Introduction
Tympanic membrane (TM) perforation produces conductive deafness and recurrent middle ear infections and later ossicular chain and cochlea vestibular damage. This serious injury can be surgically repaired by myringoplasty using a graft without the need for any further procedure in the middle ear.
Early in 1640, Banzer reported the first attempt to close a tympanic perforation using elk horn coated with pork bladder. In 1878, Berthold , introduced the term myringoplasty and performed the procedure successfully using a free skin graft. However, this important contribution was not widely accepted until 1951, when Wullstein reported on this surgical procedure in Germany.
In 1959, Storrs was the first to use temporalis fascia (TF), which has become the material used more often worldwide for this type of surgery. However, there are some disadvantages relating to the collection of this material using a retroauricular incision and positioning the graft relative to the malleus.
In 1962, Ringenberg first reported the use of a fat graft (FG) to repair tympanic perforations, including large perforations. In this series of 65 patients, 26% of perforations were larger than 30% to 40% and included some total perforations. Despite the large size of these perforations, the success rate was 86%.
Fat graft myringoplasty has advantages over that using TF or perichondrium such as the simplicity in collection, ease of positioning, and cost-effectiveness because it can be performed as an office procedure. However, FG myringoplasty is not performed often by otosurgeons mainly because of the lack of criteria for its indications in terms of the size of the perforation.
The aim of this prospective clinical study in patients given FG myringoplasty was to determine whether the size or location of the TM perforation affects the graft stability and long-term follow-up after FG myringoplasty.
Material and Methods
This prospective clinical study included patients who underwent FG myringoplasty at Hospital Naval San Carlos in San Fernando, Cadiz, Spain. Informed consent was obtained from each patient, and this clinical study was approved by our Hospital Ethical Review Committee.
The data collected for every patient were age, sex, size, and site of the perforation; duration of surgery; success of perforation closure; and audiological outcomes before and 12 months after the operation. Potential perioperative complications (eg, dizziness, bleeding) and postoperative complications (eg, middle ear infection, displacement with perforation or fat excess with granulation tissue, and TM deformity) were also recorded.
The inclusion criteria included perforation size >25% of the surface of the pars tensa of the TM, absence of infection or otorrhea at surgery, and lack of spontaneous closure at the 6-month follow-up. The exclusion criteria were the presence of cholesteatoma, attical pathology, perforation without clear margins, wet appearance of the mucosa in the tympanic cavity, presence of an acute infection, ear discharge in the 3 months before surgery, or signs of ossicular inconsistency (Figure 1).
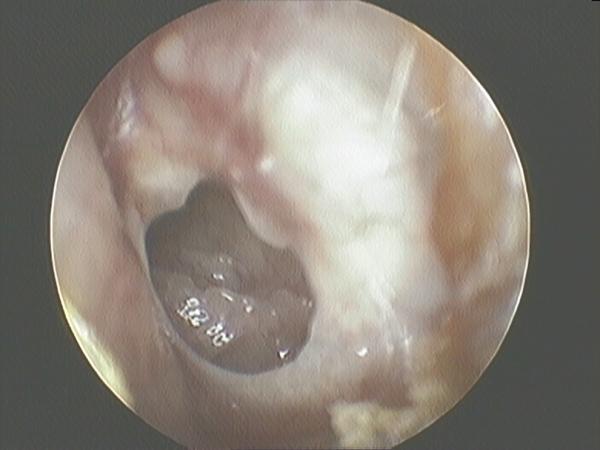
Figure 1
Perforated tympanic membrane.
All surgeries were performed with the patient under local anesthesia and sedation by the same senior otology surgeon. The ear channel and middle ear were sterilized, and local anesthesia was applied with phenol and lidocaine (Bonain’s formula) over the TM while trying to avoid contact with the middle ear mucosa for a maximum of 5 minutes.
The FG was obtained from the neck through an incision made parallel to the line of skin tension (Video 1). The graft was immersed in a chlorhexidine solution for sterilization.
Margins of the tympanic perforation were de-epithelized using a curette until bleeding occurred, with special attention to malleus handling (Video 2). The FG was positioned over the perforation like a champagne cap, without crushing and without any material covering the graft (Video 3). In cases of bilateral TM perforation, both perforations were operated using the same surgical procedure.
