxysmal positional vertigo. Auris Nasus Larynx. 2014;41(1):31–36. doi:10.1016/j.anl.2013.07.007
- 15. Perez P, Franco V, Cuesta P, Aldama P, Alvarez MJ, Mendez JC. Recurrence of benign paroxysmal positional vertigo. Otol Neurotol. 2012;33(3):437–443. doi:10.1097/MAO.0b013e3182487f78
- 16. Tanimoto H, Doi K, Nishikawa T, Nibu KI. Risk factors for recurrence of benign paroxysmal positional vertigo. J Otolaryngol Head Neck Surg. 2008;37(6):832–835. doi:10.2310/7070.2008.OA0188
- 17. Yamanaka T, Shirota S, Sawai Y, Murai T, Fujita N, Hosoi H. Osteoporosis as a risk factor for the recurrence of benign paroxysmal positional vertigo. Laryngoscope. 2013;123(11):2813–2816. doi:10.1002/lary.24099
- 18. Talaat HS, Abuhadied G, Talaat AS, Abdelaal MSS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Otorhinolaryngol. 2015;272(9):2249–2253. doi:10.1007/s00405-014-3175-3
- 19. Bhattacharyya N, Baugh RF, Orvidas L, et al. Clinical practice guideline: benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2008;139(5 Suppl 4):S47–S81. doi:10.1016/j.otohns.2008.08.022
- 20. Deeks JJ, Dinnes J, D’Amico R, et al. Evaluating non-randomised intervention studies. Health Technol Assess (Winchester, England). 2003;7(27):iii-x, 1–173. doi:10.3310/hta7270
- 21. Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ (Clin Res ed). 2003;327(7414):557–560. doi:10.1136/bmj.327.7414.557
- 22. Nunez RA, Cass SP, Furman JM. Short- and long-term outcomes of canalith repositioning for benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg. 2000;122(5):647–652. doi:10.1016/s0194-5998(00)70190-2
- 23. Del Rio M, Arriaga MA. Benign positional vertigo: prognostic factors. Otolaryngol Head Neck Surg. 2004;130(4):426–429. doi:10.1016/j.otohns.2003.12.015
- 24. Gordon CR, Levite R, Joffe V, Gadoth N. Is posttraumatic benign paroxysmal positional vertigo different from the idiopathic form? Arch Neurol. 2004;61(10):1590–1593. doi:10.1001/archneur.61.10.1590
- 25. Pollak L, Kushnir M, Shpirer Y, Zomer Y, Flechter S. Approach to benign paroxysmal positional vertigo in old age. Israel Med Assoc J. 2005;7(7):447–450.
- 26. Sunami K, Tochino R, Tokuhara Y, et al. Factors associated with recurrence of BPPV. Equilibrium Res. 2005;64(2):64–70. doi:10.3757/jser.64.64
- 27. Korres S, Balatsouras DG, Ferekidis E. Prognosis of patients with benign paroxysmal positional vertigo treated with repositioning manoeuvres. J Laryngol Otol. 2006;120(7):528–533. doi:10.1017/s0022215106000958
- 28. Pollak L, Stryjer R, Kushnir M, Flechter S. Approach to bilateral benign paroxysmal positioning vertigo. Ame J Otolaryngol Head Neck Med Surg. 2006;27(2):91–95. doi:10.1016/j.amjoto.2005.07.012
- 29. Kao CL, Hsieh WL, Chern CM, Chen LK, Lin MH, Chan RC. Clinical features of benign paroxysmal positional vertigo (BPPV) in Taiwan: differences between young and senior age groups. Arch Gerontol Geriat. 2009;49(Suppl 2):S50–S54. doi:10.1016/s0167-4943(09)70014-7
- 30. Kim BK, Kim DE, Han JH. The clinical characteristics and treatment outcome of post-traumatic BPPV. J Neurol. 2009;256:S200. doi:10.1007/s00415-009-5161-z
- 31. Guo X, Ye F, Zhang Z, Li Y, Yang X. Posttraumatic benign paroxysmal positional vertigo: analysis of 23 cases. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2010;24(16):738–739, 742.
