INTRODUCTION
Fruity and floral aromas are in high demand in the beverage industry, and there are continuous efforts to improve the aroma of beer by increasing or diversifying the fruity flavor profile. These aromas originate from flowering plants (angiosperms), which first emerged in evolution with a few species carrying small dense fruits with only one or a few seeds, and later evolved into the eudicots, the family that contains the fresh, succulent and aromatic fruits that we are so familiar with today (Friis, Pedersen and Crane ; Doyle ). Beverages made from grains, corn and rice therefore lack a fruity profile without addition of hops and fermentation with yeast. The addition of hops and, for that reason, presence of hop-derived aromas is quite variable among breweries, causing a division between the fields of hop and yeast biochemistry. Even though hundreds of aroma-active compounds have been found in beer, reviews tend to focus mainly on alcohols and esters produced by brewing yeast (Verstrepen et al. ; Saerens et al.; Pires et al.). An early work by the late Dr. Morten Meilgaard classified the aromas into eight groups, which constitute the major groups found in the ‘beer flavor wheel’ that he developed, and that divided hop and fruity flavors into two separate categories (Meilgaard, Dalgliesh and Clapperton ). The aim of this review is to include all the potentially important fruity and floral aromas in beer, including newly identified compounds derived from yeast and raw materials, that are produced in the mashing and brewing.
Aroma perception
We learn to appreciate fruits from infant childhood, both because they are rich in nutrients, vitamins, antioxidants and therefore have profound health benefits (Boeing et al.), and because of their delicious flavor. Food products typically contain hundreds of flavor-active chemical compounds that are divided based on the mechanism by which they are sensed, i.e. the ‘taste’ that is sensed by our taste buds (sour, sweet, bitter, salty and umami) (Lindemann ) and the ‘aroma’ that is the volatile fraction sensed in the olfactory bulb of the nose (Buck ; Touhara and Vosshall ). Many metabolites contribute to the aroma because they are found in concentrations above their sensory thresholds; however, only few of them provide essential contributions to the aroma profile. To determine if a compound is affecting the flavor of food, a panel of individuals first determines the sensory threshold. This is typically done by increasing the concentration until it is detected and clearly differentiated from controls without inclusion of the compound (Lawless and Heymann ). The standard method reports sensory thresholds at which 50% of the sensory panelists can detect the compound. After quantification of the compound in the food product, the ratio between the concentration and the sensory threshold, called the odor activity value (or OAV), is calculated. The OAV is a measure of how likely the aroma compound is to have an impact on the flavor profile. Compounds that are thought to make an essential contribution to the characteristics of a particular aroma are evaluated in a synthetic mixture by omitting compounds one by one. If the compound is crucial for the aroma of a fruit or beverage, it is termed a ‘character impact compound’.
The volatility and therefore the impact of the aroma compound are determined by multiple factors such as the pH, salt concentration, ethanol level, binding to fats/oils, proteins, starch and phenolic compounds (generally known as ‘the matrix effect’), as well as the temperature (Guichard ). It is therefore best to perform OAV determination based on sensory thresholds in situ (i.e. in the beer), as the overall composition (or matrix) can have a drastic effect on aroma perception. In addition, the aroma compounds can also be detected retronasally when we eat or drink. Free aroma and aroma released from sugar and amino acid-conjugated precursors by microbial and saliva enzymes in the oral cavity are detected with exhalation into the olfactory bulb of our nose (Salles et al.; Bojanowski and Hummel ). For example, it was determined that the fruity esters isoamyl acetate and isobutyl acetate are present in the breath after eating ripe banana, while the aldehyde compound (Z)-2-hexenal, which has a typical ‘green’ aroma, is found after eating unripe banana (Mayr et al.). It is evident that the combined sensory profile is influenced by many factors. For a detailed review on the impact of external stimuli, texture of the food, the composition of saliva and the mechanical impact of swallowing and chewing in relation to the differences of retronasal or direct orthonasal detection, we refer to Goldberg et al. ().
Aroma compounds with similar attributes often have additive interactions that lower the thresholds of the individual compounds. In beer, the presence of a mixture of the banana esters (isoamyl and isobutyl acetate) lowers their perception threshold and the ‘rose flavor’ higher alcohol 2-phenylethanol, a predominant aroma compound responsible for aroma of roses (Sakai et al.) that is typically found below its sensory threshold in beer, may still be detected by additive interactions with isoamyl and isobutyl alcohol if these alcohols are present at high concentrations (Meilgaard ). Moreover, in certain cases aroma compounds exert stronger than anticipated effects by interacting synergistically or antagonisticially (masking effect). For example, certain acetate and ethyl esters interact synergistically to enhance the overall fruity aroma (Lytra et al.), whereas phenylacetaldehyde, acetic acid, methional and 4-ethylphenol have been found to mask the fruitiness in red wine (San-Juan et al.). This often occurs in complex ways. For example, the ‘woody’ odor from whiskey lactone enhances the fruitiness of isoamyl acetate synergistically at lower concentrations, but masks the fruitiness at higher concentrations (Ishii et al.).
FRUITY AND FLORAL AROMAS IN BEER: CHARACTERISTIC AROMA-ACTIVE COMPOUNDS
Despite the above-mentioned factors that add complexity to the sensory perception, OAVs remain an essential concept in flavor research. We have chosen the most aroma-active compounds in beer for each group of chemicals, or examples for groups where the OAVs do not exceed 1 (mainly sesquiterpenes and lactones), but have been reported as being important due to additive or synergistic effects with related compounds. Currently, we have extensive information about the most aroma-active compounds in common fruits. They can be classified by the chemical groups they possess. We have created a database of aroma compounds in major fruit cultivars to overlay with fruity flavors, in which we identified the most aroma-active compounds based on literature, gas chromatography (GC)-olfactory studies and determination of OAVs (Supplementary data 1, Supporting Information). The major classes of fruity and floral aroma compounds in both beer and fruits are ‘higher alcohols’, i.e. alcohols with more carbon atoms than ethanol (C3 and more); ‘esters’, derivatives of fatty acids and higher alcohols or ethanol; ‘polyfunctional thiols’, thiols with other functional groups; ‘lactones’ and ‘furanones’, cyclic esters and carbohydrates with a substituted oxygen; ‘terpenoids’ (monoterpenoids (C10) and sesquiterpenoids (C15), and norisoprenoids), derivatives of isoprenoids (C5) from hops. Hop-derived ethers have also been proposed to contribute with floral notes in pilsner beer (Tressl et al. ). The latter will not be discussed in more detail as only few early studies have reported their presence. The chemical structure of the most aroma-active fruity and floral compounds in beer is shown in Figs 1–4 and discussed below. The aroma compounds in beer resemble aromas encountered in daily life (for example, fruits or flowers). These associations, called ‘sensory descriptors’, are indicated between brackets and are based on Meilgaard () unless otherwise stated.
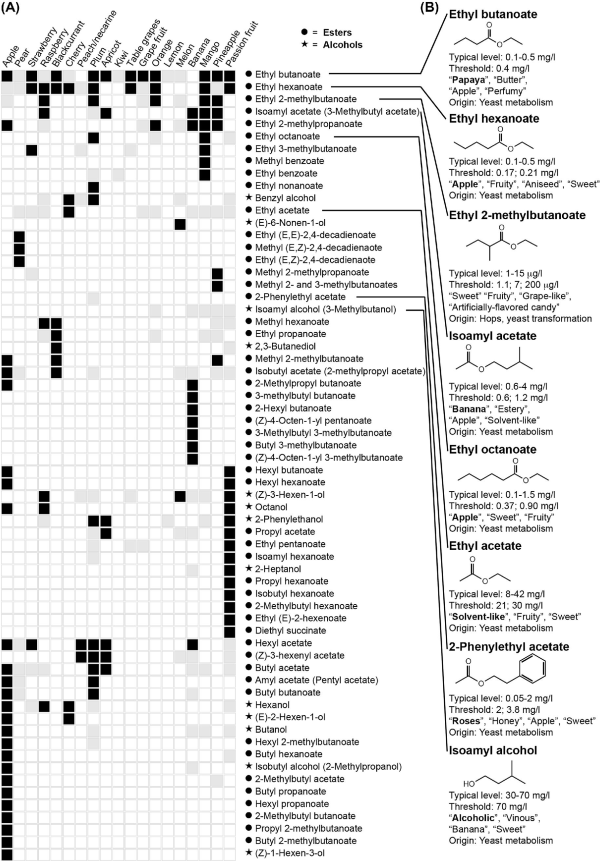
Figure 1
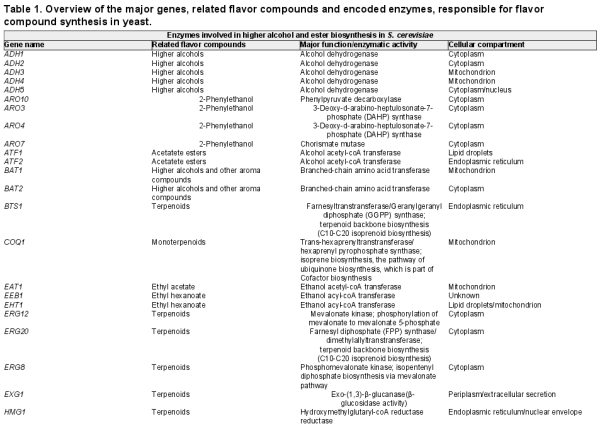
Aroma esters in fruits and beer. Their occurrence and sensory importance in fruits. (A) Based on aroma studies in fruits (see supplementary data 1 for details and references), high-impact compounds in major cultivated fruits were selected and given an arbitrary value of 1 (black). In case of compounds found above their sensory threshold, but not considered high impact, they were given an arbitrary value of 0.1 (gray). In case of multiple reports on the occurrence of a compound in a fruit, it was only considered high impact in case of concurrence in at least half of the studies. The compounds were then clustered based on appearance and impact, and a heatmap with the results was generated using BioNumerics 7.6 (clustering dendrograms are not shown). (B) Selected compounds of high importance for fruity and floral beer aroma. The typical ranges of compound levels, their sensory threshold and sensory descriptors in beer as well as the origin from either raw material or yeast are indicated below the compounds. All sensory characteristics are from Meilgaard (), except for additional sensory threshold data (1.1 μg/L) for ethyl 2-methylbutanoate, which is from Kishimoto et al. ().