During the first 3 postoperative days, daily outpatient clinic revision was performed to prevent hematoma and to reinsert any displaced grafts. After 3 days, weekly revision was performed during the first month to check and control potential infections. The first hearing test was performed 2 months after and the final test 12 months after the operation (Figure 2). After 12 months, impedanciometry was performed to ensure TM compliance.
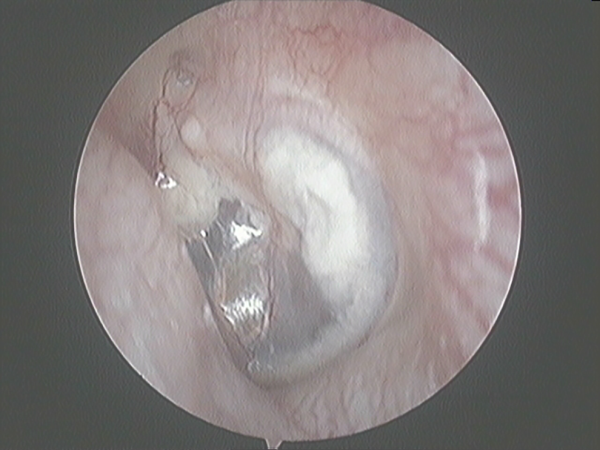
Figure 2
Tympanic membrane after one year PO.
We evaluated the results of hearing tests performed before and 12 months after surgery, following the criteria reported previously. , The hearing frequencies used were 500, 1000, 2000, and 3000 Hz. We calculated the mean air conduction (AC) threshold, bone conduction (BC) threshold, and percentages of patients with a pre- and postoperative average air–bone gap (ABG) of <20 dB.
Video-otoscopy analysis was performed using the Paint.net image analysis program (version 4.2.9; dotPDN LLC) to measure the size of the perforations as previously described. For statistical analysis, IBM SPSS Statistics (version 23.0, IBM Corp) was used.
Data were collected in a database. Nominal variables were described by their frequency distribution. Quantitative variables were assessed by calculating the arithmetic mean and standard deviation. When comparing, we have used 2-tailed paired t tests for continuous variables and the χ2 or Fisher exact test for nominal variables. For variables with a skewed distribution, the Mann-Whitney U test was used.
Results
From June 2005 to June 2019, 121 patients were enrolled in this prospective study. Their mean age was 51.1 ± 18.4 years (range, 3-78 years). No statistical significance was found in sex distribution (57 men, 64 women P = .4). Twenty-one patients had a bilateral TM perforation. A total of 142 FG myringoplasties were performed and included in this study.
The size of perforation of the pars tensa was <25% of TM in 39 (27.5%) ears, 25% to 50% of TM in 49 (34.55%) ears, 50% to 75% of TM in 34 (23.91%) ears, and 75% to 100% of TM in 20 (14.10%) ears.
Complete perforation closure was evident in 117 (82.39%) of the 142 ears after primary FG myringoplasty. Surgery failed in 25 (17.61%) ears, mostly because of microperforations affecting <10% of the surface of the eardrum. In these patients, a second attempt of FG myringoplasty was offered 12 months after the first failed surgery. This procedure was performed successfully in 13 ears. Thus, our global success rate including the results from the revision surgery was 130 (91.55%) of 142 (Table 1).
Success closing the perforation was found in 87.17% of <25%-sized perforations, 93.87 of 25% to 50%-sized perforations, 94.11% of 50% to 75%-sized perforations, and 90% of 75% to 100%-sized perforations, a nonsignificant difference (χ2 test P = .21). The closure rate did not differ significantly between anterior perforations and those in other locations. There were 4 failures in the anterior location and 9 failures in the rest of locations (Fisher exact test, P = .575).
During the follow-up, 4 (2.8%) patients developed a middle ear infection, 24 (16.9%) ears had displacement with microperforation, and 4 (2.8%) cases developed fat excess with granulation tissue and TM deformity.
Comparison of the pre- and postoperative mean AC threshold showed that the hearing was worse in 12 (8.6%) ears, unchanged in 24 (17%) ears, and improved in 106 (74.4%) ears (Figure 3).
