See the editorial comment for this article ‘Coronary flow reserve: a versatile tool for interrogating pathophysiology, and a reliable marker of cardiovascular outcomes and mortality’, by Viviany R. Taqueti, https://doi.org/10.1093/eurheartj/ehac001.
Introduction
Coronary flow reserve (CFR) describes the ratio by which coronary blood flow can be augmented by exercise, stress, or microcirculatory vasodilation. As an index of coronary disease severity, CFR has several biological and practical advantages. First, CFR offers a quantitative, global physiological interrogation of the coronary circulation, reflecting disease processes that affect both the epicardial territory and the distal coronary vasculature, including small vessels and capillaries. Second, CFR can be measured using non-invasive modalities, including echocardiography, positron emission tomography (PET), and cardiac magnetic resonance (CMR) as well as invasively with Doppler flow velocity and thermodilution. As a result, CFR measurement is now recommended by international guidelines as a diagnostic method for the identification of patients with microvascular angina who could benefit from targeted therapy.
Although extensively investigated within specific disease states, the utility of coronary flow indices as prognostic tools has not been systematically quantified across a wide range of pathologies and measurement modalities in a single, consistent analysis.
In this study, we perform a systematic review and meta-analysis of all studies reporting the impact of an abnormal CFR on all-cause mortality and major adverse cardiovascular events (MACE), across a broad range of disease processes.
Methods
Search strategy
We performed a systematic search of the MEDLINE, The Cochrane Central Register of Controlled Trials, and EMBASE databases from 1 January 2000 to 1 August 2020 for all studies in humans and written in English. The literature search strategy was designed by M.J.S.-S., R.A.-L., and R.P.
The search, carried out by M.A.K. and H.S., included the search strings (‘coronary flow reserve’ or ‘coronary flow velocity reserve’ or ‘coronary flow reserves’ or ‘coronary flow reserve velocity’ or ‘index of microcirculatory resistance’ or ‘index of microcirculatory resistance’ or ‘index of microvascular resistance’ or ‘myocardial blood flow’ or ‘myocardial flow reserve’ or ‘hyperaemic microvascular resistance’) AND (‘prognosis’ or ‘prognostic’ or ‘predictor’ or ‘survival’ or ‘mortality’ or ‘death’ or ‘outcome’ or ‘outcomes’ or ‘MACE’ or ‘major adverse cardiovascular event’ or ‘major adverse cardiovascular events’ or ‘MACEs’). C.A.R. and A.N.N. hand-searched the bibliographies of relevant selected studies, reviews, and meta-analyses to identify further eligible studies.
Inclusion and exclusion criteria
We included studies that prospectively measured invasive or non-invasive indices of coronary flow and reported a hazard ratio (HR) for all-cause mortality and/or MACE. Indices of coronary flow measurement included CFR, measured via echocardiography, PET, or invasively via Doppler or thermodilution; coronary flow velocity reserve (CFVR), measured via echocardiography; myocardial blood flow reserve (MBFR), measured via CMR; myocardial flow reserve (MFR), via CMR; and quantitative myocardial perfusion reserve (MPR), via CMR and the index of microcirculatory resistance (IMR), measured via invasive thermodilution. Indices representing the ratio between maximal coronary blood flow and resting hyperaemic coronary blood flow (CFR, CFVR, MBFR, MFR, and MPR) were considered equivalent; these studies were meta-analysed together and are collectively referred to as ‘CFR’. We performed a separate analysis of studies measuring the association of the index of microcirculatory resistance (IMR) with mortality and MACE. The definition of abnormal CFR or IMR was that used in each study [defined via receiver operating characteristic curve, median sample values, or pre-established clinically acceptable cut-offs]. Studies measuring CFR were analysed separately from those measuring IMR. Studies in which CFR was stratified into more than two bands were included in the systematic review but not the meta-analysis. Studies in which IMR was evaluated only by groups pre-determined by CFR stratification were included in the systematic review but not the meta-analysis. Abstracts were reviewed for suitability and articles retrieved accordingly. Two authors performed the search and literature screening (M.A.K. and H.S.), with disputes resolved by discussion with a third author (R.P.). If studies had overlapping first or senior author, or institution, M.F. and H.R. evaluated the published methods to look for evidence of overlapping patient cohorts (dates, disease group, modality). When this was not clear, the authors were contacted to ask for clarification.
Endpoints
The primary outcome was the HR of all-cause mortality associated with abnormal CFR. The secondary outcome was the HR of MACE associated with abnormal CFR. There were various definitions of MACE used (Supplementary material online, Table S1). We adopted the definition as used in each study, which were a combination of the following: death; non-fatal myocardial infarction; stroke; development of or hospitalization for heart failure; revascularization; arrhythmia; and (where relevant) cardiac allograft vasculopathy.
Data extraction and statistical analysis
Two authors (M.A.K. and H.S.) independently extracted the data from included studies, verified by a third author (R.P.). J.P.H., Y.A., and S.S. formulated the analysis plan.
We extracted the study population information from each study including mean age ± standard deviation (SD), follow-up duration, disease state, percentage of female population, prevalence of risk factors such as hypertension, smoking, and diabetes mellitus, and, where available, the mean CFR for abnormal and normal populations. We calculated and presented weighted means for these characteristics for the overall study population in our analysis. Where appropriate, variances were derived from SDs from each study, and mean weighted variance was used to calculate a weighted SD for the overall study population.
We extracted the event rates, HRs, and their associated 95% confidence intervals (CIs) and P-values. Where both annualized and end-of-study event rates were reported, only the end-of-study event rate was recorded. Where event rate data were missing from a manuscript, we searched any available supplementary material. Where available, we reported event rates for both mortality and MACE as weighted means, stratified by abnormal vs. normal CFR. We reported effect sizes in terms of HRs, since time-to-event data for endpoints are more methodologically robust than reporting the number of events at an arbitrary timepoint.
When data were presented as HR of abnormal vs. normal CFR, we inverted the values to present them systematically. Where studies investigated two or more populations divided by a baseline characteristic (e.g. hypertensives vs. non-hypertensives) and provided baseline characteristic data and HRs for each, they were considered as separate studies for the purposes of meta-analysis. Where continuous HRs were presented (HR per x unit change in CFR), both the HR and associated CIs were adjusted by the appropriate factor to allow systematic presentation of HRs per 0.1 unit decrease in CFR. Where continuous HRs were presented per SD change in CFR, these studies were not included in the meta-analysis but added to the systematic review. Where populations were not analysed dichotomously (e.g. in tertiles of flow reserve), the study was not included in the meta-analysis but added to the systematic review.
We performed a random-effects meta-analysis using inverse-variance weighting (expressed as HRs with 95% CI), using the natural logarithm of the HRs and their associated standard errors, using the DerSimonian and Laird method. The HRs and 95% CIs were presented as forest plots. The statistical programming environment Review Manager (RevMan) 5.4 was used for all statistical analysis.
When available, multivariate adjusted HRs were extracted and meta-analysed together with studies that only reported unadjusted HRs. We performed a sensitivity analysis of studies reporting adjusted vs. unadjusted HRs. Because multivariate adjustments varied across studies, we also performed a sensitivity analysis to explore whether the magnitude of hazard would differ when studies were grouped only within those which adjusted for commonly known risk factors such as age, sex, presence of diabetes mellitus, smoking history, dyslipidaemia, hypertension, and history of prior myocardial infarction. We included studies that did not adjust for these covariates at multivariate analysis if they were found to have no significant association with outcomes at univariate analysis.
We performed subgroup analyses of studies measuring CFR in patients with isolated coronary microvascular dysfunction (CMD). Isolated CMD was defined by an abnormal CFR with non-obstructive coronary artery disease on invasive coronary angiography or a negative stress test for myocardial ischaemia,, with no history of heart transplantation, cardiomyopathy, or aortic stenosis.
Heterogeneity was assessed using the I2 test. An I2 of <25% was considered no statistical heterogeneity, 25–50% was considered as low statistical heterogeneity, 50–75% was considered as medium statistical heterogeneity, and >75% was considered as high statistical heterogeneity. Where there was high statistical heterogeneity, subgroup analyses were performed to assess any potential differences between modalities of measurement of CFR, as well as between different presenting pathologies.