- 32. Kansu L, Avci S, Yilmaz I, Ozluoglu LN. Long-term follow-up of patients with posterior canal benign paroxysmal positional vertigo. Acta Otolaryngol. 2010;130(9):1009–1012. doi:10.3109/00016481003629333
- 33. Ahn SK, Jeon SY, Kim JP, et al. Clinical characteristics and treatment of benign paroxysmal positional vertigo after traumatic brain injury. J Trauma. 2011;70(2):442–446. doi:10.1097/TA.0b013e3181d0c3d9
- 34. Suarez H, Alonso R, Arocena M, Suarez A, Geisinger D. Clinical characteristics of positional vertigo after mild head trauma. Acta Otolaryngol. 2011;131(4):377–381. doi:10.3109/00016489.2010.534113
- 35. JY Park TL, DS Jeong KBS. Relationship between clinical features and recurrence in benign paroxysmal positional vertigo of posterior semicircular canal. Res Vestib Sci. 2011;10(2):63–67.
- 36. Liu H. Presentation and outcome of post-traumatic benign paroxysmal positional vertigo. Acta Oto-Laryngologica. 2012;132(8):803–806. doi:10.3109/00016489.2012.657359
- 37. Zhang Z, Zhang R. Clinical study of benign paroxysmal positional vertigos relevant diseases and otolith repositioning efficacy. J Apoplex Nerv Dis. 2012;29(7):648–650.
- 38. Lee JD, Park MK, Lee BD, Lee TK, Sung KB, Park JY. Abnormality of cervical vestibular-evoked myogenic potentials and ocular vestibular-evoked myogenic potentials in patients with recurrent benign paroxysmal postitional vertigo. Acta Otolaryngol. 2013;133(2):150–153. doi:10.3109/00016489.2012.723823
- 39. Yamamoto H, Sunami K, Yamane H. Clinical characteristics of posttraumatic benign paroxysmal positional vertigo. Otolaryngol Head Neck Surg (United States). 2013;149(2):P218–P219. doi:10.1177/0194599813496044a232
- 40. Zhang Yi ZY, Liu BO. Analysis of influencing factors of benign paroxysmal positional vertigo recurrence. Chinese J Stroke. 2013;8(05):351–355.
- 41. Zhou Yong GX, Li S, Xiang Yang Z. Analysis of recurrence of benign paroxysmal positional vertigo and its influencing factors. J Pract Med. 2013;29(15):2509–2511.
- 42. Babac S, Djeric D, Petrovic-Lazic M, Arsovic N, Mikic A. Why do treatment failure and recurrences of benign paroxysmal positional vertigo occur? Otol Neurotol. 2014;35(6):1105–1110. doi:10.1097/mao.0000000000000417
- 43. Chen Q, Wang X, Mao Z, Zheng Y, Liu J, Peng L. Causes of the recurrence of benign, paroxysmal positional vertigo. Chine J Phys Med Rehab. 2015;37(9):683–685.
- 44. Fang Fang DX, Jiang H. Analysis of risk factors for recurrence of benign paroxysmal positional vertigo by manual reduction. Clin Med China. 2015;31(3):211–214.
- 45. Talaat HS, Abuhadied G, Talaat AS, Abdelaal MS. Low bone mineral density and vitamin D deficiency in patients with benign positional paroxysmal vertigo. Eur Arch Otorhinolaryngol. 2015;272(9):2249–2253. doi:10.1007/s00405-014-3175-3
- 46. Wang Chunyan XF, Wang Y, Wang N, et al. Analysis of factors affecting the recurrence of benign paroxysmal positional vertigo in elderly patients. Chine J Ger. 2015;34(7):796–799.
- 47. Xuehong J. Influencing factors of recurrence of benign paroxysmal positional vertigo. Pract J Card Cereb Pneum Vasc Dis. 2015;(9):68–70.
