Introduction
A physiological connection between the nose and the genitals has long been proposed., Wilhelm Fliess (1858-1928), an otolaryngologist practicing in Berlin, was Sigmund Freud’s (1856-1939) closest friend and confidant., The theory of “reflex nasal neurosis” was published by Fliess in 1897 postulating a physiological connection between the nose and the genitals., Specific “genital spots,” according to Fliess, located on the inferior nasal turbinates played an important role in the “naso-genital” relation. Freud and Fliess elaborated on this theory in letters over the next years. Sigmund Freud, who underwent inferior turbinate surgery twice by Fliess, even referred publicist Emma Eckstein for surgery, whom he diagnosed with “nasal reflex neurosis.” The surgery ended in a disaster, resulting in recurrent nasal bleeding and a disfigured nose., Bizarre theories of Fliess of neurosis never held any scientific validity., Reports of the naso-genital relationship has since diminished in the medical literature.
Parasympathetic and sympathetic nerves innervate the vasculature and glands of the nasal mucosa with opposing actions. Parasympathetic nerves and their neurotransmitters cause mucus secretion and/or vasodilatation. On the contrary, sympathetic nerves and sympathetic neurotransmitters have little effect on mucus secretion but constrict the blood vessels of the nasal mucosa. The opposing actions of these systems most likely determine the effect of nasal patency.,
It is known that physical exercise as well as hormonal changes can have an effect on nasal airway resistance in short and in long term.- However, there are no studies investigating the effect of sexual activity on nasal breathing. This study was conducted to examine the impact of sexual activity on nasal breathing and compare the effect to nasal decongestant. Is “love” all you need to improve nasal breathing?
Material and Methods
The study received ethical approval from the institutional review board of the University of Heidelberg (Ethics committee of the Medical Faculty of Heidelberg, reference number S-385/2020). All participants signed an informed consent.
Eighteen couples (each 1 male and 1 female) enrolled in this study resulting in 36 participants. The study was conducted in 2020. All participants were health care workers and/or partners of health care workers. For evaluation of subjective nasal breathing a visual analogue scale (VAS) from 0 (no impairment) to 10 (no nasal breathing possible) was applied. For objective data, nasal resistance and flow were measured with a portable rhinometric device (Rhinomanometer 300, ATMOS Medizintechnik). The measurements were obtained at the participants’ home by themselves. The VAS and the usage of the rhinometric device were explained in detail to the participants.
Assessments of nasal function were performed at 5 specific points namely (1) before sexual activity (baseline), (2) immediately after orgasm, (3) 30 minutes, (4) 1, and (5) 3 hours after sexual climax. “Immediately” was defined as within 1 minute after sexual climax. Climax in female and male is the “sudden discharge of accumulated sexual excitement during the sexual response cycle, resulting in rhythmic muscular contractions in the pelvic region characterized by sexual pleasure.” The data were only obtained if both individuals experienced sexual orgasm. Same data were collected for the study participants on the following day after application of nasal decongestant spray (0.1% xylometazoline; 1 application per side).
Nasal Obstruction Symptom Evaluation (NOSE) questionnaires were used to assess preexisting impairments of nasal function (range 0-100, lower scores indicate better nasal breathing). The NOSE questionnaire is a validated and reliable questionnaire developed by Stewart et al, consisting of 5 questions assessing nasal obstruction within the past month. Lipan et al showed that a NOSE score of 30 best differentiates between patients with or without nasal obstruction with higher scores indicating worse obstruction. All participants also underwent prior anterior rhinoscopy by an otolaryngologist. Participants with acute or chronic rhinitis/rhinosinusitis were excluded. None of the participants had undergone prior nasal surgery or showed signs of nasal polyps.
Statistical analysis was performed with an anonymized data set using the statistical software SPSS version 23.0.00 (IBM). The difference between mean scores before and after intervention and between the 2 interventions (“sex” and “spray”) were analyzed by a t test for dependent samples or in case of less than 16 data sets with Wilcoxon test. A difference was considered statistically significant with P ≤.05.
Results
Complete data of the VAS and the NOSE score were retrieved for all 36 participants, while rhinometric data were available for 16 participants (8 couples). Average age at the time of study conduction was 32.9 (SD ± 3.9; range 26-42) years, and average NOSE score was 47.4 (SD ± 20.8; range 15-90). All participants claimed to have achieved sexual climax.