Two authors (M.A.K. and H.S.) assessed the included studies for risk of bias using the Newcastle–Ottawa quality assessment scale for cohort studies. A quality score was calculated for three major components of cohort studies: (i) selection of study groups (0–4 points), comparability of study groups (0–2 points), and determination of outcome of interest (0–3 points). A higher score represented greater methodological quality.
Results were reported in accordance with the PRISMA guideline (see Supplementary material online, Table S2) and our study was prospectively submitted on 20 August 2020 to the PROSPERO international prospective register of systematic reviews (ID 161787).
Results
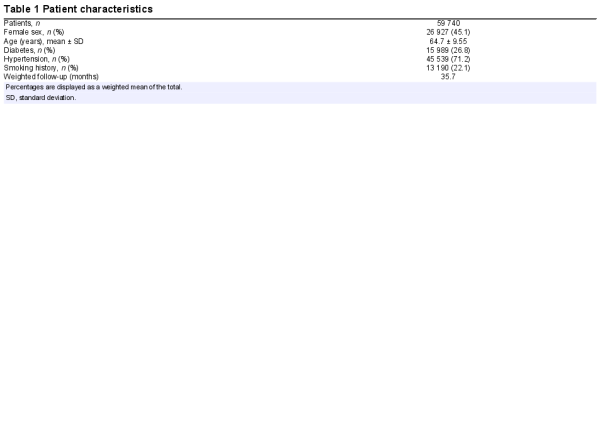
A total of 79 studies, which investigated the impact of coronary flow on the prognosis of 59 740 individuals met the inclusion criteria (Figure 1).,,, The characteristics of included studies are summarized in Supplementary material online, Table S3. Thirty-five studies including 21 307 subjects were excluded due to subject overlap in multiple studies. The patient characteristics of included studies are summarized in Table 1. Most studies included patients with proven or suspected ischaemic heart disease (58 studies including 57 613 subjects). Other common patient groups included: heart failure (7 studies including 647 subjects), heart transplants (8 studies including 784 subjects), and type 2 diabetes mellitus without symptoms of coronary artery disease (3 studies including 541 subjects). From these groups, 15 studies including 10 848 subjects were identified that met the criteria for isolated CMD. The mean follow-up duration was 35.7 months, ranging from 1 month to 150 months.
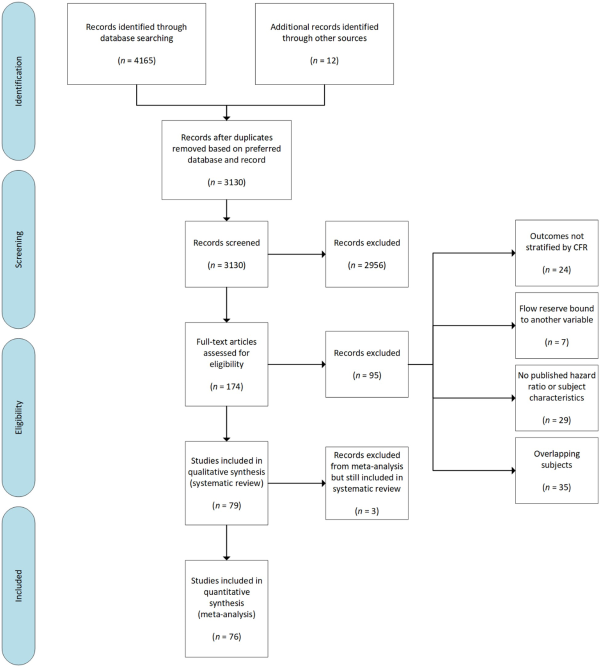
Figure 1
Search strategy and source of included studies. CFR, coronary flow reserve.
Coronary flow measurements were made using echocardiography (39 studies), PET (18 studies), CMR (4 studies), and invasive measurement (18 studies). For studies using echocardiography, the mean normal CFR was 2.61 ± 0.49, and mean abnormal CFR was 1.66 ± 0.27. For studies using invasive measurement, the mean normal CFR was 3.61 ± 1.64, and mean abnormal CFR was 2.14 ± 0.63. The composite definition of MACE varied between studies, with various combinations of mortality, non-fatal myocardial infarction, hospitalization with congestive heart failure, arrhythmia, re-transplantation, and coronary revascularization.
Sixty-three out of 79 included studies (42 667 subjects) reported data by dividing subjects dichotomously into two CFR groups (normal vs. impaired), as defined by a pre-specified clinical cut-off. The median cut-off for included studies was ≤ 2.0, ranging from 1.5 to 3.0. The mean normal and abnormal CFR across all studies were 2.70 ± 0.68 and 1.70 ± 0.32, respectively. The event rate for mortality (14 studies including 8368 patients) was 24.3% vs. 6.7% for abnormal vs. normal CFR cohorts, respectively. The event rate for MACE (28 studies including 5735 patients) was 26.4% vs. 7.9% for abnormal and normal CFR cohorts, respectively (Supplementary material online, Table S4). Twelve studies including 15 567 subjects reported HRs ‘per unit change’ in flow reserve, ranging from 0.1 units to 1 unit change in CFR.
Three studies including 782 subjects divided participants into ‘tertile’ groups of CFR, with HRs reported accordingly,, and therefore these studies were not included in the meta-analysis but remained part of our systematic review and discussion. The prognostic impact of IMR was systematically reviewed and meta-analysed separately by examining data from nine studies including 2356 subjects.
Impact of abnormal CFR on all-cause mortality and MACE
When HRs were meta-analysed, an abnormal CFR conferred a significantly increased hazard of both mortality (16 studies including 8446 subjects; HR: 3.78, 95% CI: 2.39–5.97, I2 = 88%) and MACE (60 studies including 35 498 subjects; HR: 3.42, 95% CI: 2.92–3.99, I2 = 73%) (Figure 2).
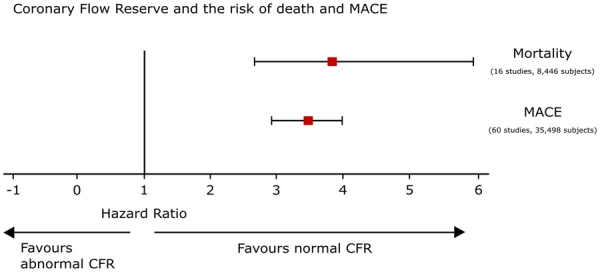
Figure 2
Coronary flow reserve and risk of death and major adverse cardiovascular events. All hazard ratios expressed after multivariable-adjusted analysis. Hazard ratios shown for the outcomes for which there are sufficient published data (i.e. at least two studies). Hazard ratios are represented by squares, and 95% confidence intervals are represented by horizontal lines.
The strength of association of CFR on mortality varied across multiple disease presentations (Figure 3), with significant hazard observed in patients with chronic coronary syndromes (7 studies including 7573 subjects; HR: 4.41, 95% CI: 2.61–7.44, I2 = 78%) and heart transplants (3 studies including 334 subjects; HR: 2.95, 95% CI: 1.81–4.82, I2 = 0%), with low inter-subgroup heterogeneity (χ2 test: 8.11, P = 0.23, I2 = 26%).
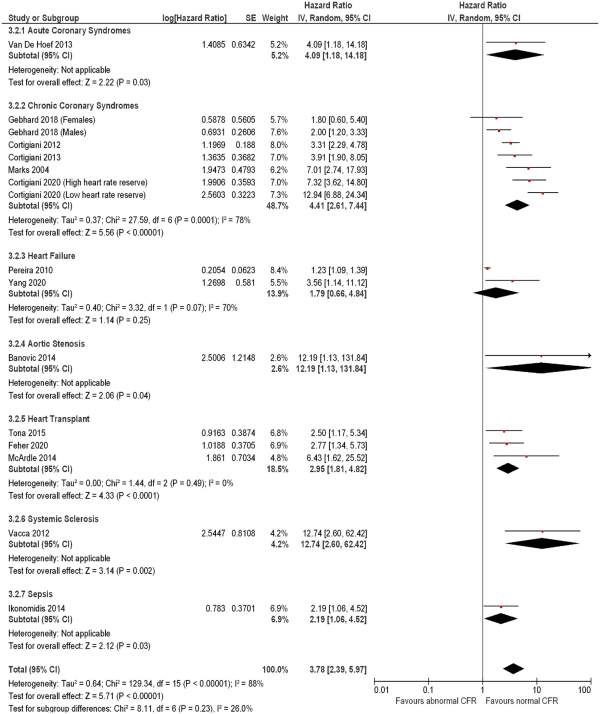
Figure 3
Forest plot showing coronary flow reserve as an indicator of all-cause mortality. Hazard ratios for individual studies are represented by squares, and 95% confidence intervals (CI) are represented by horizontal lines. Pooled estimates and their 95% confidence intervals are represented by diamonds. Subgroups are by disease presentation. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes.