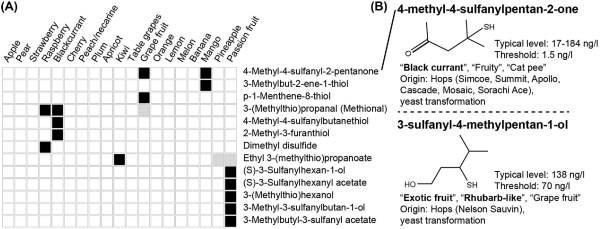
Figure 2
Polyfunctional thiols. Their occurrence and sensory importance in fruits (A) and beer (B). For more details, see the legend of Fig. 1. Sensory characteristics are from Kishimoto et al. () and Takoi et al. (). Up till now, there are no reports of occurrence of 3-sulfanyl-4-methylpentan-1-ol in fruits.
Figure 3
γ-Decalactone and strawberry furanone. Their occurrence and sensory importance in fruits (A) and beer (B). For more details, see the legend of Fig. 1. Sensory characteristics are from Meilgaard ().
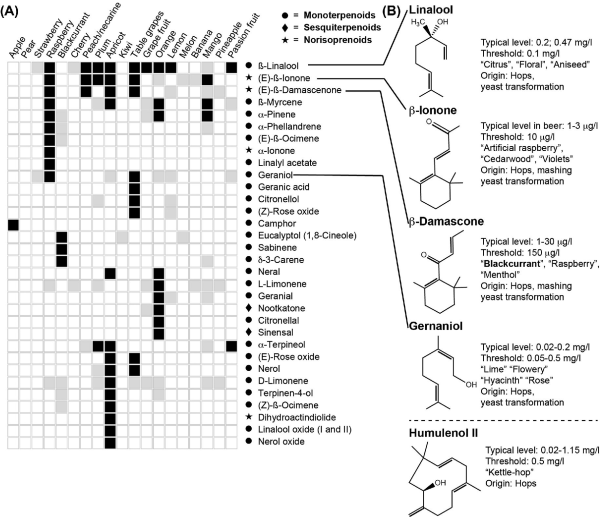
Figure 4
Mono- and sesquiterpenoids and norisoprenoids. Their occurrence and sensory importance in fruits (A) and beer (B). For more details, see the legend of Fig. 1. Sensory characteristics are from Meilgaard ().
Higher alcohols and esters
Apart from the primary metabolite ethanol, several higher alcohols are formed at different levels, of which isoamyl alcohol (Fig. 1B, descriptors: ‘alcoholic’, ‘vinous’, ‘sweet’) is the most abundant secondary metabolite and typically found well above its flavor threshold in beer. Isoamyl alcohol has also some banana flavor character and has been identified above its threshold in banana, orange, mango, pineapple and passion fruit (Fig. 1A). In addition, active amyl alcohol (2-methylbutanol) and isobutanol, which have very similar sensory properties, increase the impact of isoamyl alcohol significantly (Meilgaard ). Because of their similar properties and the high proportion of isoamyl alcohol, active amyl alcohol is often measured together in one peak using GC coupled with a flame ionization detector.
The acetate ester derivative isoamyl acetate (Fig. 1B, descriptors: ‘banana’, ‘estery’, ‘solvent-like’, ‘apple’) is one of the most important aroma compounds formed by yeast (Verstrepen et al. ) and a character impact compound in banana (Engel, Heidlas and Tressl ; Jordán et al.). In a similar fashion, isoamyl acetate is often measured together with active amyl acetate using a flame ionization detector because of similar properties. High levels of isoamyl acetate are desirable; however, it goes hand in hand with ethyl acetate (Fig. 1B, descriptors: ‘solvent-like’, ‘fruity’, ‘sweet’) that is used as a solvent in paints, lacquers and nail polish. It presents therefore a tradeoff between fruitiness and solvent-like, heavy and alcoholic aromas often found in beers fermented with high levels of sugar. Fruits do develop some ethyl acetate from fermentation by the plant cells. The levels are, however, much higher in alcoholic beverages due to the presence of large amounts of ethanol used as substrate.
Other esters also contribute significantly to the aroma of beer, particularly the so-called apple esters, ethyl hexanoate (Fig. 1B, descriptors: ‘apple’, ‘fruity’, ‘aniseed’, ‘sweet’) and ethyl octanoate, which have fruity apple aromas and are found in many fruits above sensory thresholds (Fig. 1A). In apple, pear and banana, the main characteristic aroma compounds are indeed the esters. Methyl and ethyl esters of decadienoate are powerful odorants that have a characteristic pear aroma (Rapparini and Predieri ). The aromatic ester phenylethyl acetate (Fig. 1B, descriptors: ‘roses’, ‘honey’) may also contribute to the aroma by interactions with other esters and 2-phenylethanol in beer, but the impact is less than in the aroma of wine.
Finally, the esters ethyl 2-methylpropanoate, ethyl 3-methylbutanoate and ethyl 2-methylpropanoate (Fig. 1B, descriptors: ‘sweet’, ‘fruity’, ‘grape-like’) are also high-impact hop aroma compounds (Steinhaus and Schieberle ). In addition to the terpenoid compounds, these esters were also found to be impact compounds in finished beers (Kishimoto et al.). Reports have suggested that they originate from methyl esters present in hop oils and are transesterified from the abundant methyl esters into the ethyl esters after brewing (Tressl et al. ).
Polyfunctional thiols
The best-known hop variety with a distinct thiol aroma is the New Zealand bred cultivar, Nelson Sauvin, for which the major thiol compound in beer has been identified as 3-sulfanyl-4-methyl-pentan-1-ol (Fig. 2B, descriptors: ‘exotic fruit’, ‘rhubarb-like’, ‘grapefruit’) (Takoi et al.). This thiol was also identified in significant quantities in the Hallertau Blanc and Tomahawk hop cultivars and was found at higher levels in dry-hopped beers (Gros, Nizet and Collin ; Cibaka et al.). Beer made with other hop varieties such as Simcoe, Summit and Cascade contains considerable amounts of another extremely potent thiol, 4-methyl-4-sulfanyl-2-pentanone (Fig. 2B, descriptors: ‘black currant’, ‘fruity’, ‘cats pee’) with a sensory threshold of 1.5 ng/L (or parts per trillion) (Kishimoto et al.), and Tomahawk hops contain 3-sulfanylhexan-1-ol (Gros, Nizet and Collin ), a character impact compound in passion fruit (Engel and Tressl ; Tominaga and Dubourdieu ). Notably, there is limited information in beer about the grape fruit character impact sulfur compound p-menthane-8-thiol while its oxidized derivative p-Menthane-8-thiol-2-one is a known off-flavor in aged beer and is correlated with the oxygen level (Parker ). Thus, as the importance of these hop-derived compounds is emerging, their biosynthesis pathways are gaining more attention.
Lactones and furanones
Lactones (especially γ-decalactone and δ-decalactone) are major contributors to the typical flavor of peach and apricot (Greger and Schieberle ; Dabbou et al.). Several lactones have been found in beer, among which the γ-lactones [especially γ-decalactone (Fig. 3B, descriptors: ‘apricot’, ‘peach’)] are the most prominent (Tressl, Kossa and Renner ; Tressl et al. ). They originate from malt, hops and as yeast metabolites from amino acids and hydroxylated fatty acid precursors, such as 4-oxononanoic acid, a breakdown product of oxidized linoleic acid that forms during mashing. Although they are often found below their estimated sensory thresholds in beer (OAV of 0.1–0.5), they may play an important role in providing fruity notes in beer through synergistic interactions in a similar way to what was found in model wine (Meilgaard ; Jarauta, Ferreira and Cacho ; Loscos et al.). Another important lactone is Whiskey-lactone or oak-lactone (β-methyl-γ-octalactone), which imparts ‘sweet’ and ‘coconut’ aroma in Whiskey, Gueuze and Lambic beers that are aged in oak casks (De Keersmaecker ; Mosedale and Puech ). Together with y-decalactone and y-dodecalactone that are found in Whiskey malt, the Whiskey lactone originating from oak provides for superior quality in Whiskey (Otsuka, Zenibayashi and Itoh ; Wanikawa et al.).
The furanone 4-hydroxy-2,5-dimethyl-3(2H)-furanone (or HDMF, strawberry furanone; Fig. 3B, descriptors: ‘pineapple’, ‘caramel-like’) is a high-impact compound in many fruits (Fig. 3A) and a character impact compound in strawberry, which has one of the most complex flavor profiles of all fruits (Schwab, Schaart and Rosati ). If present around its sensory threshold, it resembles ‘pineapple’ aroma in beer (Tressl, Kossa and Renner ; Sakuma et al.). However, depending on the original gravity of the wort and the brewing yeast used, it can reach higher levels during fermentation, contributing to the sweet ‘caramel-like’ aroma in beer (Sakuma et al.).