- 48. Yuan Yumei HR, Jiao Y. Analysis of related factors of benign paroxysmal positional vertigo recurrence. Chine J Prac Ner Dis. 2015;(7):97–98.
- 49. Zhou X, Yu Y, Wu Z, Liu X, Chen X. [The roles of otolith organs in the recurrence primary benign paroxysmal positional vertigo]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2015;29(18):1641–1644.
- 50. Ji-biao W. Analysis of Influencing factors on recurrence of benign paroxysmal positional vertigo after repositioning maneuver. Neural Injury Function Recon. 2016;11(2):141–144. doi:10.16780/j.cnki.sjssgncj.2016.02.015
- 51. Lv Xiaoyu JY, Zhao X. Clinical characteristics and outcome of benign paroxysmal positional vertigo. Chinese J Stroke. 2016;11(12):1023–1029. doi:10.3969/j.issn.1673-5765.2016.12.006
- 52. Picciotti PM, Lucidi D, De Corso E, Meucci D, Sergi B, Paludetti G. Comorbidities and recurrence of benign paroxysmal positional vertigo: personal experience. Int J Audiol. 2016;55(5):279–284. doi:10.3109/14992027.2016.1143981
- 53. Qian H. Clinical Features and Reccurent Risk Factors Analysis of Benign paroxysmal Positional Vertigo. Hebei Medical University; 2016.
- 54. Rhim GI. Long-term outcomes of canalith repositioning for benign paroxysmal positional vertigo: Kaplan-Meier estimate. Res Vestib Sci. 2016;15(1):17–21.
- 55. Su P, Liu YC, Lin HC. Risk factors for the recurrence of post-semicircular canal benign paroxysmal positional vertigo after canalith repositioning. J Neurol. 2016;263(1):45–51. doi:10.1007/s00415-015-7931-0
- 56. Talaat HS, Kabel AM, Khaliel LH, Abuhadied G, El-Naga HA, Talaat AS. Reduction of recurrence rate of benign paroxysmal positional vertigo by treatment of severe vitamin D deficiency. Auris Nasus Larynx. 2016;43(3):237–241. doi:10.1016/j.anl.2015.08.009
- 57. Tirelli G, Nicastro L, Gatto A, Tofanelli M. Repeated canalith repositioning procedure in BPPV: effects on recurrence and dizziness prevention. Ame J Otol. 2017;38(1):38–43. doi:10.1016/j.amjoto.2016.09.009
- 58. Yang CJ, Kim Y, Lee HS, Park HJ. Bone mineral density and serum 25-hydroxyvitamin D in patients with idiopathic benign paroxysmal positional vertigo. J Vestib Res. 2017;27(5-6):287–294. doi:10.3233/ves-170625
- 59. Chen XX, Jin ZG, Xu XR, Zhang Y, Wang CL. Risk factors for recurrence of benign paroxysmal positional vertigo in different age groups. Med J Air Force. 2018;34(2):123–126. doi:10.3969/j.issn.2095-3402.2018.02.015
- 60. Liu XW, Sun JW. [Risk factors analysis for recurrence of the idiopathic benign paroxysmal positional vertigo]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32(15):1185–1187. doi:10.13201/j.issn.1001-1781.2018.15.014
- 61. Luryi AL, Larouere MJ, Babu S, et al. Traumatic versus idiopathic benign paroxysmal positional vertigo: analysis of patient, treatment, and outcome characteristics. Otolaryngol Head Neck Surg (United States). 2018;159(1):P142–P143. doi:10.1177/0194599818785627f
- 62. Luryi AL, Lawrence J, Bojrab D, et al. Patient, disease, and outcome characteristics of benign paroxysmal positional vertigo with and without Meniere’s disease. Acta Otolaryngol. 2018;138(10):893–897. doi:10.1080/00016489.2018.1484566
- 63. Luryi AL, Lawrence J, Bojrab DI, et al. Recurrence in benign paroxysmal positional vertigo: a large, single-institution study. Otol Neurotol. 2018;39(5):622–627. doi:10.1097/mao.0000000000001800
- 64. Maslovar S, Soldo SB, Sestak A, Milinkovic K, Rogic-Namacinski J, Soldo A. 25 (OH) D3 levels, incidence and recurrence of different clinical forms of benig paroxysmal positional vertigo. Braz J Otorhinolaryngol. 2018;84(4):453–459. doi:10.1016/j.bjorl.2017.05.007
- 65. Pollak L, Huna-Baron R, Osherov M, Roni M. In whom does horizontal canal BPPV recur? Ame J Otol. 2018;39(4):410–412. doi:10.1016/j.amjoto.2018.04.003
- 66. Qin Haiyan ZM, Cai H. Analysis of influencing factors for recurrence of manual paroxysmal positional vertigo. J Clin Res. 2018;35(6):1186–1188.