The association of CFR with MACE was consistent across all disease presentations (Figure 4), with greatest hazard observed in patients with diabetes mellitus and no symptoms of ischaemic heart disease (3 studies including 541 subjects; HR: 7.47, 95% CI: 3.37–16.55, I2 = 34%) and heart failure (3 studies including 320 subjects; HR: 6.38, 95% CI: 1.95–20.90, I2 = 43%). In patients presenting with ischaemic heart disease (Figure 5), a comparable effect was seen in patients with ST-elevation myocardial infarction (STEMI) (3 studies including 529 subjects; HR: 4.35, 95% CI: 2.18–8.68, I2 = 0), non-ST-elevation myocardial infarction (NSTEMI) or unstable angina (7 studies including 886 subjects; HR: 3.76, 95% CI: 2.35–6.00, I2 = 30%), and chronic coronary syndromes (40 studies including 33 029 subjects; HR: 3.16, 95% CI: 2.64–3.78, I2 = 78%), with no inter-subgroup heterogeneity (χ2 test: 1.17, P = 0.56, I2 = 0).
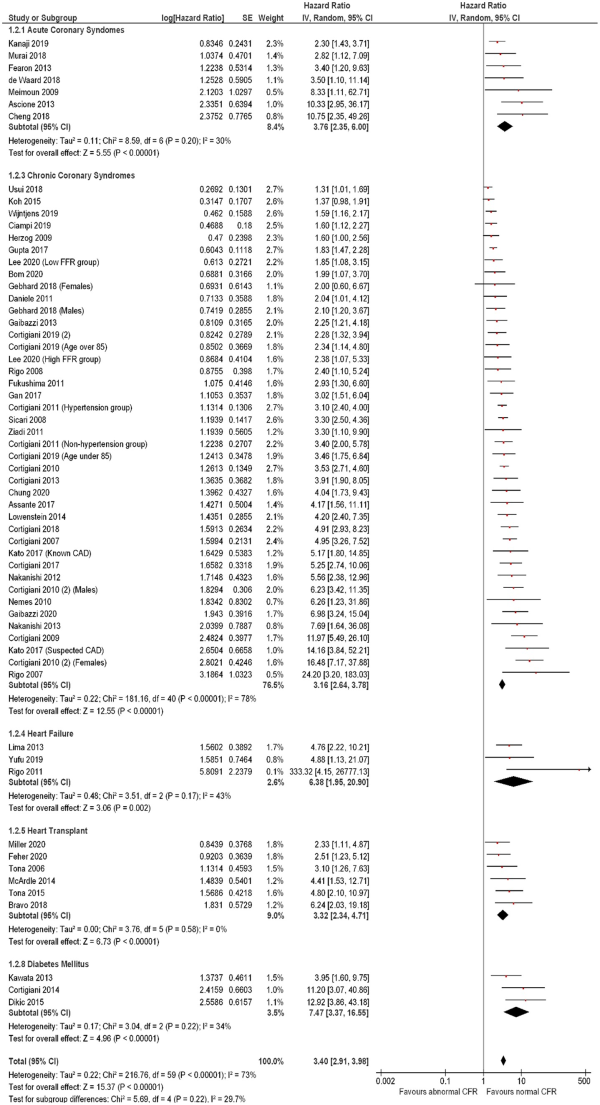
Figure 4
Forest plot showing coronary flow reserve as an indicator of major adverse cardiovascular events. Hazard ratios for individual studies are represented by squares, and 95% confidence intervals are represented by horizontal lines. Pooled estimates and their 95% confidence intervals are represented by diamonds. Subgroups are by disease presentation. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes.
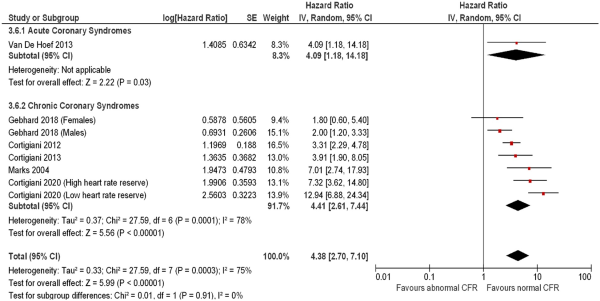
Figure 5
Forest plot showing coronary flow reserve as an indicator of mortality in patients with ischaemic heart disease. Hazard ratios for individual studies are represented by squares, and 95% confidence intervals are represented by horizontal lines. Subgroups are by disease presentation. Pooled estimates and their 95% confidence intervals are represented by diamonds. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes.
Amongst studies reporting HRs per unit change of CFR, each 0.1 unit reduction in CFR was associated with an increased hazard of mortality (4 studies including 13 809 subjects; HR per 0.1 unit reduction: 1.16, 95% CI: 1.04–1.29, I2 = 80%) (Supplementary material online, Figure S1) and MACE (8 studies including 6429 subjects; HR per 0.1 unit reduction: 1.08, 95% CI: 1.04–1.11, I2 = 74%) (Supplementary material online, Figure S2).
Impact of abnormal CFR in patients with isolated CMD
Sixteen studies including 10 848 subjects investigated CFR in patients with CMD (Supplementary material online, Table S5). Two studies including 4481 subjects investigated the impact of abnormal CFR on overall mortality (Supplementary material online, Figure S3). In this group, an abnormal CFR was associated with a significantly increased mortality hazard (HR: 5.44, 95% CI: 3.78–7.83, I2 = 0%). Fourteen studies (6367 subjects) reported the association of abnormal CFR with MACE (Supplementary material online, Figure S4). In this group, abnormal CFR was associated with a significantly increased hazard of MACE (HR: 3.56, 95% CI: 2.14–5.90, I2 = 93%).
Prognostic impact of abnormal IMR
Eight studies including 1097 subjects evaluated the prognostic impact of IMR (Supplementary material online, Table S6) with an observed association between abnormal IMR and MACE (HR: 1.15, 95% CI: 1.02–1.29, I2 = 82%) (Figure 6). This effect was not consistent across multiple disease presentations including STEMI (3 studies including 624 patients, HR: 2.03, 95% CI: 0.79–5.21, I2 = 90%), NSTEMI or unstable angina (2 studies including 655 patients, HR: 1.26, 95% CI; 0.79–2.00, I2 = 82%), chronic coronary syndromes (2 studies including 283 patients, HR: 3.99, 95% CI: 0.39–40.31, I2 = 67%), and heart transplants (1 study including 74 patients, HR: 3.93, 95% CI: 1.08–14.30). One study, including 572 subjects, reported the impact of abnormal IMR on mortality (HR; 1.56, 95% CI: 1.16–2.10).
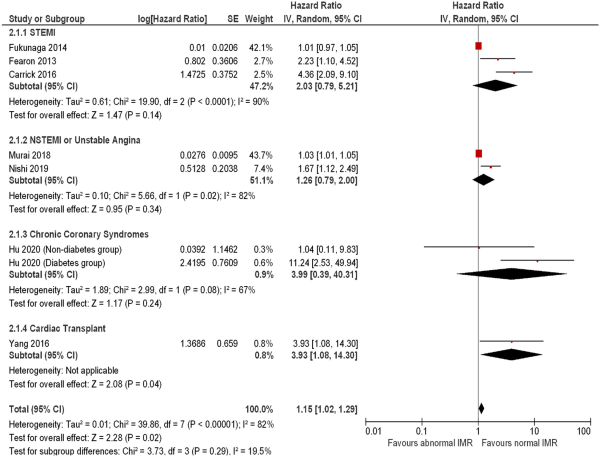
Figure 6
Forest plot showing the index of microcirculatory resistance as an indicator of major adverse cardiovascular events. Hazard ratios for individual studies are represented by squares, and 95% confidence intervals are represented by horizontal lines. Pooled estimates and their 95% confidence intervals are represented by diamonds. Subgroups are by disease presentation. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes. NSTEMI, non-ST-elevation myocardial infarction; STEMI, ST-elevation myocardial infarction.