Mono- and sesquiterpenoids and norisoprenoids
Aroma-active terpenoids originate from hops, and different varieties give a particular flavor profile. Dry hops, commonly used in the brewing process, contain 0.5–2.0% of essential oils, which consist mainly of terpene hydrocarbons and their oxygenation products (Kovačevič and Kač ). More specifically, according to Sharpe and Laws (), all known hop oils can be classified into three categories: hydrocarbons (containing monoterpenes, sesquiterpenes and aliphatic hydrocarbons), oxygenated compounds (terpene alcohols, sesquiterpene alcohols and other oxygenated compounds) and sulfur-containing compounds (thioesters, sulfides and other sulfur compounds). The quantitatively most abundant terpenoids in hops are the sesquiterpenoids, such as α-humulene (not to be confused with the hop α-acid humulone), β-caryophyllene and β-farnesene, and the monoterpenoid myrcene, which constitutes up to about 75% of hop essential oils (Sharpe and Laws ). Myrcene is an important compound in the aroma of mango (Pino and Mesa ), and early studies in lemon and lime fruits have also indicated its importance for the aroma of these fruits (Moshonas and Shaw ; Njoroge et al.). More recent studies have confirmed the importance of monoterpenes in Japanese sour citrus fruits (Tomiyama et al.; Akakabe et al.). Myrcene was shown to have a high impact in the aroma of hops; however, β-linalool (Fig. 4B, descriptors: ‘citrus’, ‘floral’, ‘aniseed’) and geraniol (Fig. 4B, descriptors: ‘lime’, ‘floral’, ‘hyacinth’, ‘rose’) seem to be even more important for the aroma of both fresh and fried hop cones (Steinhaus and Schieberle ). The content of terpenoids in the finished beers and their sensory perception depends on many factors, including not only the hop variety used, but also the point of hop addition in the brewing process, the yeast used and its enzymatic activity during fermentation as well as the ratio between terpenoids in the final beers. First of all, the hop cultivars and hopping regime during brewing have a major impact on the terpenoid content and flavor release (Kishimoto et al.; Inui et al.; Steyer, Clayeux and Lanugel ; Takoi ; Sharp, Steensels and Shellhammer ). It was found that mainly hop-derived monoterpene alcohols, including geraniol, linalool, α-terpinol, nerol and β-citronellol, contributed to the fruity, citrus and floral aromas of the final beers (Inui et al.). β-Linalool persists above its threshold in most hopped beers and is present at high levels in dry-hopped beers with up to 4.7 OAVs (Meilgaard ). It is found above its sensory threshold in strawberry, raspberry, cherry, peach, plum, apricot, grapes, citrus fruits, mango and passion fruit and can be considered a high-impact aroma compound in cherry, peach, apricot, grapes, orange and passion fruit (Fig. 4A and Supplementary data 1, Supporting Information). Thus, monoterpene-rich (primarily geraniol- and linalool-rich) hop varieties, like Cascade (Inui et al.) or Triskel (Steyer, Clayeux and Lanugel ) that brings citrus and floral notes, respectively, are used as aroma-providing hops in brewing. Most of American hop cultivars (Bravo, Mosaic, Cascade, Citra) hold a higher content of geraniol than European hop varieties (Saaz, Hallertauer Tradition and Magnum) (Takoi ). However, the absolute level of each monoterpenoid does necessarily translate into more floral and fruity beer as their sensory impacts are also influenced by the complex interactions between odor-active compounds found in beer. The flavor impression of excess linalool is perceived as more ‘fruity’ and ‘citrussy’ due to the coexistence with geraniol and β-citronellol (at the levels of only 5 μg/L) and the coexistence of all three monoterpene alcohols indeed provides a synergistic effect (Takoi et al. ). Thus, the final beer aroma depends on the final concentration and ratio of the above-mentioned compounds.
The hydrocarbon sesquiterpenes are quite hydrophobic and generally do not persist in the brewing process at high levels. Instead, they are found in higher levels as oxidized alcohol and epoxide derivatives, with a less hydrophobic nature, such as humulenol II (Fig. 4: descriptor: ‘kettle-hop’), that appear during the brewing process (Peacock and Deinzer ). They appear in quantities that are below the aroma perception thresholds of the individual compounds, but correlate with the kettle-hop aroma, and may therefore exert synergistic effects with other flavor compounds, although the details are not yet understood (Rettberg, Biendl and Garbe ).
Schieberle () and Langos, Granvogl and Schieberle () reported β-damascone (Fig. 4B: descriptors: ‘blackcurrant’, ‘raspberry’, ‘menthol’) as a high-impact aroma compound in beer using GC-O and sensory thresholds in water. However, the sensory thresholds determined in beer of 150 μg/L for β-damascone, 2.6 and 10 μg/L for α and β-ionone (Fig. 4B: descriptors: ‘artificial raspberry’, ‘cedarwood’, ‘violets’), respectively, and their presence in beer at levels around 1–30 and 1–3 μg/L (Meilgaard ) suggest that, although they contribute to the fruity and floral aroma, their importance has been overrated. These compounds are found in the hop oils and contribute to the distinction of ‘kettle-hop’ aroma (Angelino ). Therefore, they may contribute with certain fruity aromas originating from aroma hops.
Hop essential oils are highly complex mixtures, with more than 500 compounds identified, and many remaining to be identified (Roberts, Dufour and Lewis ). In addition to the compounds described above, and given that new terpenoid compounds are still being discovered in hop essential oils today (Praet et al.), additional low-quantity high-impact terpenoid compounds are likely to be identified.
BIOSYNTHESIS AND OCCURRENCE OF FRUITY AND FLORAL AROMA COMPOUNDS IN BEER
Below we will describe the molecular pathways and brewing conditions that lead to production of the fruity and floral aromas in beer. A list of major genes responsible for flavor production in Saccharomyces cerevisiae is shown in Table 1. General effectors of synthesis of a range of flavor compounds are also frequently identified because of the intertwined substrates, such as cofactors necessary for many enzymatic reactions, or general cell physiology being affected by major regulators. Primary examples are aminodeoxychorismate synthase Abz1 (Steyer et al.), necessary for folic acid production; the H+-ATPase pump Pma1, a major regulator of intracellular pH and the plasma membrane electrochemical gradient (Den Abt et al.); trehalose-6-phosphate synthase Tps1, a regulator of glycolysis (Den Abt et al.); acetyl-coA synthetase Acs1, involved in acetyl-coA formation (Rossouw, Naes and Bauer ); Gpd2, Aad6, Aad10, Aad14, Bat1, Hom1 and Thi3/Kid1 that are known to be involved in specific metabolic pathways, but also may affect general metabolism depending on the experimental conditions (Rossouw, Naes and Bauer ; Styger, Jacobson and Bauer ). Although variants in these genes may play a role in yeast strain dependency of flavor production, the molecular mechanisms that cause regulation of many flavor compounds are often affected through regulation of general cell metabolism. We will therefore describe the molecular genetics by the chemical class of the compound and highlight the general regulatory mechanisms for the cases that are well understood.
Alcohols and esters
Higher alcohols
Higher alcohol production by yeast occurs through the Ehrlich pathway either from amino acids transported over the cell membrane or through de novo biosynthesis of amino acids and their α-ketoacid intermediates. The Ehrlich pathway includes three steps: (1) deamination of the amino acid to an α-ketoacid, (2) decarboxylation and (3) reduction of aldehyde to alcohol by aldehyde reductase activity (Hazelwood et al.). The most important substrates for beer flavor are the branched-chain amino acids leucine (yielding isoamyl alcohol, 3-methylbutanol), isoleucine (yielding active amyl alcohol, 2-methylbutanol) and valine (yielding isobutanol, 2-methylpropanol). An overview of the metabolic pathways involved in higher alcohol and ester production is shown in Fig. 5.
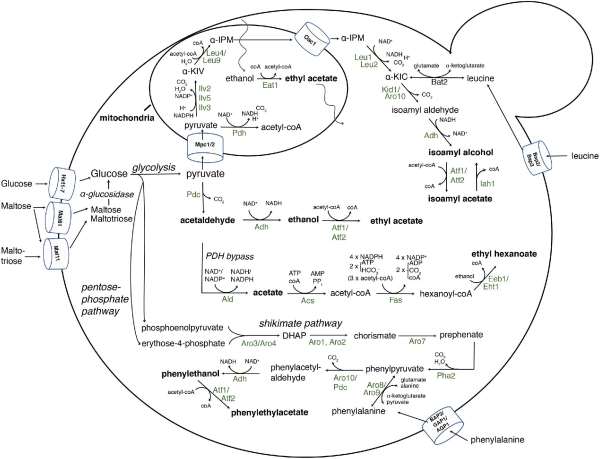
Figure 5
An overview of the yeast enzymes leading to isoamyl alcohol, 2-phenylethanol and esters during alcoholic fermentation. For simplicity, biochemical pathways leading to fusel alcohols are only shown for isoamyl alcohol and for esters only for isoamyl acetate, ethyl acetate and ethyl hexanoate. The pathways leading to active amyl alcohol and isobutanol also occur via the mitochondrial ILV (leucine-isoleucine-valine) pathway through Ilv2, 5 and 3, or via degradation of the amino acids isoleucine and valine by Bat1 and Bat2. The enzymes shown are indicated in their host organelles or in the cytosol and with the balance of the co-factors, substrates and byproducts in the biochemical reactions. Pyruvate originates from glycolysis. l-Leu4 and s-Leu4 indicate the long and short isoform present in the mitochondria and the cytosol, respectively, whereas Leu9 is a mitochondrial Leu4 paralog. α-KIV, α-ketoisovalerate; α-IPM, α-isopropylmalate; α-KIC, α-ketoisocaproate; ACS, Acetyl-coA synthase; PDH, pyruvate dehydrogenase complex; FAS, fatty acid synthase complex; PDC, pyruvate decarboxylase; ADH, alcohol dehydrogenase; ALD, acetaldehyde dehydrogenase; DAHP, 3- deoxy-D-arabino-heptulosonate-7-phosphate.