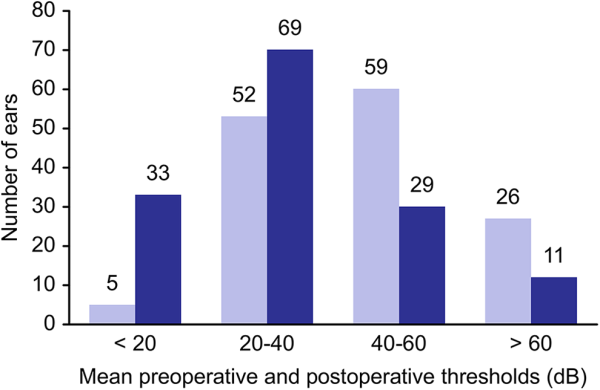
Figure 3
Hearing results after fat graft myringoplasty. Improvement in mean AC threshold.
In 33 (23.2%) ears, we obtained a final mean AC threshold of <20 dB; in 69 (48.9%) ears, we obtained a mean AC threshold between 20 and 40 dB; in 29 (20.4%) ears, we obtained a mean AC threshold between 40 and 60 dB; and finally in 11 (7.7%) ears, we obtained a mean AC threshold of >60 dB.
Preoperative mean BC threshold was 29.1 dB (5-70 dB) and did not change after surgery. Mean AC threshold was preoperatively 59.3 dB (17-95 dB) and significantly improved after surgery into 35.6 dB (10-85 dB; P < .0004). Preoperative ABG was 30.2 dB (5-70 dB) and also significantly improved into10.2 dB (5-65 dB; P < .0001; Table 2). We found no significant difference between the location of the perforation and the extent of hearing improvement (χ2 = 27.465, P = .283).
The mean preoperative ABG was 30.2 dB (5-70 dB), with 46.9% of ears at 20 to 40 dB and 9% of ears <20 dB. The mean postoperative ABG was 10.2 dB (5-65 dB), with 41.4% of ears at 20 to 40 dB and 51.7% of ears <20 dB. The ABG worsened in 5 (3.5%) ears, did not change in 32 (22.5%) ears, and improved in 105 (74.5%) ears.
The mean surgical time was 15 ± 6 min/ear (range, 8-24 minutes). No significant peri- or postoperative bleeding occurred in these patients. Temporal dizziness occurred in 14 (9.8%) patients.
Discussion
Autologous FG myringoplasty has become an accepted modality for soft tissue restoration and is being applied in many areas of surgery. Although the regenerative effects of fat grafting into the recipient bed are known, most otolaryngologists do not use FGs often and prefer other materials, such as TF or perichondrium, to close tympanic perforations. It has been demonstrated convincingly that FGs contain viable adipose stem cells (ASCs). It has also become apparent that white adipose tissue is the most suitable autologous injectable filler for correcting soft tissue defects. White adipose tissue contains large numbers of ASCs and is an excellent natural resource for repairing human tissue defects. Adipose stem cells are capable of self-renewal and have increased proliferative and multipotent differentiation capabilities. These cells also exhibit similar properties to mesenchymal stem cells (MSCs), which were first described and characterized in the bone marrow. ,
A number of nonexclusive mechanisms through which ASCs may help to repair and regenerate tissues have been postulated. In a related manner, ASCs might provide antioxidant chemicals, free radical scavengers, and chaperone/heat shock proteins at an ischemic site. Toxic substances released into the local environment would be removed, thereby promoting the recovery of the surviving cells. Recent studies have suggested that transplanted bone marrow–derived MSCs can deliver new mitochondria to damaged cells, thereby rescuing aerobic metabolism. It has also been shown that the resident ASCs within FG tissues can differentiate into adipocytes and add structure to fill the implanted tissue defect, secrete growth factors, cytokines, and chemoattractants that can stimulate angiogenesis and increase local vascularization and blood supply and inhibit the innate immune responses after tissue transplantation.
These properties have not been demonstrated in grafts involving TF or perichondrium. We believe that these factors may have contributed to our high overall success rate, which is consistent with the rates reported in other investigations , of FGs. Our series included the largest sample size studied, and we believe that this was a large enough sample to confirm the safety and effectiveness of this procedure.
Small perforations historically have been the main focus for the use of FG as the material of choice. Some authors consider FG only for perforations <25% and located in the pars tensa. - Schraff et al recommended this type of material for repair of perforations after myringotomy tubes forming 15% to 40% of the total surface of the TM. Deddens et al and Hagemann consider FG to be the material of choice for repair of small perforations, especially after grommet insertion, and others agree that this material is underused. ,
Even though TF is the most widely used material, we prefer the FG because it can be harvested more easily and faster. We also prefer the region of the neck under the ear as the donor site, where fat is more abundant, instead of the earlobe. Because of the limited quantity of fat, we believe that obtaining the FG from the earlobe is suitable only for repair of small perforations.