- 67. Tian YS, Wang SZ, Liu Y, Wang D, Guo LR. [Clinical features of the recurrence of idiopathic benign paroxysmal positional vertigo]. Lin Chung Er Bi Yan Hou Tou Jing Wai Ke Za Zhi. 2018;32(2):118–121. doi:10.13201/j.issn.1001-1781.2018.02.010
- 68. Wang Y, Xia F, Wang W, Hu W. Assessment of sleep quality in benign paroxysmal positional vertigo recurrence. Int J Neurosci. 2018;128(12):1143–1149. doi:10.1080/00207454.2018.1486835
- 69. Wei W, Sayyid ZN, Ma X, Wang T, Dong Y. Presence of anxiety and depression symptoms affects the first time treatment efficacy and recurrence of benign paroxysmal positional vertigo. Front Neurol. 2018;9:178. doi:10.3389/fneur.2018.00178
- 70. Yeo SC, Ahn SK, Lee HJ, et al. Idiopathic benign paroxysmal positional vertigo in the elderly: a long-term follow-up study. Aging Clin Exp Res. 2018;30(2):153–159. doi:10.1007/s40520-017-0763-2
- 71. Chen G, Zhao X, Yu G, Jian H, Li Y, Xu G. Otolith dysfunction in recurrent benign paroxysmal positional vertigo after mild traumatic brain injury. Acta Oto-Laryngologica. 2019;139(1):18–21. doi:10.1080/00016489.2018.1562214
- 72. Li Yujuan LP. Analysis of clinical characteristics of patients with benign paroxysmal positional vertigo of different genders. Neural Injury Function Recons. 2019;14(3):156–158. doi:10.16780/j.cnki.sjssgncj.2019.03.017
- 73. Martellucci S, Attanasio G, Ralli M, et al. Does cervical range of motion affect the outcomes of canalith repositioning procedures for posterior canal benign positional paroxysmal vertigo? Am J Otolaryngol. 2019;40(4):494–498. doi:10.1016/j.amjoto.2019.04.003
- 74. Rhim GI. Serum vitamin d and long-term outcomes of benign paroxysmal positional vertigo. Clin Exp Oto. 2019;12(3):273–278. doi:10.21053/ceo.2018.00381
- 75. Sayal NR, Cox EL, Foster N, Globerson M, Farrugia M. Analysis of patients diagnosed with benign paroxysmal positional vertigo and the corresponding incidence and patterns of electric toothbrush use. Cureus. 2019;11(9):E15. doi:10.7759/cureus.5697
- 76. Sreenivas V, Sima NH, Philip S. The role of comorbidities in benign paroxysmal positional vertigo. Ear Nose Throat J. 2019:145561319878546. doi:10.1177/0145561319878546
- 77. Su-Jin Han CYK, Dae Bo S, Mee Hyun S. Analysis of risk factors for recurrence of benign paroxysmal positional vertigo: an 11-year nationwide population-based study. Korean J Otorhinol Head Neck Surg. 2019;62(1):15–22. doi:10.3342/kjorl-hns.2017.00899
- 78. Xiaozhong J. Clinical Features and Recurrent Risk Factors Analysis of 569 patients with Benign Paroxysmal Positional Vertigo. Jilin University; 2019.