Subgroup analysis by measurement modality
Subgroup analysis of studies measuring the association of abnormal CFR on mortality revealed a consistent, preserved effect across different measurement modalities (Figure 7), with medium inter-subgroup heterogeneity (χ2 test: 5.06, P = 0.08, I2 = 60.5%). The hazard associated with abnormal CFR on mortality with invasive measurement (3 studies including 424 subjects) was HR: 4.98 (95% CI: 2.66–9.32, I2 = 0%); with echocardiography (9 studies including 7174 subjects) HR: 4.19 (95% CI: 2.14–8.21, I2 = 93%), and with PET (4 studies including 848 subjects) HR 2.35 (95% CI; 1.61–3.42, I2 = 0%). A similar effect was observed for the outcome of MACE (Figure 8), with medium heterogeneity seen in studies using CMR (3 studies including 204 subjects, HR: 4.81, 95% CI: 1.71–13.59, I2 = 74%) and echocardiography [35 studies including 24 785 subjects, HR: 4.33 (95% CI: 3.62–5.19, I2 = 60%)].
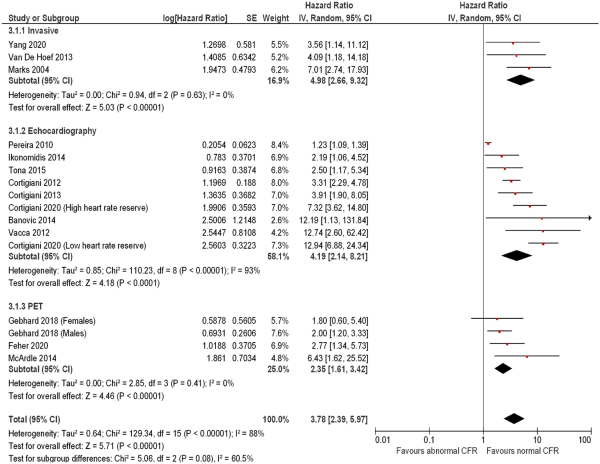
Figure 7
Forest plot showing coronary flow reserve as an indicator of mortality—subgroup analysis by measurement modality. Hazard ratios for individual studies are represented by squares, and 95% confidence intervals are represented by horizontal lines. Pooled estimates and their 95% confidence intervals are represented by diamonds. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes. CMR, cardiac magnetic resonance; PET, positron emission tomography.
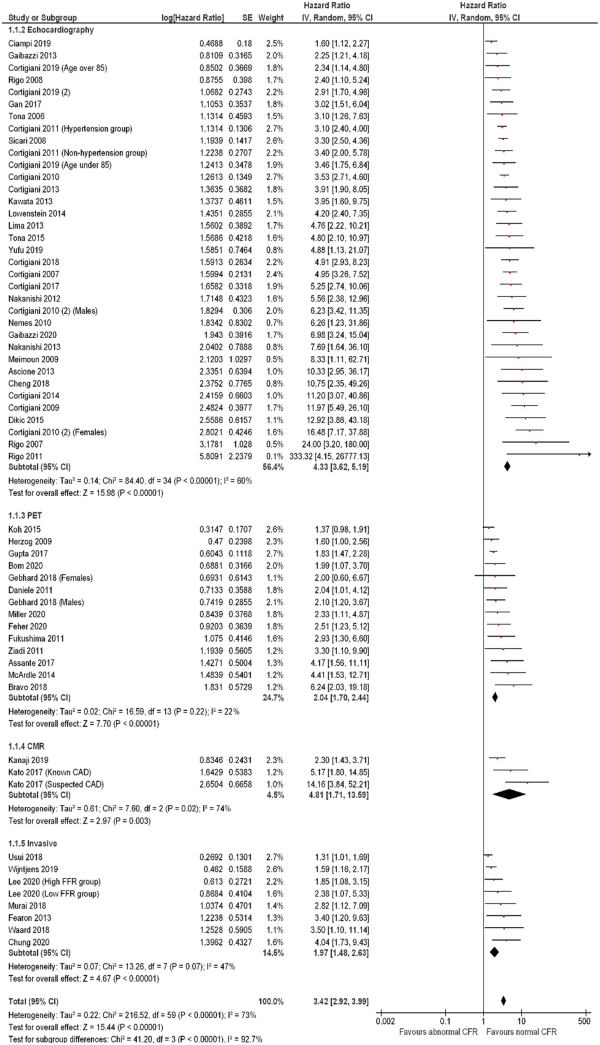
Figure 8
Forest plot showing coronary flow reserve as an indicator of major adverse cardiovascular events—subgroup analysis by measurement modality. Hazard ratios for individual studies are represented by squares, and 95% confidence intervals are represented by horizontal lines. Pooled estimates and their 95% confidence intervals are represented by diamonds. The sizes of the squares and the diamonds are proportional to the weight assigned to the relative effect sizes. CMR, cardiac magnetic resonance; PET, positron emission tomography.
There was high heterogeneity among studies of patients presenting with chronic coronary syndromes for both outcomes of mortality (I2 = 78%) and MACE (I2 = 78%). Subgroup analysis of these studies by measurement modality revealed medium inter-subgroup heterogeneity for mortality (χ2 test: 5.06, P = 0.08, I2 = 60.5%) and high inter-subgroup heterogeneity for MACE (χ2 test: 41.20, P < 0.001, I2 = 92.7%), with high heterogeneity seen in the echocardiography subgroup for both outcomes (Supplementary material online, Figures S5 and S6).
Subgroup analysis by presenting pathology for studies measuring the association of IMR with MACE showed no inter-subgroup heterogeneity (χ2 test = 3.74, P = 0.29, I2 = 19.5%).
Sensitivity analysis
On sensitivity analysis, the magnitude of hazard conferred by an abnormal CFR did not change when individual studies that adjusted for specific confounders were grouped together. When adjusted for age, sex, presence of diabetes mellitus, hypertension, dyslipidaemia, and smoking history, CFR remains associated with mortality (5 studies including 6948 subjects, HR: 5.41, 95% CI: 3.05–9.60) and MACE (27 studies including 19 296 subjects; HR: 3.75, 95% CI: 3.05–4.62). When restricted to studies additionally adjusting for prior myocardial infarction, the association of abnormal CFR with both mortality (4 studies including 6786 subjects; HR: 5.79, 95% CI: 3.00–11.19) (Supplementary material online, Figure S7) and MACE (14 studies including 14 708 subjects; HR: 3.46, 95% CI: 2.71–4.41) was preserved (Supplementary material online, Figure S8).
Sensitivity analysis for studies reporting adjusted vs. only unadjusted HRs (Supplementary material online, Figures S9 and S10) revealed a consistent association of abnormal CFR with both mortality and MACE, with no inter-subgroup heterogeneity (χ2 test: 0.12, P = 0.73, I2 = 0%).
Subgroup analysis when grouping studies adjusting for sex was limited to four studies reporting the association of abnormal CFR with MACE, including 1235 male and 1044 female subjects (Supplementary material online, Figure S11). The associated HR for males was 3.60 (95% CI: 1.24–10.44), and for females HR: 6.02 (95% CI: 0.76–47.42) with no inter-subgroup heterogeneity (χ2 test: 0.19, P = 0.66, I2 = 0%).
Discussion
In the present study, we systematically reviewed and meta-analysed studies that evaluated the association between abnormal coronary flow and clinical outcomes. We found that, across a broad range of pathologies and patient cohorts, impaired CFR was associated with an increased hazard of all-cause mortality and MACE. Normal CFR was associated with improved prognosis within all subgroups analysed, and when measured using all clinically available invasive and non-invasive modalities (Graphical Abstract).