In 1988, Terry et al used FG successfully to repair perforations comprising >50% with a 57.1% of success rate. In a series of 365 patients operated on by the same surgeon, Gibb and Chang reported on the underlay technique used to treat 206 patients and FG for the other 159 patients. The graft take rate was 91.4% in dry ears in the TF group and 89.3% in the FG group.
In 2003, Ayache et al reported the use of adipose tissue regardless of the size of the perforation and obtained a success rate of 91.1% in 45 patients. Since then, several studies ,, have used this material for repairing large perforations and have reported acceptable success rates. Other studies, for example, Saliba in 2008, have reported the use of FGs combined with hyaluronic acid. In a series of 22 consecutively repaired perforations, regardless of the size, the overall successful rate was 91%. The mean ABG improvement for the operated ears was 17 dB, and the mean time for the procedure was 10 minutes. Saliba concluded that the combination of FG with hyaluronic acid is better than FG alone and is optimal for all sizes of perforations.
Recently, Ersözlü and Gultekin reported that the use of autologous platelet gel application during FG surgery has a higher success rate tan FG alone. They considered that this combination is preferred in larger perforations as they found statistically difference in the perforation’s closure rate.
Except in children, all our procedures have been performed under local anesthesia, and most of the procedures have been performed as office-based surgery. We believe this is an advantage because of its low cost. We use a surgery room only for those patients whose comorbidities require special monitoring. In such patients, we use midazolam combined with propofol only when de-epithelizing the margins of the perforation.
Our average time of 15 ± 6 min/ear is a good indication of the ease of this procedure. This value includes the 5 to 10 minutes time waiting for the local anesthesia to become effective. We compare this with the procedure time of 10 minutes reported by Saliba, which did not include the anesthesia time. We thus consider our procedure to be valuable in optimizing resources.
In contrast to Kim et al, who reported poor hearing results with the use of large FGs to close larger perforations, we found no differences in our hearing results according to the size of the perforation. In our experience, we have found this technique to be useful for repairing perforations in all locations of pars tensa, especially those located in an anterior site because the fat in the FG is a metabolically active material that promotes cicatrization and revascularization from adjacent areas. The low rate of middle ear infection (2.8%) detected in our series may be explained by the antibacterial properties of the ASCs contained in the FG.
Even though TF is the most widely used autogenous graft material for tympanoplasty, this procedure requires a large incision, preparation of the TM flap, lifting of the annulus and the resultant discontinuation of the TM, and manipulation of middle ear structures. All of these procedures can lead to hypoesthesia of the helix, blunting of the angle of the TM, impairment of taste sensation, lateralization of the graft, and effects on acoustics. The FG inserted using a small incision is simple, and there is no risk of injuring the ear ossicles. We found no complications in our series.
In a series of 30 patients who received a FG, Ambani et al reported a success rate of 53.3%. In their series, the ABG decreased to <10 dB in 12 (40%) patients, was unchanged after the operation in 10 (33.3%) patients, and increased after the operation in 8 (26.6%) patients. A follow-up was performed at 5 months, but the patients were only examined 1 week and 1 month postoperatively. Because it is important to provide regular revision during the first week to ensure the absence of displacement of the graft, we do not recommend the FG procedure for patients for whom this revision cannot be provided.
Ambani et al also considered that in the fifth postoperative month after FG, there is a significant bulging of the TM that worsens hearing results. To avoid this potential problem, we recommend not fixing the graft over elevating the TM. In ears where scarring or granulation tissue creates a fat bulge, we recommend cutting the scarred tissue with microscissors 3 to 4 weeks after the procedure. In our experience, all of the excess fat tissue disappears after this procedure and does not influence the hearing threshold.
Recently, Shoman reported work using a palisade cartilage myringoplasty with the patient under local anesthetic in the office. He considered that the technique reported by Saliba’s results were inconsistent because of the need to obtain the FG from the neck and fill the middle ear with it. We do not share this criticism because we consider that it is easier to obtain an FG than cartilage or FT tissue. In addition, the FG must not fill the middle ear but instead should be fixed and floating on the TM.