De novo synthesis of higher alcohols starts with the common metabolic intermediate pyruvate originating from glycolysis. During the formation of higher alcohols, the mitochondrial branched-chain isoleucine-leucine-valine (ILV) pathway metabolizes pyruvate into α-ketoisovalerate through acetolactate synthase (Ilv2), acetohydroxyacid reductoisomerase (Ilv5) and dihydroxyacid dehydratase (Ilv3) (Kohlhaw ). Next, α-isopropylmalate synthase (Leu4 and Leu9) condenses the α-ketoisovalerate produced in the mitochondria with acetyl-coA and H2O to yield α-isopropylmalate. Leu4 exist in two isoforms, the full length isoform of Leu4 is targeted to the mitochondria, while the short cytosolic isoform of Leu4 is transcribed from a downstream start codon lacking the N-terminal mitochondrial target signal (Kohlhaw ). Biosynthesis of α-isopropylmalate is feedback inhibited through the C-terminal regulatory domain of Leu4 by leucine and free coenzyme A (mediated by Zn2+) (Satyanarayana, Umbarger and Lindegren ; Kohlhaw ; Koon, Squire and Baker ; Oba et al.). This inhibitory effect can be abolished by selecting for resistance to the toxic leucine analog 5,5,5-trifluoro-DL-leucine, which forces a higher flux through de novo synthesis (Casalone et al.; Cavalieri et al.). These resistant strains produced 3- to 4-fold more isoamyl alcohol in a saké fermentation (Ashida et al.), a 2-fold increase in wine fermentations (Casalone et al.), and a 2- to 3-fold increase in low alcohol beer produced with resistant S. pastorianus strains leading to a distinct banana flavor (Strejc et al.). In a similar fashion, strains that acquire resistance to DL-thiaisoleucine also produce higher levels of both active amyl alcohol and propanol from α-ketoglutarate (Fukuda et al.).
Pyruvate is also a precursor for acetaldehyde and ethanol production and for acetyl-coA, which is used as a substrate by Leu4, via the pyruvate dehydrogenase (PDH) complex and the PDH bypass pathway (Pronk, Steensma and Van Dijken ; Boubekeur et al., ; Krivoruchko et al.). Under anaerobic conditions, the E3 subunit of the mitochondrial PDH complex (encoded by the PDA1 gene) is inhibited by the reductive (high) NADH/NAD+ ratios, and the cells depend on the cytosolic PDH bypass pathways to provide the acetyl-coA (van Rossum et al.). The PDH bypass consists of the pyruvate decarboxylase (PDC), acetaldehyde dehydrogenase (ALD) and the acetyl-coA synthase genes ACS1 and ACS2 (Pronk, Steensma and Van Dijken ). The ACS1 gene is highly induced during growth with non-fermentable carbon sources and under aerobic conditions, and repressed by glucose, leaving ACS2 to provide the cytosolic acetyl-coA under anaerobic and high sugar conditions present in alcoholic fermentations (Krivoruchko et al.). Frick and Wittmann () observed that cytosolic flux into acetyl-coA was even higher during respirative growth (increased biomass) than during fermentation. They hypothesized that the PDH bypass therefore not only occurs as an anabolic reaction to provide cytosolic acetyl-coA, but also to provide acetyl-coA for the mitochondria. The formation of acetyl-coA from pyruvate is compartmentalized, as the molecule is impermeable to the inner membrane of the mitochondria, and transport of acetyl-coA therefore occurs via the carnitine shuttle (Strijbis and Distel ). Jouhten et al. () used a controlled supply of oxygen to investigate the formation of acetyl-CoA under fermentation conditions, and similarly found that the cytosolic flux of acetyl-coA was increased in more oxidative conditions. Admittedly, they did not supplement the media with L-carnitine (which cannot be synthesized by S. cerevisiae), and did therefore not include the inward flux into the mitochondria (Jouhten et al.). There appear to be very low levels of L-carnitine in barley seeds (Panter and Mudd ), which suggests that intramitochondrial production is sufficient for production of ‘normal’ levels of higher alcohols. Fluctuations in acetyl-coA levels will be discussed later, as this is also a substrate for acetate ester biosynthesis.
The second and third steps in isoamyl alcohol biosynthesis are carried out in the cytosol by isopropylmalate isomerase and β-isopropylmalate dehydrogenase encoded by LEU1 and LEU2, respectively, yielding the direct precursor of leucine, α-ketoisocaproate.
In the Ehrlich pathway, α-ketoisocaproate is produced directly by deamination of leucine by branched-chain amino acid transferase (BCAATase). There are two BCAATases involved, Bat1, located in the mitochondria, and Bat2, located in the cytosol (Eden, Simchen and Benvenisty ). They are encoded by paralogous genes that are expressed in an opposite manner during growth. BAT1 is highly expressed during logarithmic phase and repressed during stationary phase, while BAT2 is expressed during stationary phase and repressed during logarithmic phase (Eden, Simchen and Benvenisty ). Both gene products are involved in the Ehrlich pathway, and deletion or overexpression of BAT1/2 has a pronounced impact on the concentrations of higher alcohols and the overall aroma profile produced by S. cerevisiae (Eden et al.; Styger, Jacobson and Bauer ; Styger et al.). However, their role seems to be highly dependent on medium composition as Bat2 acted as the major BCAAT in laboratory yeast fermenting in synthetic medium (Eden et al.; Styger, Jacobson and Bauer ; Styger et al.), while Bat1 also affected production of higher alcohols in model wine fermentations (Lilly et al. ; Rossouw, Naes and Bauer ). Overexpression of either resulted in yeast with a fruitier fermentation profile (Lilly et al. ; Rossouw, Naes and Bauer ).
After deamination of the amino acid or de novo synthesis of the equivalent α-keto acid, they are decarboxylated and reduced to the higher alcohols. For isoamyl alcohol, α-ketoisocaproate is catabolized by the Thi3/Kid1 decarboxylase and to a lesser extent by the Aro10 aromatic decarboxylase into isoamyl aldehyde, which is subsequently reduced to isoamyl alcohol with aldehyde reductase activity of several different dehydrogenase enzymes (Adh1, 2, 3, 4 and 5, Sfa1) (Dickinson et al.; Dickinson, Salgado and Hewlins ). A similar pathway has been shown for isobutanol and active amyl alcohol with decarboxylation also carried out by the pyruvate decarboxylases Pdc1, Pdc5 and Pdc6 (Dickinson, Harrison and Hewlins ; Dickinson et al.). Saccharomyces cerevisiae strains engineered with co-compartmentalized branched chain amino acid and Ehrlich pathways in the mitochondria showed a significant increase in isobutanol production (Avalos, Fink and Stephanopoulos ). This clearly illustrates the crucial role of the mitochondria in the production of important beer aroma compounds.
Using radiolabeled [C13] valine, Oshita et al. () found that when valine was abundant, it was catabolized to isobutanol, while when its levels were scarce, the majority of isobutanol was biosynthesized de novo as by-product of the valine anabolism pathway. In both cases, the level of isobutanol at the end of the fermentation was similar. Thus, the lack of amino acids in the medium can be compensated for by biosynthesis of the substrates feeding into the Ehrlich pathway. For isoamyl alcohol, the source from which it is made depends both on the level of free leucine in the wort and the glycolytic state of the yeast. The latter was proposed to be determined by the deamination step carried out by BCAATases that require the amino-acceptor α-ketoglutarate and NADH co-factor, which are produced in high levels during glycolysis (Espinosa Vidal et al.). While the production rate of isobutanol was increased upon oxygenation, isoamyl alcohol production does not appear to be affected significantly by oxygen availability (Espinosa Vidal et al.).
2-Phenylethanol. Production of 2-phenylethanol (‘rose’ and ‘honey’ aromas) occurs via catabolism of L-phenylalanine transported from the medium into the cell and via de novo production of L-phenylalanine and intermediates in the Shikimate pathway (Fig. 5). The Shikimate pathway commences with erythrose-4-phosphate derived from the pentose phosphate pathway and phosphoenolpyruvate derived from glycolysis. They are converted into 3-deoxy-D-arabino-heptulosonate-7-phosphate (DAHP) via DAHP synthase. There are two isoforms of DAHP synthase, encoded by the ARO3 and ARO4 genes. The shikimate pathway further consists of the multifunctional enzyme Aro1 that carries out the five subsequent reactions leading to 3-enolpyruvyl-shikimate-5-phosphate, the phosphate group is removed by chorismate synthase (carbon-oxygen lyase) Aro2 to produce the final metabolite in the pathway, chorismate (Braus ). Chorismate is further processed to anthranilate for tryptophan biosynthesis or to prephenate by chorismate mutase, Aro7, for production of phenylalanine and tyrosine. Prephenate is converted into phenylpyruvate by the prephenate dehydratase Pha2 or into 4-hydroxyphenylpyruvate by the prephenate dehydrogenase Tyr1. The aromatic amino acid transferases, Aro8 and Aro9, carry out the subsequent amination that forms the end products, phenylalanine and tyrosine. The transamination reaction carried out by Aro8 and Aro9 is reversible and can therefore also mediate breakdown of phenylalanine to phenylpyruvate. The subsequent steps in the Ehrlich pathway towards 2-phenylethanol production include decarboxylation of phenylpyruvate into phenylacetylaldehyde, mainly carried out by aromatic decarboxylase Aro10 and dehydrogenation by alcohol dehydrogenase (Fig. 5). Overexpression of the branched chain amino acid transferase genes BAT1 and BAT2 could only weakly influence 2-phenylethanol levels (Lilly et al. ). This indicates that ARO8 and ARO9 are the major genes involved in deamination in the Ehrlich pathway. ARO9 and ARO10 are controlled at the transcriptional level by the aromatic amino acid biosynthesis regulator Aro80 and the amino acid starvation sensitive kinase Gcn2 (Staschke et al.; Lee and Hahn ), through mechanisms that involve both TORC1 signaling and binding of uncharged tRNAs to Gcn2 (Conrad et al.). Polygenic mapping of genes conferring high 2-phenylethyl acetate (and 2-phenylethanol) formation in beer production has been used to identify superior mutations in TOR1 and FAS2 (Trindade de Carvalho et al.). The superior TOR1 allele for 2-phenylethyl acetate production contained a nonsense mutation (E216*) that results in formation of a truncated and supposedly inactive Tor1 kinase, which is consistent with the Aro80-dependent induction of ARO9 and ARO10 upon exposure to rapamycin (Staschke et al.; Lee and Hahn ). The yields obtained are therefore dependent on the type of nitrogen source and the concentration of aromatic amino acids in the fermentation medium (Etschmann et al.; Vuralhan et al.). Surprisingly, however, ARO8 deletion enhances 2-phenylethanol production under ammonium-repressed conditions by increasing expression of ARO10 (Romagnoli et al.), encoding the major phenylpyruvate decarboxylase (Vuralhan et al., ). This underscores the high importance of ARO10 for 2-phenylethanol production.