Nasal Function Pre- and Post-Intervention
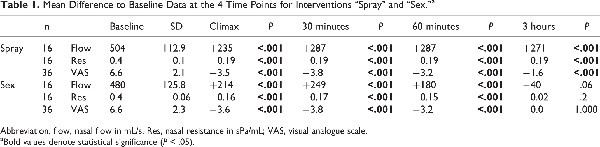
Participants showed an improved nasal breathing after sexual intercourse and after nasal spray application: mean rhinometric flow increased while resistance and VAS decreased immediately, 30 minutes, and 60 minutes post-intervention. Nasal breathing was back to the baseline level 3 hours after sexual intercourse, whereas it remained significantly improved 3 hours after application of nasal decongestant spray (Figure 1, Table 1).
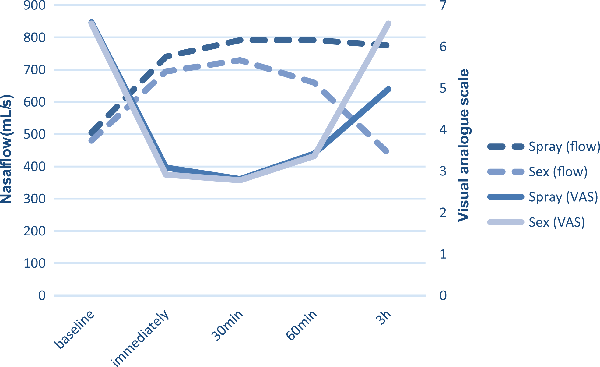
Figure 1
Nasal breathing measured by VAS and rhinometric flow before and after interventions (sexual activity and application of nasal decongestant spray). VAS indicates visual analogue scale.
Comparison at Each Time Point
Visual analogue scale scores were similar for both interventions before, immediately, 30, and 60 minutes after intervention as measured with the VAS. However, mean postcoital VAS scores were significantly higher compared to post-decongestant values at 3 hours post-intervention (P < .001; Figure 2).
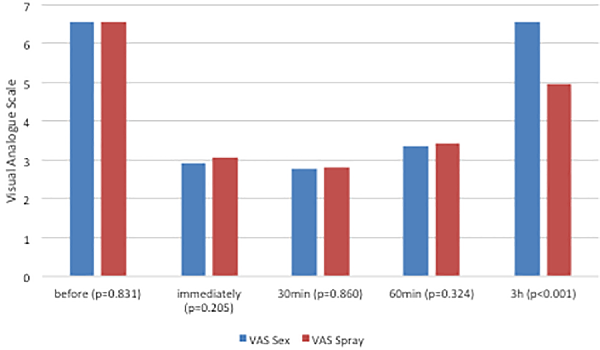
Figure 2
Comparison of nasal breathing at different time points (pre = baseline; immed = immediately after orgasm/decongestant; 30 = 30 minutes after orgasm/ decongestant; 60 = 60 minutes post orgasm/decongestant; 3 hours = 3 hours post orgasm/decongestant) measured with visual analogue scale (VAS).
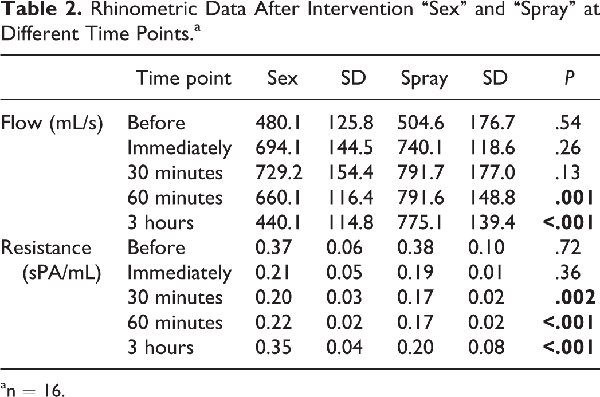
In the rhinometric data, there were no significant differences before and immediately after both interventions (Table 2). After 30 minutes, mean flow and resistance showed its peak indicating the least nasal obstruction. In the sex group, this effect decreased after 60 minutes and was back to the baseline 3 hours postcoital. In the spray group, nasal breathing remained improved after 3 hours.
