Introduction
Cerumen impaction is one of the most common ear-related disorders in patients presenting to health care facilities. It can cause blocked, painful ears and hearing loss. There are several ways of removing impacted cerumen, including manual removal, irrigation, and treatment with cerumenolytic agents. Although current evidence indicates that manual removal and irrigation have comparable effectiveness, cerumenolytic agents may be potentially more effective due to their ability to thin, soften, break up, and/or dissolve cerumen. Thus, cerumenolytic agents may reduce the time required for cerumen extraction and make the overall procedure more comfortable for patients.
A number of novel cerumenolytic agents have been developed from existing commercially available products., These have mainly comprised water- or oil-based drops, with water-based solutions being the most commonly used. Although some studies have suggested that water may be more effective than several proprietary agents, this finding is still controversial.- Salicylic acid, and potassium hydroxide solutions are economical, readily available, and easy to use at home for skin-related applications. However, the effectiveness of these solutions for cerumen dissolution is unclear. Therefore, the objective of this study was to compare the cerumen dissolution activities of (1) distilled water, (2) 7.5% sodium bicarbonate, (3) 5% potassium hydroxide, (4) 10% lactic acid, (5) 3% salicylic acid, and (6) 10% glycolic acid over a 12 hours period, using both digital photography and weighing of residual cerumen samples.
Patients and Methods
The study protocol was approved by the institutional review board of the Faculty of Medicine, Ramathibodi Hospital, Mahidol University (No: MURA2018/132). Specimens of dry impacted cerumen mechanical removal with a clean earwax instrument were obtained from patients under microscopy. None of the specimens had been previously treated with cerumenolytic agents. The sample size of 36 cerumen samples was based on previously established parameters for the 2 approaches used to evaluate cerumen dissolution (Supplemental material).
The samples were divided into equal parts weighing 50 ± 1 mg each and stored in airtight plastic bags at room temperature until required for analysis. Cerumen samples were randomized to each solution by a computerized randomization method. The generation of the randomization sequence and the allocation procedure were performed by statisticians who were not directly involved in the experimental procedures. The investigator placed the cerumen samples into test tubes containing 3 mL of the randomly allocated solutions. All tubes were incubated vertically at room temperature (approximately 25 °C, maintained with an air conditioner). Assessments of cerumen disintegration were obtained at fixed time intervals. Digital photography was used to record changes at 15 minutes, 30 minutes, 1 hour, 2 hours, and 12 hours. Undissolved cerumen remaining at 12 hours was removed from the test tubes and transferred to petri dishes; all specimens were dried in a closed room for 2 days prior to weighing. The humidity in the closed room was controlled with an air conditioner set to approximately 25 °C. The cerumen disintegration grade and the weight of the dried cerumen were recorded by an investigator and an uninvolved witness who were blinded to the assigned cerumenolytic solution. The grading of cerumen disintegration was performed in accordance with the system described by Saxby et al and Fraser.
A comparative analysis of disintegration between solutions was performed using an ordinal logistic regression model with the type of solution as the covariate. A comparative analysis of the dry cerumen weight was conducted with a mixed linear regression model with time and solution as covariates. The level of statistical significance was set at P < .05. All data analyses were performed with Stata version 14.0 (Stata Corp).
Results
Cerumen Disintegration
Representative digital photographs depicting cerumen disintegration are shown in Figure 1. Greater turbidity can be seen in the tubes containing potassium hydroxide and sodium bicarbonate (Figure 1). The degree of cerumen disintegration over time was evaluated to determine the speed of disintegration. Potassium hydroxide was the fastest-acting cerumenolytic agent, causing moderate dissolution within 30 minutes. Potassium hydroxide and sodium bicarbonate showed moderate disintegration at 1 hour. However, the action of potassium hydroxide was much faster, causing substantial disintegration at 2 hours. Sodium bicarbonate exhibited similar results after 12 hours. Distilled water and lactic acid were equally effective, with both causing moderate disintegration at 2 hours, and substantial cerumen disintegration after 12 hours. Glycolic acid and salicylic acid caused no visible changes in the cerumen sample within the first 30 minutes, only mild disintegration was observed after 12 hours.