The three aromatic amino acids, phenylalanine, tyrosine and tryptophan, exert feedback inhibition on multiple enzymes of the pathway. The phenylalanine-dependent DAHP synthase, Aro3, is sensitive to feedback inhibition by phenylalanine, while the tyrosine-dependent DAHP synthase, Aro4, is feedback inhibited by tyrosine (Fukuda, Watanabe and Asano ; Etschmann et al.; Tzin et al.; Zhang et al.). In a similar fashion to the LEU4 mutants in the leucine biosynthesis pathway, the inhibitory effects exerted on Aro3 and Aro4 by the end products, phenylalanine and tyrosine, can be released by selecting for resistance to fluorinated phenylalanine analogs (Fukuda, Watanabe and Asano ; Fukuda et al.). These mutants produced three to six times elevated levels of 2-phenylethanol in Sake fermentation trials. The fluorinated phenylalanine analogs p-fluoro-DL-phenylalanine and o-fluoro-DL-phenylalanine have also been used for selection of ARO4 and TYR1 mutants that produced up to 20-fold more 2-phenylethanol and 2-phenylethyl acetate than the wild-type wine yeast (Cordente et al. ). The isolated ARO4 mutants contained mutations in specific amino acid residues that release Aro4 from suppression by tyrosine (Hartmann et al.) leading to higher levels of the three aromatic amino acids intracellularly, whereas mutants in TYR1 accumulate phenylalanine extracellularly and show reduced intracellular tyrosine formation, indicating a lower activity of the prephenate dehydrogenase (Cordente et al.). The TYR1 mutants showed the highest increase in 2-phenylethanol levels, suggesting that the flux from prephenate to phenylpyruvate and the release of feedback inhibition on Aro4 by lower intracellular tyrosine levels are determining factors for the production of 2-phenylethanol.
Ester biosynthesis in yeast
Acetate ester biosynthesis in yeast. The word ‘ester’ was introduced probably as a reference to ethyl acetate, acetic ether, or in German: essig-ether (Gmelin ). In its most basic form, ethyl acetate is formed by a dehydration reaction between acetic acid and ethanol with the loss of a water molecule, or degraded into acetic acid and ethanol in hydrated acidic or alkaline conditions (Riemenschneider and Bolt ). Nevertheless, early studies of ester formation in brewing conditions concluded that acetate ester formation during brewing was not simply due to spontaneous esterification of acetate and alcohols, but instead involved enzymatic activity of S. cerevisiae through the energy rich thioester acetyl-coA (Nordström , ; Howard and Anderson ). This led to believe that acetyl-coA, an important metabolite in many pathways in living cells, was the determining factor for their production. However, it was observed that factors such as addition of unsaturated fatty acids and provision of oxygen, which inhibit acetate ester production, do not lower the levels of acetyl-coA or change the affinity constants for acetate synthase enzyme(s), highlighting possible involvement of other factors, such as the availability or expression level of the AATase as a major factor for their production (Malcorps et al.).
Surprisingly, most acetate esters are formed by the activity of a single alcohol acetyl-coA transferase (AATase) enzyme, encoded by the ATF1 gene (Fujii et al.; Fujii, Yoshimoto and Tamai ; Verstrepen et al. ). Because of the importance of the ATF1 gene for the aroma profile of beer, saké and wine, its regulation during fermentation has been investigated in detail. The gene is expressed at low levels in brewing yeast with a peak at 12–36 h into the beer fermentation, when the yeast is still actively growing and fermenting under anaerobic conditions (Verstrepen et al.). Beer made with lager yeast engineered with a constitutively overexpressed ATF1 gene contained four times more isoamyl acetate and six times more ethyl acetate compared to a wild-type strain and showed a very distinct fruity (‘banana’ and ‘pineapple’) and solvent-like character (Verstrepen et al. 2003). Thus, the limiting factor for ester production is not only the level of the alcohols, but to an even greater extent the expression level of the AATase enzyme. Interestingly, all the amyl esters in banana are formed during the ripening phase after ethylene treatment (Marriott ; Engel, Heidlas and Tressl ), which is accompanied by increased flux through glycolysis, increased levels of leucine and isoleucine (together with the higher alcohols), and induced expression of the banana AATase BanAAT gene (Seymour ; Jayanty et al.), thus indicating that fruit ripening potentially has some resemblance with the rapid formation of isoamyl acetate by yeast during fermentation. Also, in addition to the major AATase gene, ATF1, S. cerevisiae and S. pastorianus contain a paralogous gene, ATF2, which plays a minor role in the production of acetate esters during fermentation. Overexpression of ATF2 in a laboratory yeast with a low basal level of AATase activity causes a significant increase in the production of isoamyl acetate and ethyl acetate (Verstrepen et al. ), while its overexpression in wild-type beer, saké and wine yeasts has more subtle effects (Verstrepen et al. ; Lilly et al. ; Sahara et al.). This led to the proposal of using an ATF2 overexpression strain for saké production in order to avoid production of overly solvent-like and banana-like flavors (Sahara et al.). Recently, an ethanol acetyl-coA transferase, Eat1, has been isolated from Wickerhamomyces anomalus (Kruis et al.). Saccharomyces cerevisiae contains a mitochondrial EAT1 ortholog (YGR015C), which has been shown to enhance ethyl acetate production when overexpressed in aerobic conditions. Polygenic analysis of ethyl acetate production in the absence of the ATF1 gene led to discovery of natural variants of EAT1 and SNF8 having a mutation that causes an early termination of translation (eat1K179fs and snf8E148*), and which lower ethyl acetate production (Holt et al. ). However, modification of three unrelated yeast strains (wine, sake and ale yeast) showed highly strain-dependent effects of the two mutations, which underscores the importance of evaluating such mutations in the proper strain background, both for breeding and genetic engineering.
Substrate availability. The affinity constant (Km) of the major AATase (Atf1) for isoamyl alcohol (measured in partially purified cell extracts) was 25 mM (∼2204 mg/L) (Malcorps and Dufour ), which is roughly 20-fold higher than the average substrate level. This substrate limitation is consistent with the fact that there is generally a good correlation between isoamyl alcohol and isoamyl acetate levels during the course of a yeast fermentation (Calderbank and Hammond ; Quilter et al.). However, the variability in acetate ester production cannot only be explained by the availability of the higher alcohols (or ethanol), and the influence of acetyl-coA has been poorly explored in S. cerevisiae. The Atf1 enzyme contains transmembrane domains which fix the protein in the membrane of lipid droplets that bud out from the endoplasmic reticulum (ER), whereas Atf2 is localized in the membranes of the ER (Verstrepen et al.; Tiwari, Koffel and Schneiter ). Therefore, both enzymes depend on the cytosolic pool of acetyl-coA, which is generated by the PDH bypass (discussed previously for higher alcohols). Acetyl-coA metabolism has been engineered in Escherichia coli with significantly improved isoamyl acetate production (Vadali et al.; Vadali, Bennett and San ).
The cytosolic level of acetyl-coA is regulated by phosphorylation of acetyl-CoA carboxylase Acc1 by Snf1/AMPK kinase, which inactivates the enzyme and limits the production of malonyl-coA from acetyl-coA and CO2, resulting in higher levels of acetyl-coA (Zhang, Galdieri and Vancura ). The enhanced acetyl-coA pool goes hand in hand with increased acetylation of histones and other acetylated proteins. During initiation of growth, the acetyl-coA levels increase from ∼3 to ∼30 μM in exponentially growing cell cultures, which might represent the metabolic state of the cell in acetylation/deacetylation reactions (Cai and Tu ; Cai et al.; Weinert et al.). The Atf1 enzyme has an affinity (Km) for acetyl-coA of 25 μM (Malcorps and Dufour ), but since the cytosolic pool of acetyl-coA has not been measured in the course of alcoholic fermentation, it remains unclear whether fluctuations in its concentration significantly affect AATase productivity.
ATF1 orthologs in Saccharomyces sensu stricto species and S. pastorianus. An S. eubayanus ortholog of the ATF1 gene (designated Lg-ATF1) is also present in the S. cerevisae/eubayanus hybrid lager yeast S. pastorianus. The activity of the Lg-ATF1 encoded enzyme towards production of isoamyl acetate is lower than that of the S. cerevisiae ATF1 encoded enzyme. Its overexpression in commercial lager yeast leads to a 2-fold increase of isoamyl acetate in synthetic YP medium (Verstrepen et al. ). The kinetic properties of Atf1 from S. uvarum and S. kudriavzevii have been determined after overexpression in an S. cerevisiae laboratory strain in absence of the known AATase genes, ATF1 and ATF2, and the isoamyl hydrolase gene, IAH1 (Stribny, Querol and Perez-Torrado ). The affinity constants (Km) of isoamyl alcohol for the Atf1 orthologs in S. uvarum and S. kudriavzevii were 92.9 and 57.4 mM vs. 32.3 mM for S. cerevisiae with the same experimental setup. With the close homology of 99% similar amino acids between the S. uvarum and S. eubayanus ATF1 gene products, we can speculate that the Lg-ATF1 gene product in lager yeast has similar properties. Interestingly, 2-phenylethyl acetate production by S. cerevisiae in wine fermentations was 2-fold higher when the S. cerevisiae ATF1 ORF was replaced with ORFs from either S. uvarum or S. kudriavzevii, which illustrates some diversity in the enzyme specificities, even between Saccharomyces sensu stricto species.
The complex regulation of Atf1. The yeast AATase activity is affected by a range of different fermentation conditions, including carbon to nitrogen (C/N) ratio, sugar content of the wort, CO2/hydrostatic pressure, fermentation temperature, levels of unsaturated fatty acids and oxygen, and pitching rate (Engan ; Calderbank and Hammond ; Verstrepen et al. ; Dekoninck et al.). The molecular basis for the regulation of ATF1 transcript levels by unsaturated fatty acids, oxygen and nutrients is based on both direct and indirect evidence discussed below, while the effect of CO2/hydrostatic pressure is discussed separately. A simplified model of the complex regulation of ATF1 is shown in Fig. 6.