Conclusion
Fat grafts are easy to obtain and can be obtained from several parts of the body. All are suitable for myringoplasty. In office FG myringoplasty, in adult and pediatric patients with variable perforation sizes, is a well-tolerated procedure with very satisfactory clinical results. Fat graft myringoplasty is fast, cost-effective and as well suited to office-based surgery.
Acknowledgments
The authors want to thank his invaluable assistance in the preparation of this manuscript to Prof Jose Manuel Revuelta Emeritous Surgery Professor from Cantabria’s University.
Authors’ Note All authors had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Dr Rodriguez, Dr O’Connor, and Dr Plaza had significant contribution in data collection and writing and editing assistance. Dra Rodriguez and Dr Valdeon assisted specially in the translation and statistics. Dra Garcia had significant contribution in statistics. Author Elena Rodriguez Posadas is now affiliated with Pulmonology Department, Hospital Santa Maria del Puerto, Puerto de Santa Maria, Cadiz, Spain. Dra Maria Teresa Garcia Iriarte is now affiliated with Otorhinolaryngology Department, Hospital Universitario Virgen de Valme, Sevilla, Spain.
Declaration of Conflicting Interests The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding The author(s) received no financial support for the research, authorship, and/or publication of this article.
Carlos O’Connor Reina
https://orcid.org/0000-0002-1670-4235
Supplemental Material Supplemental material for this article is available online.
References
- 1. Gil Carcedo LM. Otologia. In. Otologia. Buenos Aires: Editorial Panamericana; 2004:97–99.
- 2. Suarez C. Tratado de otorrinolaringologia y cirugia de cabeza y cuello. Tomo II. In: Buenos Aires MMP, ed. 2007:1461.
- 3. Berthold E. Uber Myringoplastik. Med-chir Cent. 1879;14(195):207.
- 4. Sarkar S. A review on the history of tympanoplasty. Indian J Otolaryngol Head Neck Surg. 2013;65(suppl 3):S455–S460. doi:10.1007/s12070-012-0534-5
- 5. Wullstein H. Die extratympanale endocranielle Fensterung bei chronischer Otitis media und Labyrinthinnendruck-Storung im Vergleich zur typischen Fensterung am seitlichen Bogengange. Ztschr Laryng Rhin Otol. 1951;30(5):203–216.
- 6. Storrs L. Myringoplasty with the use of fascia grafts. Arch Otolaryngol Head Neck Surg. 1969;74(1):45–49.
- 7. Vartiainen E, Nuutinen J. Success and pitfalls in myringoplasty: follow up study of 404 cases. Am J Otolaryngol. 1993;14(3):301–305.
- 8. Ringenberg JC. Closure of tympanic membrane perforations by the use of fat. Laryngoscope. 1978;88(6):982–993.
- 9. Gun T, Sozen T, Fatih O, Erdem O, Bayar N, Cingi C. Influence of size and site of perforation on fat graft myringoplasty. Auris Nasus Larynx. 2014;41(6):507–512. doi:10.1016/j.anl.2014.08.004
- 10. Frade González C, Castro Vilas C, Cabanas Rodríguez E, Elhendi W, Vaamonde Lago P, Labella Caballero T. Factores pronósticos del resultado anatómico y funcional de las miringoplastias. Acta Otorrinolaringol Esp. 2002;53(10):729–735. doi:10.1016/s0001-6519(02)78369-6
- 11. Committee on Hearing and Equilibrium. Committee on Hearing and Equilibrium guidelines for the evaluation of results of treatment of conductive hearing loss. Otolaryngol Head Neck Surg. 1995;113(3):186–187. doi:10.1016/S0194-5998(95)70103-6
- 12. Kim D, Park S, Yeo SW, et al. Clinical efficacy of fat-graft myringoplasty for perforations of different sizes and locations. Acta Otolaryngol. 2011;131(1):22–26. doi:10.3109/00016489.2010.499881
- 13. Rinker BD, Vyas KS. Do stem cells have an effect when we fat graft? Ann Plast Surg. 2016;76(suppl 4):S359–S363.