In order to evaluate any influence from preexisting nasal function impairment, separate analyses were done for NOSE scores below and above 30 according to Lipan et al. A NOSE score of 30 best differentiates between patients with or without nasal obstruction with higher scores indicating worse obstruction. Nine participants had NOSE scores below 30. They showed significantly lower mean VAS scores (3.0) in the baseline measurement than the 27 persons above 30 (7.74; P < .001), indicating less nasal obstruction.
Improvements were more tempered in the group with a NOSE score below 30 (no nasal obstruction). In this group, the VAS improved slightly for both interventions immediately and 30 minutes post-intervention (from 3 to 2.7 to 2.3 for sex and 3.6 to 3.2 to 3.4 for spray). The VAS rose 1 and 3 hours after intervention (3.2 and 3.7 for both groups) indicating more nasal obstruction as shown in Figure 3. However, all improvements were not statistically significant (P > .05).
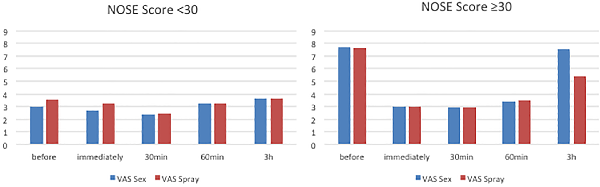
Figure 3
Average VAS score for patients with NOSE score under 30 (no nasal obstruction) and over 30 (nasal obstruction). NOSE indicates Nasal Obstruction Symptom Evaluation; VAS, indicates visual analogue scale.
Patients with a NOSE score over 30 (nasal obstruction) showed significant improvement in the spray group (all VAS post-intervention measurements were significantly improved compared to VAS baseline P < .05). In the sex group, all measurements improved significantly compared to VAS baseline (P < .001) except after 3 (P = 0.35) hours. Rhinometric data showed similar results (improvement at time points immediately, 30, 60 minutes after sex and spray [P = .003], and no improvement in either group after 3 hours, P = .29 and .78 for resistance and flow).
Discussion
We report improved nasal breathing after sexual intercourse for up to 60 minutes and to the same extent as application of nasal decongestant as measured with subjective VAS. This was confirmed by objective rhinometric data as mean nasal flow increased while resistance decreased immediately, 30 minutes, and 60 minutes post-intervention. Three hours after sexual intercourse, nasal breathing was back to the baseline level while it remained improved for longer after nasal decongestant. The effect was significant in patients with some preexisting nasal obstruction (NOSE score >30).
Although Sigmund Freud and Wilhelm Fliess already described a physiological connection between the nose and the genital area a long time ago,-, this is the first exploratory study investigating sexual activity with climax and its impact on nasal breathing and patency. The strength of this study is the use of both subjective (VAS) and objective (rhinometric) measurements at different time points. Comparison to nasal decongestants were also very informative.
This study however has major limitations. We were not able to collect rhinometric data in all participants. This could be due to the participants’ inability to focus on the device before and immediately after intercourse. The participants were all health care professionals indicating that our study group does not represent an equally distributed population. Our relatively high mean NOSE score and average VAS at baseline suggest that we selected participants complaining of nasal obstruction. This may be the reason why they agreed to conduct in our study causing a selection bias in our study population. The collection of data by the participants may not be reliable. As the rhinometric measurements were obtained at the participants’ home by themselves, the compliance with the guidelines cannot be guaranteed. Acoustic rhinometric evaluation would have been ideal, but a portable version was unavailable. Also, the results of this study, though interesting, may not be generalizable.
Stimuli leading to changes in nasal breathing include physical exercise, temperature, alterations of body position, and hormonal changes—neurologic syndromes and dentistry also have an effect on nasal reflexes.,-, An increase in nasal patency with exercise is well known and described in the literature.,- Hanci et al described a decrease in nasal resistance after exercise in swimmers, runners, and handball players. To our knowledge, sexual activity and its effect on nasal breathing has never been investigated. Studies investigating nasal function and exercise have mainly assessed isometric exercises., Wilde et al reported that isotonic exercise causes a drop in nasal resistance and may have a nasal decongestant effect. Depending on the sexual exercise, one may experience isotonic or isometric contractions. Investigating different intercourse positions and its effect on nasal patency would certainly be an interesting future study.