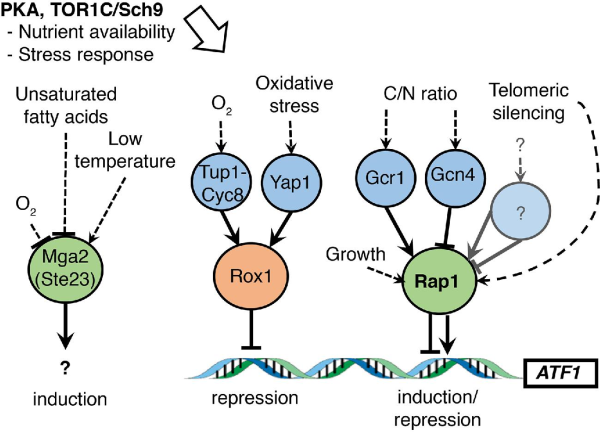
Figure 6
Simplified model of induction/repression of ATF1 gene expression via multiple binding partners. The Rox1 oxygen repressor and Rap1 inducer/repressor are essential for ATF1 expression (Fujiwara et al.). The ATF1 promoter does not contain transcription factor binding sites for Gcn4, Gcr1, Yap1 or Cyc8-Tup1. Instead, we propose that Rap1 and Rox1 regulate expression through indirect interaction with repressors and inducers. The Rap1 interactions with Gcr1 and Gcn4 can induce and repress transcription, respectively (Tornow et al.; Joo et al.), whereas Rox1 interaction with Cyc8-Tup1 is known to repress transcription (Smith and Johnson ). Yap1 has been shown to interact with Rox1 and repress the transcription of the high-affinity iron transporter gene FET4 (Caetano et al.). The major upstream PKA and Sch9 FGM pathways have also been found to participate in control of ATF1 expression (Fujiwara et al.; Verstrepen et al. ). They may regulate the availability of Rap1 itself or induction/repression of yet unknown Rap1 interaction partners.
The multifaced activator/repressor Rap1. Some of the multiple effects can be explained by the protein kinase A (PKA) and Sch9-mediated fermentable growth medium (FGM) pathways, which have been shown to affect the expression of ATF1 (Fujiwara et al.; Verstrepen et al. ). A truncated TOR1 allele was shown to enhance the levels of acetate esters, and in particular the ‘rose’ and ‘honey’-like ester phenylethyl acetate (Trindade de Carvalho et al.). Tor1 is a well-known regulator of nitrogen catabolite repression genes (Conrad et al.) and is therefore likely to affect the expression of the ATF1 gene. However, it is difficult to interpret these results in direct relation to fermentation conditions, as strains attenuated or disrupted in these pathways are known to be affected in broad stress-related responses. A more targeted approach was used by Fujiwara et al. (), which showed that the repressor-activator protein Rap1 is responsible for both transcriptional activation in anaerobic conditions and repression by unsaturated fatty acids. Rap1 is an essential transcription factor that regulates silencing and chromosome end maintenance by recruiting Sir and Rif proteins to the telomeres through its C-terminal RCT protein interaction domain (Chen et al.). Interestingly, Rap1 and the nitrogen catabolite repression transcription factor Gcn4 interact directly and repress ribosomal protein expression under nitrogen starvation (Joo et al.), which could also explain why low nitrogen content hampers ATF1 expression. The transcription factor Gcr1 also interacts directly with Rap1, and is able to induce genes of the glycolytic pathway (Tornow et al.), which explains why ATF1 is upregulated in high gravity fermentations although it does not contain a Gcr1 binding site. Based on the observation that Rap1 can bind directly to zinc finger transcription factors and repress or induce expression of target genes, it is possible that many of the effects can be explained through a coupled interaction. This raises the possibility that other stress response zinc-finger transcription factors, such as Msn2/Msn4, could alter Rap1 transcription efficiency, explaining the complex regulation of the ATF1 gene.
In addition to the possible coupled interactions, Rap1 availability is itself regulated by external factors such as oxygen (Dastidar et al.). Interestingly, transcriptional changes occur in cells that stop growing, which disperses Rap1 from telomeric loci to bind to other parts of the genome (Platt et al.). As Rap1 is a major regulator of ribosomal protein expression, which consumes a large fraction of the cellular energy production (Lieb et al.), it can be regarded as one of the general activators of growth. A rule of thumb among brewers says that vigorous production of acetate esters is a good indicator of the happiness of the yeast. This conclusion fits with the role of Rap1 in induction/repression of ATF1 transcription and with the effect of stress responses that negatively affect ATF1 transcription.
Activation by Mga2. Early research revealed that the melting point of the unsaturated fatty acids is correlated with the repression of ATF1 transcription in a similar way as OLE1 fatty acid desaturase expression (Fujiwara et al.). Ole1 transforms the saturated fatty acids into Δ9-unsaturated acids (such as oleic acid) that are required for growth. The paralogous transcription factors Mga2 and Spt23 are necessary for induction of OLE1 transcription. They have complementing functions as an mga2Δ spt23Δ double deletion strain is auxotrophic for unsaturated fatty acids as opposed to the single deletion strains (Zhang, Skalsky and Garfinkel ; Chellappa et al.; Kandasamy et al.). Apart from being tightly regulated by unsaturated fatty acids, Mga2 activation is repressed by oxygen and induced in response to low temperatures (Nakagawa et al.). Yeast responds to cold by increasing membrane fluidity as an adaptation to control membrane rigidness, whereas oxygen limitation requires upregulation of OLE1 for more efficient desaturase activity (Aguilar and de Mendoza ). Cold induction of Mga2 fits with increased fruitiness of beers produced at low temperature. However, the temperature effect on acetate ester production is strain dependent (Verstrepen et al. ), and fits with counteraction by decreased growth rate via regulation by Rap1.
Mga2 and Spt23 are tethered in their inactive forms to the membrane of the ER via a C-terminal hydrophobic domain. Activation occurs by proteolytic cleavage of the C-terminal domain after which they enter the nucleus and activate their transcription targets (Hoppe et al.; Rape et al.). The processing and release of Mga2 and Spt23 from the ER into the nucleus is strongly inhibited by unsaturated fatty acids (Hoppe et al.). Transcriptional activation by the soluble Mga2 factor, but not Spt23, retains repression by unsaturated fatty acids even without the C-terminal transmembrane domain (Chellappa et al.). Thus, different roles have been assigned to Spt23 and Mga2, in which Mga2 regulates both expression and degradation of the OLE1 transcript (Kandasamy et al.). Only re-addition of soluble Mga2 could restore the transcript to wild-type levels (Chellappa et al.), indicating that Mga2 plays a major role.
A mutant Mga2 allele with a premature stop codon (Ser706*) was shown to release ATF1 mRNA transcription from repression by unsaturated fatty acids (Takahashi et al.). The Mga2 mutation was identified in Saké yeasts that produce 2.6-fold more isoamyl acetate after acquiring resistance to the antibiotic aureobasidin A (Takahashi et al.). The mechanism of action of this truncated form of Mga2 could occur in multiple ways. For example, it could cause a permanent nuclear localization through which it could activate its targets constitutively.
Repression by Rox1. The strong repression of the ATF1 gene by oxygen occurs mainly through the Rox1 transcription factor (Fujii et al.; Fujiwara et al.). Rox1 is itself regulated by another transcription factor Hap1, which responds to oxygen through sensing of the heme level in the cells (Kastaniotis and Zitomer ). When oxygen is abundant, Hap1 upregulates Rox1 and represses targets that are not required in aerobic conditions. For efficient repression by Rox1, the Ssn6-Tup1 repressor complex binds to Rox1 (and other DNA binding proteins) and facilitates gene repression (Smith and Johnson ). YAP1 mutants with a truncated Yap1 C-terminus have been isolated, which showed up to 3-fold elevated production of isoamyl acetate in wine fermentations (Cordente et al.). These mutants were isolated based on cerulenin resistance. Yap1 is a zinc finger transcription factor that is induced under oxidative stress (Gulshan, Thommandru and Moye-Rowley ). Acetic acid induces an oxidative stress response in S. cerevisiae by several different mechanisms, which is necessary for cell survival, and in which Yap1 is a central regulator (Semchyshyn et al.; Morano, Grant and Moye-Rowley ). It has been shown that Rox1 represses gene expression of the FET4 high-affinity iron transporter gene through interaction with Yap1 (Caetano et al.). A yap1Δ strain also produced higher transcript levels of Rox1, indicating several modes of inhibition.
The inhibition of Atf1 by CO2 and hydrostatic pressure. One of the most pressing issues for brewers is the lack of isoamyl acetate production in the very tall cylindroconical fermentors used in commercial breweries. The amount of isoamyl acetate produced by yeast AATase activity is negatively correlated with the depth of the fermentor. This is due to the high levels of dissolved CO2 present under high hydrostatic pressure and to the physical agitation effect by the CO2 bubbles accumulating at high depths (Vrieling ; Meilgaard ). CO2 is essential as substrate in low levels during rapid fermentation as it feeds into the TCA cycle through the anaplerotic reactions and for the biosynthesis of fatty acids, purines and the charged amino acids aspartate and glutamate (Aguilera et al. ). However, at higher levels, it inhibits growth, fusel alcohol production and, to an even larger extend, the formation of acetate esters (Renger, Hateren and Luyben ; Shen et al.). When the yeast is subjected to high CO2 pressure for about 30 h, the uptake of any remaining branched chain amino acids through the cell membrane is reduced (Knatchbull and Slaughter ). This is correlated with lower production of fusel alcohols and esters as well as higher diacetyl production, increased cell size and lower viability, but it is not directly correlated with the ethanol production rate, at least not when the CO2 overpressure remains limited (e.g. 1 bar CO2 pressure) (Knatchbull and Slaughter ; Slaughter, Flint and Kular ). The growth inhibitory effects of CO2 are to some extend caused by direct inhibition of metabolic enzymes, lowering of the intracellular pH and impairing mitochondrial function once the CO2 (and bicarbonate) is inside the cells (Jones and Greenfield ; McIntyre and McNeil ; Aguilera et al. ; Garcia-Gonzalez et al.). As a consequence of the combined effects caused by high CO2 pressure, the cells have lower ATP levels upon long-term exposure (Richard, Guillouet and Uribelarrea ), but the major molecular biological factors involved in growth inhibition and reduction of the AATase activity have not yet been clarified.