- 79. Strupp M, Arbusow V. Acute vestibulopathy. Curr Opin Neurol. 2001;14(1):11–20. doi:10.1097/00019052-200102000-00003
- 80. Schuknecht HF. Cupulolithiasis. Arch Otol. 1969;90(6):765–778. doi:10.1001/archotol.1969.00770030767020
- 81. Hall SF, Ruby RR, McClure JA. The mechanics of benign paroxysmal vertigo. J Otol. 1979;8(2):151–158.
- 82. Chau AT, Menant JC, Hubner PP, Lord SR, Migliaccio AA. Prevalence of vestibular disorder in older people who experience dizziness. Front Neurol. 2015;6:268. doi:10.3389/fneur.2015.00268
- 83. von Brevern M, Radtke A, Lezius F, et al. Epidemiology of benign paroxysmal positional vertigo: a population based study. J Neurol Neuro Psychiatry. 2007;78(7):710–715. doi:10.1136/jnnp.2006.100420
- 84. Vibert D, Kompis M, Hausler R. Benign paroxysmal positional vertigo in older women may be related to osteoporosis and osteopenia. Ann Otol Rhinol Laryngol. 2003;112(10):885–889. doi:10.1177/000348940311201010
- 85. Riggs BL, Khosla S, Melton LJ 3rd. A unitary model for involutional osteoporosis: estrogen deficiency causes both type I and type II osteoporosis in postmenopausal women and contributes to bone loss in aging men. J Bone Miner Res. 1998;13(5):763–773. doi:10.1359/jbmr.1998.13.5.763
- 86. Hughes I, Thalmann I, Thalmann R, Ornitz DM. Mixing model systems: using zebrafish and mouse inner ear mutants and other organ systems to unravel the mystery of otoconial development. Brain Res. 2006;1091(1):58–74. doi:10.1016/j.brainres.2006.01.074
- 87. Campos A, Crespo PV, Garcia JM, Sanchez-Quevedo MC, Ciges M. The crystalline pattern of calcium in different topographical regions of the otoconial membrane. an electron probe and spectroscopic diffraction study. Acta Otolaryngol. 1999;119(2):203–206. doi:10.1080/00016489950181675
- 88. Balatsouras DG, Koukoutsis G, Fassolis A, Moukos A, Apris A. Benign paroxysmal positional vertigo in the elderly: current insights. Clin Int Aging. 2018;13:2251–2266. doi:10.2147/CIA.S144134
- 89. Korres S, Balatsouras DG, Kaberos A, Economou C, Kandiloros D, Ferekidis E. Occurrence of semicircular canal involvement in benign paroxysmal positional vertigo. Otol Neurotol. 2002;23(6):926–932. doi:10.1097/00129492-200211000-00019
- 90. von Brevern M, Seelig T, Neuhauser H, Lempert T. Benign paroxysmal positional vertigo predominantly affects the right labyrinth. J Neurol Neurosurg Psychiatry. 2004;75(10):1487–1488. doi:10.1136/jnnp.2003.031500
- 91. Cohen HS, Kimball KT, Stewart MG. Benign paroxysmal positional vertigo and comorbid conditions. ORL. 2004;66(1):11–15. doi:10.1159/000077227
- 92. D’Silva LJ, Staecker H, Lin J, et al. Retrospective data suggests that the higher prevalence of benign paroxysmal positional vertigo in individuals with type 2 diabetes is mediated by hypertension. J Vestib Res. 2016;25(5-6):233–239. doi:10.3233/VES-150563
- 93. Yoda S, Cureoglu S, Yildirim-Baylan M, et al. Association between type 1 diabetes mellitus and deposits in the semicircular canals. Otolaryngol Head Neck Surg. 2011;145(3):458–462. doi:10.1177/0194599811407610
- 94. Jauregui-Renaud K, Aranda-Moreno C, Herrera-Rangel A. Utricular hypofunction in patients with type 2 diabetes mellitus. Acta Otorhinolaryngol Ital. 2017;37(5):430–435. doi:10.14639/0392-100x-1243
- 95. Moriarty B, Rutka J, Hawke M. The incidence and distribution of cupular deposits in the labyrinth. Laryngoscope. 1992;102(1):56–59. doi:10.1288/00005537-199201000-00011
- 96. Lopez-Escamez JA, Gamiz MJ, Finana MG, Perez AF, Canet IS. Position in bed is associated with left or right location in benign paroxysmal positional vertigo of the posterior semicircular canal. Am J Otolaryngol. 2002;23(5):263–266. doi:10.1053/ajot.2002.124199
- 97. Wada M, Naganuma H, Tokumasu K, Hashimoto S, Ito A, Okamoto M. Arteriosclerotic changes as background factors in patients with peripheral vestibular disorders. Int Tinnitus J. 2008;14(2):131–134.