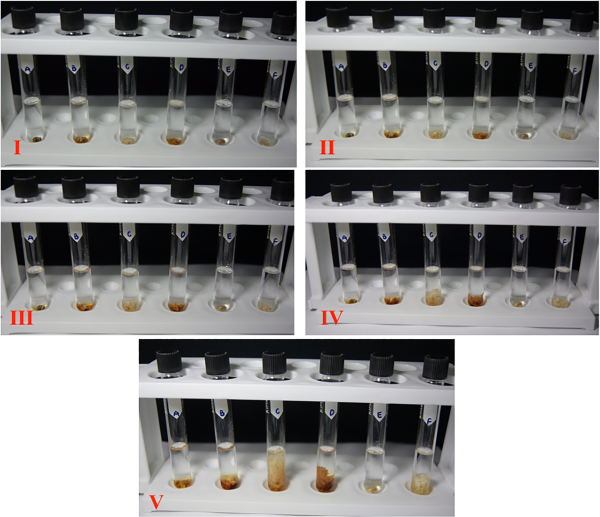
Figure 1
Degree of cerumen disintegration in 6 different solutions in one batch.
The median grades assigned to the solutions over time showed that the potassium hydroxide solution was the most effective since it yielded a different grade at each time interval and caused substantial disintegration after 12 hours. Both distilled water and sodium bicarbonate were approximately equally effective in dispersing the cerumen, whereas lactic acid achieved a smaller degree of disintegration. Salicylic acid and glycolic acid did not cause any disintegration (Figure 2).
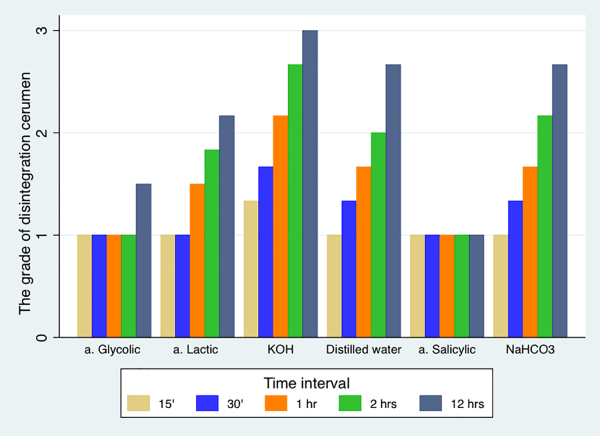
Figure 2
The cerumen disintegration grades of each test solution at specific time intervals.
The dissolution activities of the solutions were compared via 2 ordinal logistic regression models that assigned distilled water and sodium bicarbonate as the reference groups (Table 1).
In the first model with distilled water as the reference group, potassium hydroxide had the highest odds ratio (OR) for disintegration (OR = 273.237; 95% CI: 0.203-367 470.4) while that for sodium bicarbonate was lower (OR = 1.129; 95% CI: 0.002-850.341); this difference did not reach statistical significance. Lactic acid had a much lower effect on cerumen disintegration than distilled water (OR = 0.017, 95% CI: <0.001-18.415); however, this was not statistically significant. Both salicylic acid and glycolic acid had significantly less dissolution activity than distilled water.
In the second model with sodium bicarbonate as the reference group, potassium hydroxide exhibited a dissolution activity that was approximately 242 times higher (OR = 242.078. 95% CI: 0.183-319 851.9) than sodium bicarbonate, though this was not statistically significant. Lactic acid and distilled water had ORs less than 1, indicating that their disintegration activities were lower than that of sodium bicarbonate; nevertheless, this was not statistically significant. Both salicylic acid and glycolic acid had a significantly lower activity than sodium bicarbonate.
Assessments Based on Undissolved Cerumen Weight
The associations between the cerumenolytic solutions and weights of residual undissolved cerumen samples are shown in Table 2. Cerumen samples subjected to distilled water (reference group) had a mean weight of 0.030 ± 0.011 g after drying. Only sodium bicarbonate had a negative coefficient (−0.001), indicating a greater effectiveness in reducing cerumen weight compared to distilled water; however, this was not statistically significant. On the other hand, potassium hydroxide and lactic acid yielded positive coefficients of <0.001 and 0.008, respectively; these results were not statistically significant. The mean cerumen weights for glycolic acid and salicylic acid were significantly higher than that for distilled water (P < .05), indicating the lack of effectiveness of these 2 cerumenolytic agents.