A lead into a better understanding of the molecular-genetic mechanisms involved in suppression of ester production may come from the Sch9 and cAMP/PKA pathways that have been implicated in ATF1 expression regulation (Fujiwara et al.; Verstrepen et al. ) and CO2 sensing. Saccharomyces cerevisiae can sense CO2 and downregulate the enzyme carbonic anhydrase to limit bicarbonate levels under CO2 exposure. This occurs through sphingolipid Pkh1/2-mediated activation (phosphorylation) of the Sch9 protein kinase, with subsequent activation (phosphorylation) of the Cst6 transcription factor, responsible for expression of carbonic anhydrase (Pohlers et al.). On the other hand, high CO2 levels activate adenylate cyclase (Cyr1) in Candida albicans, where it acts as a chemosensor for virulence in the human body (Klengel et al.). An activated Cyr1 enzyme will produce a surge in cyclic AMP (cAMP) which can thus explain CO2-mediated activation of PKA (Vandamme, Castermans and Thevelein ). However, Jungbluth, Mosch and Taxis () could not find direct evidence for Cyr1 being regulated by CO2/bicarbonate in S. cerevisiae, and while CO2 exposure causes filamentous growth (hypha formation) in some Candida species, it does not have this effect in S. cerevisiae (no pseudohyphae formation), suggesting that different pathways are involved in the two species (Gancedo ; Vandamme, Castermans and Thevelein ) The mitochondrial CO2/cAMP/PKA pathway is a well-characterized regulatory pathway in mammalian cells (Valsecchi et al.), and has been investigated in S. cerevisiae where a mitochondrial spike in cAMP levels upon bicarbonate exposure led to the proposition that mitochondrially localized Cyr1/PKA is responsive to CO2 during non-fermentative growth (Hess et al.). The involvement of the cAMP/PKA pathway in sensing CO2 pressure in industrial anaerobic fermentation conditions is therefore not yet clear.
The biological function of Atf1. Atf2 has been shown to function primarily in the sterol detoxification cycle together with the deacetylase Say1 (Tiwari, Koffel and Schneiter ). On the other hand, a phenotype has not been detected for the ATF1 null mutant except for strongly reduced acetate ester production. Acetate ester production can have an impact on the dispersal of yeasts in nature. This was shown by purging the headspace from semi-anaerobic fermenting yeast cultures into a closed gas box containing fruit flies. The fruit flies showed a higher frequency of gathering towards the headspace gas originating from a yeast with artificially engineered overexpression of the ATF1 gene (Christiaens et al.), and were especially attracted to ethyl acetate. It has been known for a long time that there are many non-Saccharomyces yeasts typically isolated in much higher titers from fruits and other sugar-rich natural habitats that produce much higher quantities of ethyl acetate (Tabachnick and Joslyn ; Rojas et al.; Sabate et al.; Fleet ; Raspor et al.; Li et al.). For example, the corn sap beetle was highly attracted by W. anomalus (Pichia anomala) that produces abnormally high levels of ethyl acetate, even exceeding that of an ATF1 overexpression strain in S. cerevisiae (Nout and Bartelt ). The production of esters by the Pichia and Saccharomyces genera must be affected differently in nature, as the Saccharomyces genes are repressed by oxygen while Pichia is an aerobic yeast that produces esters at high levels in the presence of oxygen, a condition typically encountered in most natural niches. In addition, a range of volatile aroma-active metabolites, including acetic acid and beer phenolic off-flavors, such as 4-vinylguaiacol, also function as attractants for fruit flies (Drosophila melanogaster) (Dzialo et al.). It remains elusive whether the Atf1 enzyme performs other biological functions besides the production of acetate esters. Interestingly, the S. cerevisiae Atf1 AATase has very broad substrate specificity and can acetylate long-chain fatty alcohols (Ding et al.). In mammalian cells, the non-volatile acetate ester 12-O-Tetradecanoylphorbol-13-acetate disrupts actin filaments and increases mucin secretion, which increases the extracellular viscosity and adhesiveness, through regulation by the protein kinase C (PKC)-MAPK kinase pathway (Shiba, Sasaki and Kanno ; Hong, Forstner and Forstner ; Lee et al.; Bansil and Turner ). Yeast mutants resistant to aureobasidin A and producing increased isoamyl acetate have been isolated (Takahashi et al.). Aureobasidin A disrupts cortical actin patches and inhibits inositol phosphorylceramide synthase AUR1, which triggers signaling through the cell-wall-integrity PKC pathway (Endo et al.; Zhong, Murphy and Georgopapadakou ; Jesch et al.). In S. cerevisiae, the integral membrane mucin Msb2 serves to stimulate pseudohyphal growth via MAPK activation (Cullen et al.) and inhibition of sphingolipid synthesis via PKC-MAPK induces telomeric silencing (Lee et al.). Both pathways are poorly characterized under anaerobic fermentation conditions. A yet unexplored possibility is that Atf1 acetylates a yet unidentified lipid messenger, which regulates a subtle phenotype or a phenotype that is only prominent under specific natural conditions. More studies should be carried out especially under anaerobic conditions to investigate the unresolved issues concerning Atf1 functionality in S. cerevisiae.
Selection for improved acetate ester production and esterase activity. Isolation of strains resistant to various drugs has been employed to improve the production of acetate esters and increase the fruity aroma of saké. As previously mentioned, the toxic leucine analog 5,5,5-trifluoroleucine has been used to increase the isoamyl alcohol level, which also leads to a higher isoamyl acetate level. It is, however, not desirable to have high levels of isoamyl alcohol as it also gives a heavy (‘alcoholic’, ‘vinous’, ‘sweet’) aroma. Isoprenoid anticancer drug 1-farnesylpyridinium (Hirooka et al.), copper (Hirooka et al.), ergosterol inhibitor econazole (Asano et al.), monofluoroacetate (Watanabe et al.) and isoamyl monochloroacetate (Watanabe, Nagai and Kondo ) have all been used specifically for improvement of the isoamyl alcohol to acetate conversion ratio. These compounds work either by increasing the AATase activity or by reducing esterase catalyzed degradation. The major esterase in S. cerevisiae, isoamyl acetate hydrolase encoded by the IAH1 gene, has been cloned and characterized (Fukuda et al., ). Loss of function of this gene significantly increases the yield of isoamyl acetate and to a lesser extent that of the other acetate esters. It is therefore the balance between AATase and esterase activities that determines the final level of acetate esters produced by brewing yeast (Fukuda et al.).
Ester biosynthesis and role of the fatty acid synthase complex. The apple esters ethyl hexanoate and ethyl octanoate are present above their threshold in certain beers and thus contribute to the fruity aroma. Ethyl hexanoate, the most prominent apple ester in beer, is produced by the ethanol acyl-coA transferases Eht1 and Eeb1 that condense ethanol with a medium-chain fatty acid charged with coenzyme A (acyl-coA), with Eeb1 being particularly active (Saerens et al.). Moreover, an allele of the phospholipase B gene, PLB2, with in vitro acyltransferase activity (Merkel et al.), was isolated by polygenic analysis of ethyl ester production, and confirmed to be a strong effector of ethyl octanoate production by gene disruption (Steyer et al.). Reverse esterase activity has also been observed with the purified Iah1 enzyme, but only for ethyl hexanoate formation (Kuriyama et al.).
The expression levels of Eht1 and Eeb1 do not show a strong correlation with the final levels of ethyl hexanoate (Saerens et al.), which is consistent with the finding that the bottleneck of their formation is the level of the acyl-coA substrate (Saerens et al.). Ethyl hexanoate has received considerable interest in saké brewing because it is a key aroma compound in high-quality saké. Yeast selected for cerulenin resistance showed 3.7-fold increased production of ethyl hexanoate in saké fermentations (Akada et al.). The mutation responsible for cerulenin resistance (Gly1250Ser) was found to be in the FAS2 encoded subunit of the fatty acid synthase (FAS) complex (Inokoshi et al.). The cerulenin resistance conferring mutation, which is located in the middle of the 3-ketosynthase domain, may compromise cycling of the fatty acids and confer leakage of medium-chain fatty acid substrates, such as hexanoic acid, from the FAS complex. This specific mutation, causing enhanced production of ethyl hexanoate, has been engineered into saké yeasts currently in commercial use (Aritomi et al.). Furthermore, a FAS2 allele isolated from an Ale yeast has been shown to improve acetate ester production in a way that appears to be unrelated to cerulenin resistance (Trindade de Carvalho et al.). The molecular mechanisms of mutations leading to the altered acetate ester production have not been elucidated.
Non-Saccharomyces yeasts with superior ester profiles. Non-Saccharomyces yeast (often termed non-conventional yeast) contains an untapped reservoir of flavor production that can enhance or complement the flavor profiles of Saccharomyces yeasts. In particular, co- or sequential fermentations with P. kluyverii or Cyberlindnera fabianii and brewing yeast shows potential for enhancing the ester profile (van Rijswijck et al.; Holt et al. ). While P. kluyverii produces a very high level of acetate esters (resembling a Saccharomyces ATF1 overexpression strain), C. fabianii forms a high level of ethyl esters (‘apple’ like). However, the high levels of acetate esters were repressed in consecutive fermentations with brewing yeast, leading to the hypothesis that esterases are activated upon fermentation with S. cerevisiae (Holt et al. ). A better understanding of how they interact in co- and sequentially inoculated cultures will therefore not only be interesting in an ecological context, but might also be beneficial for development of compatible strains.