- 98. Zhang D, Zhang S, Zhang H, et al. Evaluation of vertebrobasilar artery changes in patients with benign paroxysmal positional vertigo. Neuroreport. 2013;24(13):741–745. doi:10.1097/WNR.0b013e328364b948
- 99. Hemenway WG, Lindsay JR. Postural vertigo due to unilateral sudden partial loss of vestibular function. Ann Otol Rhinol Laryngol. 1956;65(3):692–706. doi:10.1177/000348945606500311
- 100. Yang X, Yang B, Wu M, et al. Association between serum uric acid levels and benign paroxysmal positional vertigo: a systematic review and meta-analysis of observational studies. Front Neurol. 2019;10:91. doi:10.3389/fneur.2019.00091
- 101. Celikbilek A, Gencer ZK, Saydam L, Zararsiz G, Tanik N, Ozkiris M. Serum uric acid levels correlate with benign paroxysmal positional vertigo. Euro J Neurol. 2014;21(1):79–85. doi:10.1111/ene.12248
- 102. Jeong SH, Choi SH, Kim JY, Koo JW, Kim HJ, Kim JS. Osteopenia and osteoporosis in idiopathic benign positional vertigo. Neurology. 2009;72(12):1069–1076. doi:10.1212/01.wnl.0000345016.33983.e0
- 103. Mikulec AA, Kowalczyk KA, Pfitzinger ME, Harris DA, Jackson LE. Negative association between treated osteoporosis and benign paroxysmal positional vertigo in women. J Laryn Otol. 2010;124(4):374–376. doi:10.1017/S002221510999209X
- 104. Jeong S-H, Kim J-S, Shin JW, et al. Decreased serum vitamin D in idiopathic benign paroxysmal positional vertigo. J Neurol. 2013;260(3):832–838. doi:10.1007/s00415-012-6712-2
- 105. Buki B, Ecker M, Junger H, Lundberg YW. Vitamin D deficiency and benign paroxysmal positioning vertigo. Med Hypo. 2013;80(2):201–204. doi:10.1016/j.mehy.2012.11.029
- 106. Saeed BMN, Omari AF. Climatic variations and benign paroxysmal positional vertigo. J Otol. 2016;11(1):33–37. doi:10.1016/j.joto.2016.03.002
- 107. Meghji S, Murphy D, Nunney I, Phillips JS. The seasonal variation of benign paroxysmal positional vertigo. Otol Neurotol. 2017;38(9):1315–1318. doi:10.1097/mao.0000000000001534
- 108. Korpon JR, Sabo RT, Coelho DH. Barometric pressure and the incidence of benign paroxysmal positional vertigo. Am J Otolaryngol. 2019;40(5):641–644. doi:10.1016/j.amjoto.2019.05.016
- 109. Karatas A, Acar Yuceant G, Yuce T, Haci C, Cebi IT, Salviz M. Association of benign paroxysmal positional vertigo with osteoporosis and vitamin d deficiency: a case controlled study. J Int Adv Otol. 2017;13(2):259–265. doi:10.5152/iao.2016.2640
- 110. Yang CJ, Kim Y, Lee HS, Park HJ. Bone mineral density and serum 25-hydroxyvitamin D in patients with idiopathic benign paroxysmal positional vertigo. J Vestib Res. 2018;27(5-6):287–294. doi:10.3233/ves-170625