Coronary flow and prognosis
On average, we found that an abnormal CFR conferred subjects a 3.7 times increased risk of death, based on 16 studies including 8446 patients. This finding is important because overall mortality is a bias-resistant and clinically relevant outcome. Furthermore, we found that an abnormal CFR conferred subjects a 3.4 times increased hazard of cardiovascular events, even when multiple other known prognostic markers, such as age, hypertension, diabetes mellitus, dyslipidaemia, smoking history, and prior myocardial infarction, were adjusted for in individual studies. We found that, on average, every 0.1 unit reduction in CFR is associated with a 16% increase in hazard of death. Although 0.1 units is pragmatically a small gradation in the individual patient, this finding demonstrates that CFR represents a continuum of risk, with lower levels predisposing patients to more adverse clinical outcomes.
Our findings are consistent across a broad range of disease groups, which importantly include both established cardiovascular pathologies and subjects at risk of vascular disease, such as those with diabetes mellitus. In addition, a preserved CFR appears to have a protective role against adverse clinical outcomes even amongst subjects whose pathologies have a less known vascular link, such as systemic sclerosis.
Because our study sample was intentionally drawn from multiple disease groups with a wide risk profile, overall heterogeneity was high. Importantly, however, subgroup analysis by pathology and measurement modality demonstrated a consistent association between abnormal CFR and poorer prognosis, with low inter-group heterogeneity and similar magnitude of HRs (Figures 3and4). Perhaps expectedly, higher heterogeneity was observed in studies of patients chronic coronary syndromes (arguably a heterogeneous population with a wider range of risk), when compared with those presenting with STEMI and NSTEMI (Figure 5). Importantly, the magnitude of association between CFR and outcomes remained comparable between groups.
We also observed a variable effect size across modalities, with higher magnitudes of hazard seen in studies using echocardiography, when compared with invasively measured CFR (Figures 7and8). Speculatively, this difference in effect size may again be attributable to different risk profiles of samples recruited (and therefore different overall mean CFR), as echocardiography is more widely used as a screening tool, when compared with invasive flow assessment.
Finally, our overall findings remain unaltered when studies are grouped amongst those that adjusted for specific confounders (Supplementary material online, Figures S7 and S8). This strongly suggests that the prognostic role of CFR is independent of other known vascular risk factors, such as age, sex, diabetes mellitus, dyslipidaemia, hypertension, smoking history, and prior myocardial infarction.
Prognostic impact of the IMR
A specific analysis involving studies that evaluated the prognostic role of IMR has also found a protective role of preserved microvascular function, although to a lesser magnitude than CFR (8 studies including 1097 patients; MACE HR: 1.02, 95% CI: 1.02–1.29). Subgroup analyses in patients with STEMI (HR: 2.03, 95% CI: 0.79–5.21), NSTEMI or unstable angina (HR: 1.26, 95% CI: 0.79–2.00), and chronic coronary syndromes (HR: 3.99, 95% CI: 0.39–40.31) did not reach significance. This could potentially be explained by the smaller number of patients in such studies. Lee et al. grouped 867 subjects by CFR and reported HRs for the association of IMR with MACE. IMR was found to be prognostic only in a subgroup of patients with abnormal CFR (HR: 2.87, 95% CI: 1.48–5.59).
Only one study reported a HR for the association of hyperaemic microvascular resistance (HMR) with MACE. De Waard et al. demonstrated that HMR, a Doppler-based measure of microvascular function, had greater prognostic association with MACE per 1 unit change (HR: 1.55, 95% CI: 1.18–2.04) than CFR (HR: 0.36, 95% CI: 0.12–1.09). These findings have been reinforced by Toya et al., who demonstrated a comparable risk of MACE associated with unit changes in CFR and HMR. Consequently, further studies are required to clarify the prognostic association of HMR with mortality and MACE.
Implications for clinical practice
Assessment of coronary flow is already recommended in clinical guidelines as a diagnostic modality for patients with angina, to allow targeted therapy to those individuals without obstructive epicardial coronary artery disease. Our findings strongly support such recommendations and suggest that coronary flow assessment using multiple measurement modalities could be expanded to other patient cohorts and pathologies.
Numerous studies already support a strategy of aggressive risk-factor modification in patients with stable coronary artery disease. Although it has not been proven that improvement in coronary flow is one of the mechanisms by which medical interventions offer benefit to patients, our findings support such a pathophysiological mechanism. Multiple disease states are associated with impaired coronary flow (including diabetes mellitus, hypercholesterolaemia, and hypertension) and many interventions known to be of prognostic benefit in such patients also augment coronary flow, such as antihypertensive,, glucose-lowering, and lipid-lowering agents., Notably, we found that in patients with isolated CMD, an abnormal CFR confers subjects a five times increased hazard of death and 3.5 times increased hazard of cardiovascular events, supporting the findings recently reported by Gdowski et al. These findings are also consistent with Brainin et al., who quantified the cardiovascular risk associated with non-endothelial-dependent coronary vascular dysfunction using echocardiography (relative risk: 4.58, 95% CI: 3.58–5.87) and PET (relative risk: 2.44, 95% CI: 1.80–3.30).
Whilst the results of the COURAGE and ISCHEMIA studies have shown that epicardial revascularization is not associated with significant reduction in MACE in patients with stable coronary artery disease, our findings build upon accumulated evidence supporting the idea that MACE risk reduction should be targeted beyond epicardial coronary disease. The overall functional health of the coronary circulation appears to be a more reversible target for vascular therapies, via populational lifestyle modification as well as pharmacological interventions. Our results also demonstrate that the association between reduced coronary flow and increased risk of mortality and MACE is consistent across multiple disease processes and all available diagnostic modalities, suggesting that coronary flow should be more routinely used in a broader group of pathologies for the identification of those at higher risk of cardiovascular events.
Limitations
This study-level meta-analysis aggregated heterogeneous populations across different disease states and measurement modalities. It precluded detailed analysis of temporal relationships or sub-groups, which would have been afforded by analysis of comprehensive patient-level data. All included studies published observational data and were necessarily at high risk of bias due to a lack of randomization and lack of blinding in all studies (Supplementary material online, Table S7). These studies are also at risk of publication bias, since only the positive studies are likely to be reported. Studies also used different cut-off values for normal CFR, as well as different definitions of MACE. Therefore, establishing a universal relationship between a specific CFR cut-off and specific adverse events is not possible from our study. Several studies did not publish a HR for dichotomous data, instead providing HR per unit change in CFR, which could not be part of our main analysis. However, these studies provided an arguably even more important insight into the progressive increase in hazard with degree of abnormality of CFR. The overall heterogeneity of our study was high; however, after multiple subgroup analyses across different pathologies and measurement modalities, we demonstrated a consistent hazard of mortality and MACE associated with abnormal CFR. In addition, we meta-analysed HRs of individual studies that did not all adjust for an identical set of variables. Our sensitivity analysis indicates that this did not affect the overall findings.
Conclusions
In patients with established cardiovascular disease, and in those at risk of developing it, impaired coronary flow is strongly associated with an increased hazard of death and MACE. Clinicians should incorporate coronary flow measurement more routinely as a diagnostic and risk stratification tool, to target strict vascular modification therapies to those at higher risk.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
The authors are grateful for the infrastructural support from the National Institute of Health Research (NIHR) Biomedical Research Centre based at Imperial College Healthcare NHS Trust and Imperial College London.
Funding
J.H. was supported by the Wellcome Trust (212183/Z/18/Z). P.D.M. was funded by the Wellcome Trust [214567/Z/18/Z]. For the purpose of Open Access, the author has applied a CC BY public copyright licence to any Author Accepted Manuscript version arising from this submission. A.N.N. was supported by the National Insitute for Health Research Academy. M.F. (MR/V001620/1) and C.A.R. (MR/S021108/1) were supported by the Medical Research Council. D.F. (FS 04/079), M.J.S.-S. (FS/14/27/30752), and R.P. (FS/11/46/28861) were supported by the British Heart Foundation.
Conflict of interest: M.A.K. has nothing to declare. H.S. reports an educational grant from Amgen. J.P.H. has nothing to disclose. H.R. has nothing to disclose. M.F. has nothing to disclose. A.N.N. has nothing to disclose. C.A.R. has nothing to disclose. M.J.S.-S. has nothing to disclose. S.S. reports speaker’s bureau fees from Philips, Pfizer, AstraZeneca, and an educational grant from Medtronic. Y.A. has nothing to disclose. R.A.-L. reports speaker’s fee from Philips Volcano. G.C. has nothing to disclose. J.M. reports a patent in iFR technology for assessment of coronary stenoses with royalties paid to Imperial Innovations. R.P. reports consultancy fees from Philips. All other authors have nothing to declare.