Several studies have focused on the duration of exercises’ effects on nasal breathing.- These report a decrease in nasal resistance up to 30 minutes after exercise. Strohl et al showed that nasal resistance returned to baseline after 30 minutes and was lowest when measured between the first and the fifth minute. Our study showed improvements for up to 60 minutes, although diminishing slowly after 30 minutes. Sympathetic reflexes are active in the nasal mucosa, and α-adrenergic agonists decrease mucosal thickness and increase nasal patency. Sexual intercourse is not normal physical exercise. Sexual arousal plus the climax at the end trigger not just sympathetic reflexes but also parasympathetic ones. The interaction is not fully understood but maybe the reason for prolonged nasal patency. A study to further investigate this point would be a masturbation control group and a sexual intercourse without orgasm group. All of our participants reported sexual climax. Whether multiple female orgasms would further increase nasal patency or if the maximal improvement occurs after a single orgasm is an interesting question for future exploration.
Studies suggest that nasal airflow resistance decreases with intensity but not duration of exercise. We did not investigate the duration of sexual intercourse in our cohort; however, extrapolating the results of previous exercise studies, one might suggest that sexual intercourse duration is not as important as actually just “doing it.”
Other interesting studies have reported links between specific physical activities and nasal function. Hasegawa et al reported that breath holding for 30 seconds decreased nasal resistance. Immersion of both feet in warm water (42 °C) may have a transient positive effect on nasal resistance. Performing 5 minutes of axillary pressure (crutch reflex) leads to contralateral increase in nasal vasoconstriction. Jang et al showed that in nasal septal deviation, the mucosal response is more prominent in the concave nasal cavity. Incorporating these findings could potentially synergistically improve nasal function even more.
If further studies prove that masturbation alone has similar positive effect, there might be a potential natural substitution for nasal decongestant application in some cases. Headaches secondary to sinus problems might benefit from such “natural means.” Freud and Fliess “naso-genital” reflexes may not be to due genital spots located on the nasal turbinate. However, this study does suggest a physiological link between sexual activity and nasal function. Such link warrants further exploration if only for the interesting findings it is bound to produce.
Conclusion
Sexual intercourse with climax improves nasal breathing to the same degree as application of nasal decongestant for up to 60 minutes as measured with subjective VAS. This was confirmed by objective rhinometric data mean as nasal flow increased while resistance decreased immediately, 30 minutes, and 60 minutes post-intervention. Three hours after sexual intercourse, nasal breathing was back to the baseline level in the “sex group,” whereas after application of nasal decongestant spray, nasal breathing was still significantly improved. Only participants having nasal obstruction (NOSE score >30) showed improvement after sex.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Olcay Cem Bulut
https://orcid.org/0000-0002-6999-2074
References
- 1. Baraniuk JN, Merck SJ. Nasal reflexes: implications for exercise, breathing, and sex. Curr Allergy Asthma Rep. 2008;8(2):147–153. doi:10.1007/s11882-008-0025-7
- 2. Mackenzie J. Article IV. Irritation of the sexual apparatus as an etiological factor in the production of nasal disease. 1. Am J Med Sci. 1884;87(174):360–365.
- 3. Fliess W.Die beziehungen zwischen nase und weiblichen geschlechtsorganen. In Ihrer Biologischen Bedeutung Dargestellt. na; 1897.
- 4. Young AR. Freud’s friend fliess. J Laryngol Otol. 2002;116(12):992–995.
- 5. Freud S. Letter from Freud to Fliess, February 4, 1888. The Complete Letters of Sigmund Freud to Wilhelm Fliess, 1887-1904. The Belknap Press of Harvard University; 1985:18–21.
- 6. Canning BJ. Neurology of allergic inflammation and rhinitis. Curr Aller Asthma Rep. 2002;2(3):210.
- 7. Sarin S, Undem B, Sanico A, Togias A. The role of the nervous system in rhinitis. J Aller Clin Immunol. 2006;118(5):999–1014.