- 14. Ambani KP, Gangwani RW, Bhavya BM, Vakharia SD, Katarkar AU. A comparative study between fat myringoplasty and temporalis fascia tympanoplasty in moderate to large central perforation of pars tensa of tympanic membrane. Int J Otorhinolaryngol Head Neck Surg. 2017;3(4):997. doi:10.18203/issn.2454-5929.ijohns20174321
- 15. Yuping R, Kolinin MG, Li Y. Cell, stem cells and regenerative medicine fat grafting with adipose stem cells: the successes and challenges. Sciforschen. 2015;12:1–4.
- 16. Fraser JK, Wulur I, Alfonso Z, Hedrick MH. Fat tissue: an underappreciated source of stem cells for biotechnology. Trends Biotechnol. 2006;24(4):150–154.
- 17. Ogawa R, Mizuno H, Watanabe A, Migita M, Hyakusoku H, Shimada T. Adipogenic differentiation by adipose-derived stem cells harvested from GFP transgenic mice—including relationship of sex differences. Biochem Biophys Res Commun. 2004;319(2):511–517. doi:10.1016/J.BBRC.2004.05.021
- 18. Gimble JM, Katz AJ, Bunnell BA. Adipose-derived stem cells for regenerative medicine. Circ Res. 2007;100(9):1249–1260. doi:10.1161/01.RES.0000265074.83288.09
- 19. Ayache S, Braccini FF, Facon F, Thomassin JM. Adipose graft: an original option in myringoplasty. Otol Neurotol. 2003;24(2):158–164.
- 20. Ringenberg JC. Fat graft myringoplasty. Laryngoscope. 1962;72:188–192.
- 21. Deddens AE, Muntz HR Lusk RP. Adipose myringoplasty in children. Laryngoscope. 1993;103(2):216–219.
- 22. Liew L, Daudia A, Narula A. Synchronous fat plug myringoplasty and tympanostomy tube removal in management of refractory otorrhoea in younger patients. Int J Pediatr Otorhinolaryngol. 2002;66(3):291–296.
- 23. Terry RM, Bellini MJ, Clayton MI, Gandhi AG. Fat graft myringoplasty—a prospective trial. Clin Otolaryngol Allied Sci. 1988;13(3):227–229.
- 24. Goodman WS. Tympanoplasty: areolar tissue graft. Laryngoscope. 1971;81(11):1819–1825.
- 25. Gross CW, Bassila M, Lazar R, Long TE, Stagner S. Adipose plug myringoplasty: an alternative to formal myringoplasty techniques in children. Otolaryngol Head Neck Surg. 1989;101(6):617–620.
- 26. Schraff S, Markham J, Welch C, Darrow DH, Derkay CS. Outcomes in children with perforated tympanic membrane after tympanostomy tube placement: results using a pilot treatment algorithm. Am J Otolaryngol. 2006;27(4):238–243.
- 27. Hagemann M. Tympanoplastik mit autologem Fettgewebe. Laryngorhinootologie. 2003;82(6):393–396. doi:10.1055/s-2003-40537
- 28. Ozgursoy OB, Yorulmaz I. Fat graft myringoplasty: a cost effective but underused procedure. J Laryngol Otol. 2005;119(4):277–279.
- 29. Gibb AG, Chang SK. Myringoplasty (A review of 365 operations). J Laryngol Otol. 1982;96(10):915–930.
- 30. Fiorino F, Barbieri F. Fat graft myringoplasty after unsuccessful tympanic membrane repair. Eur Arch Otorhinolaryngol. 2007;264(10):1125–1128. doi:10.1007/s00405-007-0323-z
- 31. Alzahrani M, Saliba I. Hyaluronic acid fat graft myringoplasty vs fat patch fat graft myringoplasty. Eur Arch Otorhinolaryngol. 2015;272(8):1873–1877. doi:10.1007/s00405-014-2982-x
- 32. Saliba I. Hyaluronic acid fat graft myringoplasty: how we do it. Clin Otolaryngol. 2008;33(6):610–614.
- 33. Ersözlü T, Gultekin E. A comparison of the autologous platelet-rich plasma gel fat graft myringoplasty and the fat graft myringoplasty for the closure of different sizes of tympanic membrane perforations. Ear Nose Throat J. 2020;99(5):331–336. doi:10.1177/0145561319900388
- 34. Shoman NM. Clinical and audiometric outcomes of palisade cartilage myringoplasty under local anesthetic in an office setting. Am J Otolaryngol. 2019;40(4):482–486. doi:10.1016/j.amjoto.2019.03.015