- 111. Vibert D, Sans A, Kompis M, et al. Ultrastructural changes in otoconia of osteoporotic rats. Audio Neuro. 2008;13(5):293–301. doi:10.1159/000124277
- 112. Bahmad F Jr, DePalma SR, Merchant SN, et al. Locus for familial migrainous vertigo disease maps to chromosome 5q35. Ann Otol Rhinol Laryngol. 2009;118(9):670–676. doi:10.1177/000348940911800912
- 113. Amin FM, Asghar MS, Hougaard A, et al. Magnetic resonance angiography of intracranial and extracranial arteries in patients with spontaneous migraine without aura: a cross-sectional study. Lancet Neurol. 2013;12(5):454–461. doi:10.1016/s1474-4422(13)70067-x
- 114. Thomsen LL. Investigations into the role of nitric oxide and the large intracranial arteries in migraine headache. Cephalalgia. 1997;17(8):873–895. doi:10.1046/j.1468-2982.1997.1708873.x
- 115. Tepper D. Migraine associated vertigo. Headache. 2015;55(10):1475–1476. doi:10.1111/head.12704
- 116. Evans RW, Ishiyama G. Migraine with transient unilateral hearing loss and tinnitus. Headache. 2009;49(5):756–758. doi:10.1111/j.1526-4610.2008.01075.x
- 117. Neri M, Frustaci A, Milic M, et al. A meta-analysis of biomarkers related to oxidative stress and nitric oxide pathway in migraine. Cephalalgia. 2015;35(10):931–937. doi:10.1177/0333102414564888
- 118. Gucluturk MT, Unal ZN, Ismi O, Cimen MB, Unal M. The role of oxidative stress and inflammatory mediators in benign paroxysmal positional vertigo. J Int Adv Otol. 2016;12(1):101–105. doi:10.5152/iao.2015.1412
- 119. Ciancarelli I, Tozzi-Ciancarelli MG, Spacca G, Di Massimo C, Carolei A. Relationship between biofeedback and oxidative stress in patients with chronic migraine. Cephalalgia. 2007;27(10):1136–1141. doi:10.1111/j.1468-2982.2007.01398.x
- 120. Shimomura T, Kowa H, Nakano T, et al. Platelet superoxide dismutase in migraine and tension-type headache. Cephalalgia. 1994;14(3):215–218; discussion 181. doi:10.1046/j.1468-2982.1994.014003215.x
- 121. Tuncel D, Tolun FI, Gokce M, Imrek S, Ekerbicer H. Oxidative stress in migraine with and without aura. Biol Trace Element Res. 2008;126(1-3):92–97. doi:10.1007/s12011-008-8193-9
- 122. Ishiyama A, Jacobson KM, Baloh RW. Migraine and benign positional vertigo. Ann Otol Rhinol Laryngol. 2000;109(4):377–380. doi:10.1177/000348940010900407
- 123. Karlberg M, Hall K, Quickert N, Hinson J, Halmagyi GM. What inner ear diseases cause benign paroxysmal positional vertigo? Acta Oto-Laryngologica. 2000;120(3):380–385. doi:10.1080/000164800750000603
- 124. Katsarkas A, Kirkham TH. Paroxysmal positional vertigo--a study of 255 cases. J Otolaryngol. 1978;7(4):320–330.