- 8. Bhutta MF. Sex and the nose: human pheromonal responses. J Royal Soc Med. 2007;100(6):268–274. doi:10.1177/014107680710000612
- 9. Ellegård E, Karlsson G. Nasal congestion during the menstrual cycle. Clin Otolaryngol Allied Sci. 1994;19(5):400–403.
- 10. Forsyth R, Cole P, Shephard R. Exercise and nasal patency. J Appl Physiol Respir Environ Exerc Physiol. 1983;55(3):860–865.
- 11. Haeggström A, Östberg B, Stjerna P, Graf P, Hallén H. Nasal mucosal swelling and reactivity during a menstrual cycle. Orl. 2000;62(1):39–42.
- 12. Philpott CM, Alami ME, Murty GE. The effect of the steroid sex hormones on the nasal airway during the normal menstrual cycle 1. Clin Otolaryngol Allied Sci. 2004;29(2):138–142.
- 13. Winn P. Dictionary of Biological Psychology. Routledge; 2003.
- 14. Stewart MG, Witsell DL, Smith TL, Weaver EM, Yueh B, Hannley MT. Development and validation of the Nasal Obstruction Symptom Evaluation (NOSE) scale. Otolaryngol Head Neck Surg. 2004;130(2):157–163. doi:10.1016/j.otohns.2003.09.016
- 15. Lipan MJ, Most SP. Development of a severity classification system for subjective nasal obstruction. JAMA Facial Plast Surg. 2013;15(5):358–361.
- 16. Chester AC. The nose and sex: the nasogenital reflex revisited. J Royal Soc Med. 2007;100(11):489–490. doi:10.1177/014107680710001105
- 17. Fonseca MT, Machado JA, Pereira SA, Pinto KM, Voegels RL. Effects of physical exercise in nasal volume. Braz J Otorhinolaryngol. 2006;72(2):256–260. doi:10.1016/s1808-8694(15)30065-3
- 18. Fonseca MT, Voegels RL, Pinto KM. Evaluation of nasal volume by acoustic rhinometry before and after physical exercise. Am J Rhinol. 2006;20(3):269–273. doi:10.2500/ajr.2006.20.2863
- 19. Richerson HB, Seebohm PM. Nasal airway response to exercise. J Allergy. 1968;41(5):269–284. doi:10.1016/0021-8707(68)90032-4
- 20. Hanci D, Altun H, Sahin E, Aydin S. Nasal response after exercise in swimmers, runners and handball players. ENT Updates. 2016;6(2):64–69.
- 21. Jang YJ, Lee JH, Jang JY. Acoustic rhinometric evaluation of the nasal response to exercise in patients with nasal septal deviation. Clin Otolaryngol Allied Sci. 2000;25(5):423–427. doi:10.1046/j.1365-2273.2000.00390.x
- 22. Wilde AD, Cook JA, Jones AS. The nasal response to isometric exercise. Clin Otolaryngol Allied Sci. 1995;20(4):345–357. doi:10.1111/j.1365-2273.1995.tb00056.x
- 23. Konno A, Togawa K, Itasaka Y. Neurophysiological mechanism of shrinkage of nasal mucosa induced by exercise. Auris Nasus Larynx. 1982;9(2):81–90. doi:10.1016/s0385-8146(82)80004-7
- 24. Lacroix JS, Correia F, Fathi M, Grouzmann E. Post-exercise nasal vasoconstriction and hyporeactivity: possible involvement of neuropeptide Y. Acta Oto-Laryngol. 1997;117(4):609–613. doi:10.3109/00016489709113446
- 25. Strohl KP, Arnold JL, Decker MJ, Hoekje PL, Doershuk CF, Stern RC. The nasal response to exercise in patients with cystic fibrosis. Rhinology. 1992;30(4):241–248.
- 26. Hasegawa M, Kern EB. The effect of breath holding, hyperventilation, and exercise on nasal resistance. Rhinology. 1978;16(4):243–249.
- 27. Wilde AD. The effect of cold water immersion on the nasal mucosa. Clin Otolaryngol Allied Sci. 1999;24(5):411–413. doi:10.1046/j.1365-2273.1999.00275.x
- 28. Wilde AD, Jones AS. The nasal response to axillary pressure. Clin otolaryngol Allied Sci. 1996;21(5):442–444. doi:10.1046/j.1365-2273.1996.00823.x