Data availability
The data underlying this article are available on reasonable request to the corresponding author.
References
- 1. Gould KL, Lipscomb K. Effects of coronary stenoses on coronary flow reserve and resistance. Am J Cardiol1974;34:48–55.
- 2. Kern MJ, Bach RG, Mechem CJ et al Variations in normal coronary vasodilatory reserve stratified by artery, gender, heart transplantation and coronary artery disease. J Am Coll Cardiol1996;28:1154–1160.
- 3. Kern MJ, Lerman A, Bech JW et al; American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Physiological assessment of coronary artery disease in the cardiac catheterization laboratory: a scientific statement from the American Heart Association Committee on Diagnostic and Interventional Cardiac Catheterization, Council on Clinical Cardiology. Circulation2006;114:1321–1341.
- 4. Cortigiani L, Rigo F, Gherardi S et al Coronary flow reserve during dipyridamole stress echocardiography predicts mortality. JACC Cardiovasc Imaging2012;5:1079–1085.
- 5. Herzog BA, Husmann L, Valenta I et al Long-term prognostic value of 13N-ammonia myocardial perfusion positron emission tomography added value of coronary flow reserve. J Am Coll Cardiol2009;54:150–156.
- 6. Indorkar R, Kwong RY, Romano S et al Global coronary flow reserve measured during stress cardiac magnetic resonance imaging is an independent predictor of adverse cardiovascular events. JACC Cardiovasc Imaging2019;12:1686–1695.
- 7. Gould KL, Kirkeeide RL, Buchi M. Coronary flow reserve as a physiologic measure of stenosis severity. J Am Coll Cardiol1990;15:459–474.
- 8. Knuuti J, Wijns W, Saraste A et al; ESC Scientific Document Group. 2019 ESC Guidelines for the diagnosis and management of chronic coronary syndromes: the Task Force for the diagnosis and management of chronic coronary syndromes of the European Society of Cardiology (ESC). Eur Heart J2020;41:407–477.
- 9. Gdowski MA, Murthy VL, Doering M, Monroy-Gonzalez AG, Slart R, Brown DL. Association of isolated coronary microvascular dysfunction with mortality and major adverse cardiac events: a systematic review and meta-analysis of aggregate data. J Am Heart Assoc2020;9:e014954.
- 10. Cortigiani L, Ciampi Q, Rigo F, Bovenzi F, Picano E, Sicari R. Prognostic value of dual imaging stress echocardiography following coronary bypass surgery. Int J Cardiol2019;277:266–271.
- 11. Gupta A, Taqueti VR, van de Hoef TP et al Integrated noninvasive physiological assessment of coronary circulatory function and impact on cardiovascular mortality in patients with stable coronary artery disease. Circulation2017;136:2325–2336.
- 12. Green R, Cantoni V, Acampa W et al Prognostic value of coronary flow reserve in patients with suspected or known coronary artery disease referred to PET myocardial perfusion imaging: a meta-analysis. J Nucl Cardiol2021;28:904–918.
- 13. DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials1986;7:177–188.
- 14. The Cochrane Collaboration. Review Manager (RevMan). Computer Program. 5.4th ed.; 2020.
- 15. Camici PG, Crea F. Coronary microvascular dysfunction. N Engl J Med2007;356:830–840.
- 16.
- 17. Moher D, Liberati A, Tetzlaff J, Altman DG, Group P; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Int J Surg2010;8:336–341.
- 18. Anantharam B, Janardhanan R, Hayat S et al Coronary flow reserve assessed by myocardial contrast echocardiography predicts mortality in patients with heart failure. Eur J Echocardiogr2011;12:69–75.
- 19. Ascione L, Carlomagno G, Sordelli C et al Dipyridamole coronary flow reserve stratifies prognosis in acute coronary syndrome patients without left anterior descending disease. Eur Heart J Cardiovasc Imaging2013;14:858–864.
- 20. Assante R, Acampa W, Zampella E et al Prognostic value of atherosclerotic burden and coronary vascular function in patients with suspected coronary artery disease. Eur J Nucl Med Mol Imaging2017;44:2290–2298.
- 21. Banovic M, Bosiljka VT, Voin B et al Prognostic value of coronary flow reserve in asymptomatic moderate or severe aortic stenosis with preserved ejection fraction and nonobstructed coronary arteries. Echocardiography2014;31:428–433.
- 22. Bom MJ, van Diemen PA, Driessen RS et al Prognostic value of [15O]H2O positron emission tomography-derived global and regional myocardial perfusion. Eur Heart J Cardiovasc Imaging2020;21:777–786.
- 23. Bravo PE, Bergmark BA, Vita T et al Diagnostic and prognostic value of myocardial blood flow quantification as non-invasive indicator of cardiac allograft vasculopathy. Eur Heart J2018;39:316–323.
- 24. Britten MB, Zeiher AM, Schächinger V. Microvascular dysfunction in angiographically normal or mildly diseased coronary arteries predicts adverse cardiovascular long-term outcome. Coron Artery Dis2004;15:259–264.
- 25. Carrick D, Haig C, Carberry J et al Microvascular resistance of the culprit coronary artery in acute ST-elevation myocardial infarction. JCI Insight2016;1:e85768.
- 26. Cheng R, Zhu X, Li Y, Bai X, Xue L, Wei L. Coronary flow reserve in non-infarcted myocardium predicts long-term clinical outcomes in patients undergoing percutaneous coronary intervention. Yonsei Med J2018;59:252–257.
- 27. Choi KH, Lee JM, Kim SR et al Prognostic value of the index of microcirculatory resistance over serum biomarkers in cardiac amyloidosis. J Am Coll Cardiol2020;75:560–561.
- 28. Chung JH, Lee KE, Lee JM et al Effect of sex difference of coronary microvascular dysfunction on long-term outcomes in deferred lesions. JACC Cardiovasc Interv2020;13:1669–1679.
- 29. Ciampi Q, Zagatina A, Cortigiani L et al Functional, anatomical, and prognostic correlates of coronary flow velocity reserve during stress echocardiography. J Am Coll Cardiol2019;74:2278–2291.
- 30. Cortigiani L, Ciampi Q, Carpeggiani C, Bovenzi F, Picano E. Prognostic value of heart rate reserve is additive to coronary flow velocity reserve during dipyridamole stress echocardiography. Arch Cardiovasc Dis2020;113:244–251.
- 31. Cortigiani L, Ciampi Q, Lombardo A, Rigo F, Bovenzi F, Picano E. Age- and gender-specific prognostic cutoff values of coronary flow velocity reserve in vasodilator stress echocardiography. J Am Soc Echocardiogr2019;32:1307–1317.
- 32. Cortigiani L, Gherardi S, Faggioni M et al Dual-imaging stress echocardiography for prognostic assessment of high-risk asymptomatic patients with diabetes mellitus. J Am Soc Echocardiogr2017;30:149–158.
- 33. Cortigiani L, Huqi A, Ciampi Q, Bombardini T, Bovenzi F, Picano E. Integration of wall motion, coronary flow velocity, and left ventricular contractile reserve in a single test: prognostic value of vasodilator stress echocardiography in patients with diabetes. J Am Soc Echocardiogr2018;31:692–701.
- 34. Cortigiani L, Rigo F, Galderisi M et al Diagnostic and prognostic value of Doppler echocardiographic coronary flow reserve in the left anterior descending artery in hypertensive and normotensive patients. Heart2011;97:1758–1765.
- 35. Cortigiani L, Rigo F, Gherardi S et al Prognostic implication of Doppler echocardiographic derived coronary flow reserve in patients with left bundle branch block. Eur Heart J2013;34:364–373.
- 36. Cortigiani L, Rigo F, Gherardi S, Bovenzi F, Picano E, Sicari R. Implication of the continuous prognostic spectrum of Doppler echocardiographic derived coronary flow reserve on left anterior descending artery. Am J Cardiol2010;105:158–162.