- 125. Yetiser S. Co-existence of benign paroxysmal positional vertigo and Meniere’s syndrome. J Int Adv Otol. 2017;13(1):65–68. doi:10.5152/iao.2016.2906
- 126. Yamane H, Sunami K, Iguchi H, Sakamoto H, Imoto T, Rask-Andersen H. Assessment of Meniere’s disease from a radiological aspect - saccular otoconia as a cause of Meniere’s disease? Acta Otolaryngol. 2012;132(10):1054–1060. doi:10.3109/00016489.2012.680980
- 127. Morita N, Cureoglu S, Nomiya S, et al. Potential cause of positional vertigo in Meniere’s disease. Otol Neurotol. 2009;30(7):956–960. doi:10.1097/MAO.0b013e3181b24368
- 128. Shim DB, Kim JH, Park KC, Song MH, Park HJ. Correlation between the head-lying side during sleep and the affected side by benign paroxysmal positional vertigo involving the posterior or horizontal semicircular canal. Laryngoscope. 2012;122(4):873–876. doi:10.1002/lary.23180
- 129. Sato G, Sekine K, Matsuda K, Takeda N. Effects of sleep position on time course in remission of positional vertigo in patients with benign paroxysmal positional vertigo. Acta Otolaryngol. 2012;132(6):614–617. doi:10.3109/00016489.2012.655860
- 130. Dornhoffer JL, Colvin GB. Benign paroxysmal positional vertigo and canalith repositioning: clinical correlations. Am J Otol. 2000;21(2):230–233. doi:10.1016/s0196-0709(00)80014-9
- 131. Gross EM, Ress BD, Viirre ES, Nelson JR, Harris JP. Intractable benign paroxysmal positional vertigo in patients with Meniere’s disease. Laryngoscope. 2000;110(4):655–659. doi:10.1097/00005537-200004000-00022
- 132. Parnes LS, McClure JA. Free-floating endolymph particles: a new operative finding during posterior semicircular canal occlusion. Laryngoscope. 1992;102(9):988–992. doi:10.1288/00005537-199209000-00006
- 133. Motin M, Keren O, Groswasser Z, Gordon CR. Benign paroxysmal positional vertigo as the cause of dizziness in patients after severe traumatic brain injury: diagnosis and treatment. Brain Injury. 2005;19(9):693–697. doi:10.1080/02699050400013600
- 134. Dix MR, Hallpike CS. The pathology, symptomatology and diagnosis of certain common disorders of the vestibular system. Ann Otol Rhinol Laryngol. 1952;61(4):987–1016. doi:10.1177/000348945206100403
- 135. Katsarkas A. Benign paroxysmal positional vertigo (BPPV): idiopathic versus post-traumatic. Acta Otolaryngol. 1999;119(7):745–749. doi:10.1080/00016489950180360
- 136. Riga M, Bibas A, Xenellis J, Korres S. Inner ear disease and benign paroxysmal positional vertigo: a critical review of incidence, clinical characteristics, and management. Int J Otolaryngol. 2011;2011:709469. doi:10.1155/2011/709469
- 137. Hong SM, Park DC, Yeo SG, Cha CI. Vestibular evoked myogenic potentials in patients with benign paroxysmal positional vertigo involving each semicircular canal. Am J Otolaryngol. 2008;29(3):184–187. doi:10.1016/j.amjoto.2007.07.004
- 138. Yang WS, Kim SH, Lee JD, Lee WS. Clinical significance of vestibular evoked myogenic potentials in benign paroxysmal positional vertigo. Otol Neurotol. 2008;29(8):1162–1166. doi:10.1097/MAO.0b013e31818a0881
- 139. von Brevern M, Schmidt T, Schonfeld U, Lempert T, Clarke AH. Utricular dysfunction in patients with benign paroxysmal positional vertigo. Otol Neurotol. 2006;27(1):92–96. doi:10.1097/01.mao.0000187238.56583.9b
- 140. Han DG, Kim DJ. The evolutionary hypothesis of benign paroxysmal positional vertigo. Med Hypo. 2019;134:109445. doi:10.1016/j.mehy.2019.109445