Discussion
In this study, we compared the effectiveness of 6 different solutions in dissolving human cerumen specimens. These solutions included 7.5% sodium bicarbonate (which is commonly used in Thai hospitals), distilled water (which was used as a control agent), 5% potassium hydroxide, 10% lactic acid, 3% salicylic acid, and 10% glycolic acid. On the basis of photographic evidence of cerumen disintegration at specific time intervals, potassium hydroxide was shown to be the fastest-acting agent, as it caused moderate disintegration in just 30 minutes and substantial disintegration at 2 hours. Lactic acid was identified as a new effective cerumenolytic agent, and it exhibited an activity equal to distilled water. Both distilled water and lactic acid solution achieved moderate disintegration at 2 hours and nearly substantial disintegration after 12 hours.
The sodium bicarbonate solution caused moderate disintegration at 1 hour and substantial disintegration at 12 hours. Our findings were consistent with previous studies in which both distilled water and sodium bicarbonate were shown to cause substantial cerumen disintegration at 12 hours., Sodium bicarbonate was also found to be a superior cerumenolytic agent in a study conducted by Fraser, who compared the ability of aqueous and oil-based agents to disintegrate cerumen over 3 days. Whereas sodium bicarbonate completely disintegrated the specimens after 3 days, the other oil-based products were ineffective. Nevertheless, our results slightly differ from those of an in vitro study by Chalishazar and Williams, who reported that distilled water was more rapidly acting and effective than 5% sodium bicarbonate. This difference may be attributed to the higher concentration (7.5%) of sodium bicarbonate solution used in our study.
By assessing the degree of cerumen disintegration over 5 specific time intervals, we found that potassium hydroxide and sodium bicarbonate had a more rapid effect than the other agents; this supports their potential application in outpatient settings. On the other hand, agents such as distilled water and lactic acid (which could substantially dissolve the cerumen at 12 hours) may be more suitable for cases of complicated cerumen impaction, wherein the patient could self-administer these agents at home and return to the clinic after 1 to 2 days for removal of the dissolved cerumen. Although the use of distilled water as a cerumenolytic agent may offer financial benefits for both patients and the health care system, prolonged exposure of the external ear canal to water may predispose patients to otitis externa.
In this study, potassium hydroxide and lactic acid were identified as new potential agents that can be used to partially dissolve impacted cerumen and facilitate subsequent removal. Potassium hydroxide is an alkali, which is known to dissolve keratin; therefore, it may be used to partially disperse the dense keratin plug (before syringing or another procedure) in cases of keratosis obturans and external canal cholesteatoma, which are difficult to treat with conventional methods. Potassium hydroxide may also be used to facilitate the removal of debris or desquamated epithelium in a large postoperative cavity after radical canal wall down mastoidectomy. However, caution is required with the use of potassium hydroxide for cerumen removal, due to the potential for irritant reactions of varying intensity. Nevertheless, no prior studies have assessed side effects associated with the application of potassium hydroxide on the external ear canal skin. Thus, additional in vivo studies are required to evaluate both the effectiveness of cerumen disintegration and potential adverse events associated with different potassium hydroxide concentrations.
Lactic acid is an alpha hydroxyl acid, and a 10% formulation has been shown to cause mild skin exfoliation. Although lactic acid achieved good results in the present study, with substantial cerumen disintegration after 12 hours, in vivo studies are required to determine the appropriate concentration for therapeutic use and evaluate potential adverse effects on the external ear canal.
Although sodium bicarbonate has a long track record of clinical efficacy as a cerumenolytic agent and has been the standard treatment used at our institution, our results suggest that potassium hydroxide may be the most effective agent for dissolving cerumen, followed by sodium bicarbonate, distilled water, and lactic acid. Although the results of these comparisons were not statistically significant, this may have been attributed to the imprecision associated with the use of only 3 grades to classify the degree of disintegration.