- 37. Cortigiani L, Rigo F, Gherardi S et al Prognostic effect of coronary flow reserve in women versus men with chest pain syndrome and normal dipyridamole stress echocardiography. Am J Cardiol2010;106:1703–1708.
- 38. Cortigiani L, Rigo F, Gherardi S, Galderisi M, Bovenzi F, Sicari R. Prognostic meaning of coronary microvascular disease in type 2 diabetes mellitus: a transthoracic Doppler echocardiographic study. J Am Soc Echocardiogr2014;27:742–748.
- 39. Cortigiani L, Rigo F, Gherardi S et al Additional prognostic value of coronary flow reserve in diabetic and nondiabetic patients with negative dipyridamole stress echocardiography by wall motion criteria. J Am Coll Cardiol2007;50:1354–1361.
- 40. Cortigiani L, Rigo F, Sicari R, Gherardi S, Bovenzi F, Picano E. Prognostic correlates of combined coronary flow reserve assessment on left anterior descending and right coronary artery in patients with negative stress echocardiography by wall motion criteria. Heart2009;95:1423–1428.
- 41. Daniele S, Nappi C, Acampa W et al Incremental prognostic value of coronary flow reserve assessed with single-photon emission computed tomography. J Nucl Cardiol2011;18:612–619.
- 42. de Waard GA, Fahrni G, de Wit D et al Hyperaemic microvascular resistance predicts clinical outcome and microvascular injury after myocardial infarction. Heart2018;104:127–134.
- 43. Dikic M, Tesic M, Markovic Z et al Prognostic value of calcium score and coronary flow velocity reserve in asymptomatic diabetic patients. Cardiovasc Ultrasound2015;13:41.
- 44. Farhad H, Dunet V, Bachelard K, Allenbach G, Kaufmann PA, Prior JO. Added prognostic value of myocardial blood flow quantitation in rubidium-82 positron emission tomography imaging. Eur Heart J Cardiovasc Imaging2013;14:1203–1210.
- 45. Fearon WF, Low AF, Yong AS et al Prognostic value of the Index of Microcirculatory Resistance measured after primary percutaneous coronary intervention. Circulation2013;127:2436–2441.
- 46. Feher A, Srivastava A, Quail MA et al Serial assessment of coronary flow reserve by rubidium-82 positron emission tomography predicts mortality in heart transplant recipients. JACC Cardiovasc Imaging2020;13:109–120.
- 47. Fukunaga M, Fujii K, Kawasaki D et al Thermodilution-derived coronary blood flow pattern immediately after coronary intervention as a predictor of microcirculatory damage and midterm clinical outcomes in patients with ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv2014;7:149–155.
- 48. Fukushima K, Javadi MS, Higuchi T et al Prediction of short-term cardiovascular events using quantification of global myocardial flow reserve in patients referred for clinical 82Rb PET perfusion imaging. J Nucl Med2011;52:726–732.
- 49. Gaibazzi N, Picano E, Suma S et al Coronary flow velocity reserve reduction is associated with cardiovascular, cancer, and noncancer, noncardiovascular mortality. J Am Soc Echocardiogr2020;33:594–603.
- 50. Gaibazzi N, Rigo F, Lorenzoni V et al Comparative prediction of cardiac events by wall motion, wall motion plus coronary flow reserve, or myocardial perfusion analysis: a multicenter study of contrast stress echocardiography. JACC Cardiovasc Imaging2013;6:1–12.
- 51. Gan LM, Svedlund S, Wittfeldt A et al Incremental value of transthoracic Doppler echocardiography-assessed coronary flow reserve in patients with suspected myocardial ischemia undergoing myocardial perfusion scintigraphy. J Am Heart Assoc2017;6:e004875.
- 52. Gebhard C, Fiechter M, Herzog BA et al Sex differences in the long-term prognostic value of (13)N-ammonia myocardial perfusion positron emission tomography. Eur J Nucl Med Mol Imaging2018;45:1964–1974.
- 53. Hu X, Zhang J, Lee JM et al Prognostic impact of diabetes mellitus and index of microcirculatory resistance in patients undergoing fractional flow reserve-guided revascularization. Int J Cardiol2020;307:171–175.
- 54. Ikonomidis I, Makavos G, Nikitas N et al Coronary flow reserve is associated with tissue ischemia and is an additive predictor of intensive care unit mortality to traditional risk scores in septic shock. Int J Cardiol2014;172:103–108.
- 55. Kanaji Y, Yonetsu T, Hamaya R et al Prognostic value of phase-contrast cine-magnetic resonance imaging-derived global coronary flow reserve in patients with non-ST-segment elevation acute coronary syndrome treated with urgent percutaneous coronary intervention. Circ J2019;83:1220–1228.
- 56. Kato S, Saito N, Nakachi T et al Stress perfusion coronary flow reserve versus cardiac magnetic resonance for known or suspected CAD. J Am Coll Cardiol2017;70:869–879.
- 57. Kawata T, Daimon M, Hasegawa R et al Prognostic value of coronary flow reserve assessed by transthoracic Doppler echocardiography on long-term outcome in asymptomatic patients with type 2 diabetes without overt coronary artery disease. Cardiovasc Diabetol2013;12:121.
- 58. Knott KD, Seraphim A, Augusto JB et al The prognostic significance of quantitative myocardial perfusion: an artificial intelligence-based approach using perfusion mapping. Circulation2020;141:1282–1291.
- 59. Koh AS, Murthy VL, Sitek A et al Left atrial enlargement increases the risk of major adverse cardiac events independent of coronary vasodilator capacity. Eur J Nucl Med Mol Imaging2015;42:1551–1561.
- 60. Konerman MC, Lazarus JJ, Weinberg RL et al Reduced myocardial flow reserve by positron emission tomography predicts cardiovascular events after cardiac transplantation. Circ Heart Fail2018;11:e004473.
- 61. Lee JM, Choi KH, Doh JH et al Long-term patient prognostication by coronary flow reserve and index of microcirculatory resistance: international registry of comprehensive physiologic assessment. Korean Circ J2020;50:890–903.
- 62. Lima MF, Mathias W Jr, Sbano JC et al Prognostic value of coronary and microvascular flow reserve in patients with nonischemic dilated cardiomyopathy. J Am Soc Echocardiogr2013;26:278–287.
- 63. Lowenstein JA, Caniggia C, Rousse G et al Coronary flow velocity reserve during pharmacologic stress echocardiography with normal contractility adds important prognostic value in diabetic and nondiabetic patients. J Am Soc Echocardiogr2014;27:1113–1119.
- 64. Marks DS, Gudapati S, Prisant LM et al Mortality in patients with microvascular disease. J Clin Hypertens (Greenwich)2004;6:304–309.
- 65. Mc Ardle BA, Davies RA, Chen L et al Prognostic value of rubidium-82 positron emission tomography in patients after heart transplant. Circ Cardiovasc Imaging2014;7:930–937.
- 66. Meimoun P, Benali T, Elmkies F et al Prognostic value of transthoracic coronary flow reserve in medically treated patients with proximal left anterior descending artery stenosis of intermediate severity. Eur J Echocardiogr2009;10:127–132.
- 67. Miller RJH, Manabe O, Tamarappoo B et al Comparative prognostic and diagnostic value of myocardial blood flow and myocardial flow reserve after cardiac transplantation. J Nucl Med2020;61:249–255.
- 68. Monroy-Gonzalez AG, Tio RA, de Groot JC et al Long-term prognostic value of quantitative myocardial perfusion in patients with chest pain and normal coronary arteries. J Nucl Cardiol2019;26:1844–1852.
- 69. Murai T, Yonetsu T, Kanaji Y et al Prognostic value of the index of microcirculatory resistance after percutaneous coronary intervention in patients with non-ST-segment elevation acute coronary syndrome. Catheter Cardiovasc Interv2018;92:1063–1074.
- 70. Nakanishi K, Fukuda S, Shimada K et al Prognostic value of coronary flow reserve on long-term cardiovascular outcomes in patients with chronic kidney disease. Am J Cardiol2013;112:928–932.
- 71. Nakanishi K, Fukuda S, Shimada K et al Impaired coronary flow reserve as a marker of microvascular dysfunction to predict long-term cardiovascular outcomes, acute coronary syndrome and the development of heart failure. Circ J2012;76:1958–1964.