In this study, we also compared the efficacies of different agents by directly measuring the weight of the undissolved cerumen. The use of sodium bicarbonate resulted in the greatest weight reduction. This finding differs from that reported in an in vitro study by Saxby et al, who noted that distilled water produced the greatest reduction in cerumen weight. As explained earlier, this difference could be explained by the use of a higher sodium bicarbonate concentration in the present study. The different methods used to process cerumen samples may also have been a factor. Saxby et al collected cerumen samples and mixed them into homogeneous balls with a uniform shape and size. Although sodium bicarbonate had higher odds of weight reduction, fast disintegration of the specimen with potassium hydroxide could be of interest for usage in outpatient settings, as the clinician may start an attempt to remove the specimen mechanically. As cerumen samples consist of different component layers which may affect the process of disintegration, future studies should attempt to use extracted cerumen samples in their original shape and size, in order to evaluate the actual effectiveness of cerumenolytic agents.
In summary, the current study showed that glycolic acid and salicylic acid caused no visible signs of cerumen disintegration and resulted in very little reduction in dry weight. In contrast, the self-administration of distilled water by patients at home is an economical, readily available, and safe approach for cerumen removal. Potassium hydroxide and sodium bicarbonate may be useful in outpatient settings, as they can reduce the time required for cerumen removal and optimize the subsequent use of ear suction. Future in vivo studies with large sample sizes are required to confirm the results of this in vitro study, and to evaluate the safety and determine the optimal duration, frequency, and concentration of cerumenolytic agent application.
Conclusion
The results of this study suggest that 5% potassium hydroxide may be the fastest-acting cerumenolytic agent, followed by sodium bicarbonate, distilled water, and lactic acid. In assessments based on the weight of undissolved cerumen, all 4 of these agents showed equivocal effectiveness at 12 hours. However, 10% glycolic acid and 3% salicylic acid showed no visible signs of cerumen disintegration and caused very little reduction in dry weight.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Nguyen Quynh Anh
https://orcid.org/0000-0002-6161-9179
Supplemental Material Supplemental material for this article is available online.
References
- 1. Roland PS, Smith TL, Schwartz SR, et al. Clinical practice guideline: cerumen impaction. Otolaryngol Head Neck Surg. 2008;139(3 suppl 2):S1–S21.
- 2. Clegg AJ, Loveman E, Gospodarevskaya E, et al. The safety and effectiveness of different methods of earwax removal: a systematic review and economic evaluation. Health Technol Assess. 2010;14(28):1–192.
- 3. Loveman E, Gospodarevskaya E, Clegg A, et al. Ear wax removal interventions: a systematic review and economic evaluation. Br J Gen Pract. 2011;61(591):e680–e683.
- 4. Schwartz SR, Magit AE, Rosenfeld RM, et al. Clinical practice guideline (update): earwax (cerumen impaction). Otolaryngol Head Neck Surg. 2017;156(1_suppl):S1–S29.
- 5. Eekhof JA, de Bock GH, Le Cessie S, Springer MP. A quasi-randomised controlled trial of water as a quick softening agent of persistent earwax in general practice. Br J Gen Pract. 2001;51(469):635–637.
- 6. Nanda MS. Role of water as a quick ear wax softener before wax removal under microscope. Otolaryngol Online J. 2015;5(3):8.
- 7. Bellini MJ, Terry RM, Lewis FA. An evaluation of common cerumenolytic agents: an in-vitro study. Clin Otolaryngol Allied Sci. 1989;14(1):23–25.
- 8. Saxby C, Williams R, Hickey S. Finding the most effective cerumenolytic. J Laryngol Otol. 2013;127(11):1067–1070.
- 9. Arif T. Salicylic acid as a peeling agent: a comprehensive review. Clin Cosmet Investig Dermatol. 2015;8:455–461.
- 10. Ueda S, Mitsugi K, Ichige K, et al. New formulation of chemical peeling agent: 30% salicylic acid in polyethylene glycol. Absorption and distribution of 14C-salicylic acid in polyethylene glycol applied topically to skin of hairless mice. J Dermatol Sci. 2002;28(3):211–218.
- 11. Kose O, Ozmen I, Arca E. An open, comparative study of 10% potassium hydroxide solution versus salicylic and lactic acid combination in the treatment of molluscum contagiosum in children. J Dermatolog Treat. 2013;24(4):300–304.
- 12. Fraser JG. The efficacy of wax solvents: in vitro studies and a clinical trial. J Laryngol Otol. 1970;84(10):1055–1064.
- 13. Chalishazar U, Williams H. Back to basics: finding an optimal cerumenolytic (earwax solvent). Br J Nurs. 2007;16(13):806–808.