- 72. Nemes A, Balázs E, Pintér S, Csanády M, Forster T. Long-term prognostic significance of coronary flow velocity reserve in patients with significant coronary artery disease not involving the left anterior descending coronary artery (results from the SZEGED study). Echocardiography2010;27:306–310.
- 73. Nishi T, Murai T, Ciccarelli G et al Prognostic value of coronary microvascular function measured immediately after percutaneous coronary intervention in stable coronary artery disease: an international multicenter study. Circ Cardiovasc Interv2019;12:e007889.
- 74. Patel KK, Spertus JA, Chan PS et al Myocardial blood flow reserve assessed by positron emission tomography myocardial perfusion imaging identifies patients with a survival benefit from early revascularization. Eur Heart J2020;41:759–768.
- 75. Pepine CJ, Anderson RD, Sharaf BL et al Coronary microvascular reactivity to adenosine predicts adverse outcome in women evaluated for suspected ischemia results from the National Heart, Lung and Blood Institute WISE (Women’s Ischemia Syndrome Evaluation) study. J Am Coll Cardiol2010;55:2825–2832.
- 76. Pereira VF, de Carvalho Frimm C, Rodrigues AC, Cúri M. Coronary reserve impairment prevents the improvement of left ventricular dysfunction and adversely affects the long-term outcome of patients with hypertensive dilated cardiomyopathy. J Am Soc Hypertens2010;4:14–21.
- 77. Rigo F, Ciampi Q, Ossena G, Grolla E, Picano E, Sicari R. Prognostic value of left and right coronary flow reserve assessment in nonischemic dilated cardiomyopathy by transthoracic Doppler echocardiography. J Card Fail2011;17:39–46.
- 78. Rigo F, Sicari R, Gherardi S, Djordjevic-Dikic A, Cortigiani L, Picano E. Prognostic value of coronary flow reserve in medically treated patients with left anterior descending coronary disease with stenosis 51% to 75% in diameter. Am J Cardiol2007;100:1527–1531.
- 79. Rigo F, Sicari R, Gherardi S, Djordjevic-Dikic A, Cortigiani L, Picano E. The additive prognostic value of wall motion abnormalities and coronary flow reserve during dipyridamole stress echo. Eur Heart J2007;29:79–88.
- 80. Sicari R, Rigo F, Gherardi S, Galderisi M, Cortigiani L, Picano E. The prognostic value of Doppler echocardiographic-derived coronary flow reserve is not affected by concomitant antiischemic therapy at the time of testing. Am Heart J2008;156:573–579.
- 81. Tio RA, Dabeshlim A, Siebelink HM et al Comparison between the prognostic value of left ventricular function and myocardial perfusion reserve in patients with ischemic heart disease. J Nucl Med2009;50:214–219.
- 82. Tona F, Caforio AL, Montisci R et al Coronary flow velocity pattern and coronary flow reserve by contrast-enhanced transthoracic echocardiography predict long-term outcome in heart transplantation. Circulation2006;114:49–55.
- 83. Tona F, Osto E, Famoso G et al Coronary microvascular dysfunction correlates with the new onset of cardiac allograft vasculopathy in heart transplant patients with normal coronary angiography. Am J Transplant2015;15:1400–1406.
- 84. Usui E, Murai T, Kanaji Y et al Clinical significance of concordance or discordance between fractional flow reserve and coronary flow reserve for coronary physiological indices, microvascular resistance, and prognosis after elective percutaneous coronary intervention. EuroIntervention2018;14:798–805.
- 85. Vacca A, Montisci R, Garau P et al Prognostic impact of coronary microcirculation abnormalities in systemic sclerosis: a prospective study to evaluate the role of non-invasive tests. Arthritis Res Ther2013;15:R8.
- 86. van de Hoef TP, Bax M, Meuwissen M et al Impact of coronary microvascular function on long-term cardiac mortality in patients with acute ST-segment-elevation myocardial infarction. Circ Cardiovasc Interv2013;6:207–215.
- 87. Wijntjens GWM, van Lavieren MA, van de Hoef TP et al Pressure-derived estimations of coronary flow reserve are inferior to flow-derived coronary flow reserve as diagnostic and risk stratification tools. Int J Cardiol2019;279:6–11.
- 88. Yang HM, Khush K, Luikart H et al Invasive assessment of coronary physiology predicts late mortality after heart transplantation. Circulation2016;133:1945–1950.
- 89. Yang JH, Obokata M, Reddy YNV, Redfield MM, Lerman A, Borlaug BA. Endothelium-dependent and independent coronary microvascular dysfunction in patients with heart failure with preserved ejection fraction. Eur J Heart Fail2020;22:432–441.
- 90. Yufu K, Kondo H, Shinohara T et al Assessment of coronary flow reserve predicts long-term outcome of responders to cardiac resynchronization therapy. Heart Vessels2019;34:763–770.
- 91. Ziadi MC, Dekemp RA, Williams KA et al Impaired myocardial flow reserve on rubidium-82 positron emission tomography imaging predicts adverse outcomes in patients assessed for myocardial ischemia. J Am Coll Cardiol2011;58:740–748.
- 92. Rahman H, Demir OM, Ryan M et al Optimal use of vasodilators for diagnosis of microvascular angina in the cardiac catheterization laboratory. Circ Cardiovasc Interv2020;13:e009019.
- 93. Toya T, Corban MT, Park JY et al Prognostic impact and clinical outcomes of coronary flow reserve and hyperemic microvascular resistance. EuroIntervention2021;17:569–575.
- 94. Maron DJ, Hochman JS, Reynolds HR et al; ISCHEMIA Research Group. Initial invasive or conservative strategy for stable coronary disease. N Engl J Med2020;382:1395–1407.
- 95. Pedersen TR, Kjekshus J, Berg K et al ; Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). 1994. Atheroscler Suppl2004;5:81–87.
- 96. Yusuf S, Sleight P, Pogue J, Bosch J, Davies R, Dagenais G; Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med2000;342:145–153.
- 97. Nahser PJ Jr, Brown RE, Oskarsson H, Winniford MD, Rossen JD. Maximal coronary flow reserve and metabolic coronary vasodilation in patients with diabetes mellitus. Circulation1995;91:635–640.
- 98. Galderisi M, Rigo F, Gherardi S et al The impact of aging and atherosclerotic risk factors on transthoracic coronary flow reserve in subjects with normal coronary angiography. Cardiovasc Ultrasound2012;10:20.
- 99. Galderisi M, Cicala S, Caso P et al Coronary flow reserve and myocardial diastolic dysfunction in arterial hypertension. Am J Cardiol2002;90:860–864.
- 100. Motz W, Strauer BE. Improvement of coronary flow reserve after long-term therapy with enalapril. Hypertension1996;27:1031–1038.
- 101. Kamezaki F, Tasaki H, Yamashita K et al Angiotensin receptor blocker improves coronary flow velocity reserve in hypertensive patients: comparison with calcium channel blocker. Hypertens Res2007;30:699–706.
- 102. Sundell J, Laine H, Nuutila P et al The effects of insulin and short-term hyperglycaemia on myocardial blood flow in young men with uncomplicated Type I diabetes. Diabetologia2002;45:775–782.
- 103. Cerit L, Duygu H, Gulsen K, Gunsel A. Effect of statins on coronary blood flow after percutaneous coronary intervention in patients with stable coronary artery disease. Neth Heart J2017;25:258–263.
- 104. Baller D, Notohamiprodjo G, Gleichmann U, Holzinger J, Weise R, Lehmann J. Improvement in coronary flow reserve determined by positron emission tomography after 6 months of cholesterol-lowering therapy in patients with early stages of coronary atherosclerosis. Circulation1999;99:2871–2875.
- 105. Brainin P, Frestad D, Prescott E. The prognostic value of coronary endothelial and microvascular dysfunction in subjects with normal or non-obstructive coronary artery disease: a systematic review and meta-analysis. Int J Cardiol2018;254:1–9.
- 106. Boden WE, O’Rourke RA, Teo KK et al; COURAGE Trial Research Group. Optimal medical therapy with or without PCI for stable coronary disease. N Engl J Med2007;356:1503–1516.