Polyfunctional thiols
Polyfunctional thiols (thiols with other functional groups, e.g. alcohol groups) have been investigated thoroughly in wine making as they are character impact aroma compounds responsible for the varietal tropical character of Sauvignon Blanc white wines, especially originating from New Zealand (Dubourdieu and Tominaga ). However, these compounds have also been identified in commercial lager beers (Vermeulen et al.). The high-impact 4-methyl-4-sulfanyl-2-pentanone exists in the free form in considerable quantities in some aroma hops (Kishimoto et al.). As shown previously in studies on white wine, a large fraction of thiols are released from their non-aroma active cysteinylated and glutathionylated precursors also during beer fermentations with yeast (Cibaka et al., ). The levels of thiol-conjugates vary strongly in the different hop cultivars, leading to a significant difference in the final ratio and a unique thiol profile in beer. Typically, the release of thiols has been studied first with a commercially available tryptophanase enzyme with strong carbon-sulfur β-lyase (CS β-lyase) activity towards cysteine-S-conjugates. Indeed, tryptophanase treatment could release the polyfunctional thiol 3-sulfanylhexan-1-ol (a key compound in passion fruit and grapefruit aroma) from extracts of aroma and dual-purpose hops (Gros, Tran and Collin ; Cibaka et al.). When this enzyme is overexpressed in yeast, it leads to a very significant increase in release of the polyfunctional thiols in wine fermentations, suggesting that one of the bottlenecks is the CS β-lyase activity (Swiegers et al.). This is dependent on the precursor thiol compound. For example, free thiol is released from cysteine-S-4-methyl-4-sulfanyl-2-pentanone by the CS β-lyase Irc7 (Roncoroni et al.), while its release from cysteine-S-3-sulfanylhexan-1-ol appears to be a polygenic trait with multiple enzymes involved. The S. cerevisiae cystathionine β-lyase Str3 and the Irc7 enzymes have only residual activities towards cysteine-S-3-sulfanylhexan-1-ol while having other preferred natural substrates (Holt et al.; Roncoroni et al.). However, when these enzymes are overexpressed, they do lead to a significant increase in the free thiol level (Holt et al.; Roncoroni et al.) and thus illustrate the hidden capabilities of metabolic enzymes in flavor release. The release of thiols from cysteine conjugated precursors in grape must, linked to IRC7 gene regulation, is repressed by the presence of favorable nitrogen sources (ammonium) and highly induced by nitrogen catabolite repression (Subileau et al.; Thibon et al.). Its expression level, and consequently the release of polyfunctional thiols, can be enhanced by introduction of a naturally occurring mutation in the nitrogen catabolite repression regulator gene URE2 (Dufour et al.). Interestingly, the IRC7 gene is present in two isoforms, with the majority of S. cerevisiae strains (75%) containing an inactive 38-bp truncation (Belda et al.), while other members of the Saccharomyces sensu stricto species contain the full-length gene. In S. cerevisae, the IRC7 locus is situated 4.5 kb from the right-end telomere on chromosome 6 and is subjected to Sir2-dependent subtelomeric silencing through deacetylation of N-terminal lysines in H3 and H4 histones (Imai et al.; Ehrentraut et al.). Silencing defects caused by mutations in Sir2, Sir3 or Sir4 causes increased expression levels of the IRC7 gene (Ehrentraut et al.; Samel, Rudner and Ehrenhofer-Murray ). Interestingly, IRC7 is located approximately 10 kb away from the telomere end in S. uvarum, S. arboricola and S. eubayanus. These species have additional subtelomeric genes in comparison to S. cerevisiae. This observation is consistent with the finding that an S. uvarum strain produces high levels of thiols compared to S. cerevisiae wine strains (Knight et al.). The level of subtelomeric silencing may therefore play an important role in interspecies hybrids, such as S. pastorianus. Issues with sulfury off-flavors (e.g. H2S or DMS) have not been reported in wine trials with strains containing the URE2 mutation or in experiments with high and constitutive expression of the carbon-sulfur β-lyase genes IRC7 or STR3. Fermentation trials with beer medium are needed to exclude any potential side effects in these mutants. Moreover, the use of non-Saccharomyces yeasts has also shown great potential for thiol release (Anfang, Brajkovich and Goddard ; Zott et al.), in particular, commercialized P. kluyverii yeast has been patented for enhancing thiol release in co- and sequential beer fermentations (Saerens and Swiegers ).
In addition to the cysteine-S-conjugates, the thiols 3-sulfanyl-4-methyl-pentan-1-ol and 3-sulfanyl-4-methylpentan-1-ol (an important aroma thiol in Nelson Sauvin hop) were found to be present mainly in the form of glutathionylated precursors in hops, which explains their release during fermentation (Cibaka et al.). The biochemical conversion of glutathionylated precursors into free thiols occurs via the γ-glutamyltranspeptidase Ecm38, which splits the dipeptide conjugated intermediate into cysteine conjugates (Cordente, Capone and Curtin ; Santiago and Gardner ). Thus, the CS β-lyases are also necessary for the hydrolysis of glutathione conjugates. Finally, the oligopeptide and glutathione transporter Opt1 and the general amino acid permease Gap1 have been identified as strong effectors of thiol release, which indicates their involvement in transport over the cell membrane (Subileau et al.,; Cordente, Capone and Curtin ; Santiago and Gardner ).
Biosynthesis of S-conjugate precursors in hops
The biosynthesis of glutathionylated precursors in grapevine is believed to occur through a glutathione-S-transferase, which condenses reactive and stress-induced aldehydes (such as trans-2-hexenal) with glutathione (to from glutathione-S-3-hexan-1-ol) (Thibon et al.). Formation of the glutathione-S-conjugated precursor in grapevine leaves was induced by UV radiation, drought stress and botrytis infections, which correlates with the expression of the two glutathione-S-transferase genes, VvGST3 and VvGST4, that were shown to form glutathione-S-3-hexan-1-ol in vitro with the substrates trans-2-hexenal and glutathione (Kobayashi et al.). The high UV index in the southern hemisphere is believed to induce high levels of polyfunctional thiols and their precursors in Sauvignon Blanc grapes from New Zealand, although post-harvest UV treatments of defrosted grapes do not appear to affect the levels (Parish-Virtue et al.). Clearly, more research is necessary to conclusively determine the impact of UV radiation. The formation of glutathione-S-3-hexan-1-ol is induced in grapevine cultivated in high nitrogen conditions. However, the transcript levels of the major grapevine glutathione-S-transferase enzymes were not induced under this condition, which suggests the presence of other yet unidentified enzymes responsible for their formation under high nitrogen conditions (Helwi et al.).
Lactones and furanones
Lactones in beer
The formation of γ-lactones is dependent on the yeast strain (Loscos et al.) and the composition of the wort, particularly the fatty acid levels originating from both malt and hops. Biological formation of lactones typically starts with oxidation of a fatty acid substrate by lipoxygenases (LOX), which introduces a hydroxy group from hydrogen peroxide in a specific position, and subsequent breakage of the hydroxy acid in the β-oxidation pathway (Romero-Guido et al.; Joo and Oh ; El Hadi et al.). Next, the shortened fatty acid is released from coenzyme-A and forms a ring structure by esterification of the hydroxy group with the carboxylic acid (called lactonization). Lactonization is partly dependent on the yeast strain used during the fermentation and is favored at low pH in the fermentation media by spontaneous lactonization of fatty acids excreted from the cell (occurs naturally around pH 3.5) (Muller, Kepner and Webb ; Endrizzi et al.). Lactonization in beer is therefore likely to occur either by heating to form Maillard products during mashing and kilning of the malt or by enzymatic catalysis in the wort. During Whiskey production, high concentrations of lactic acid bacteria can occur spontaneously. These bacteria can cross-feed hydroxylated oleic and palmitoleic acid to distillers and brewer's yeast, which transforms these compounds into γ-decalactone and γ-dodecalactone (‘peach’, ‘fatty’, ‘butter’) (Wanikawa, Hosoi and Kato ).
Barley contains two LOX enzymes (LOX-1 and LOX-2), of which only LOX-2 is active during germination in the malting process (Yu et al.). The activity of this LOX enzyme is not only responsible for the formation of γ-nonalactone that contributes with ‘sweet’ and ‘coconut’ aroma, but also of trans-2-nonenal, a ‘cardboard’ off-flavor, that is formed mainly by hydroxylated fatty acid degradation during aging in the bottle and negatively affects flavor stability (Baert et al.). Therefore, LOX-negative barley strains have been constructed by the Tsingtao brewery (work commenced by Heineken and Carlsberg); they lack the LOX genes, do not form significant amounts of trans-2-nonenal, and result in beer with an increased shelf-live (Yu et al.). γ-Nonalactone also originates from hop precursors (Sakuma et al.), but with no active barley LOX enzyme, the conversion is dependent on autooxidation (Baert et al.). The fatty acids linoleic and linolenic acid are released during mashing and subsequently hydroxylated, which indicates both lipase and LOX activities (Kobayashi et al.). The LOX enzymes are heat-labile and are partly inactivated during the mashing (Kobayashi et al.; Baert et al.).
The formation of γ-butyrolactone has been studied in film-forming yeasts (S. fermentati), and derives from the metabolism of the amino acid glutamate, resulting in the formation of 4-oxobutyric acid (Muller, Kepner and Webb ; Wurz, Kepner and Webb ). The formation of lactones has been particularly well studied in the lipophilic yeast Yarrowia lipolytica that produces much higher levels than S. cerevisiae (Wache et al.). Also, it was shown with radiolabeling experiments that γ-decalactone and γ-dodecalactone could be produced from oleic acid (C18) in Sporobolomyces odorus, which is consistent with both oleic and linolenic as the natural precursors (Haffner and Tressl ).
Furanones formed during malting and mashing
Strawberry furanone is formed as a Maillard product during kilning of malt and mash boiling and is found in higher concentrations in roasted malt varieties (Schieberle ; Slaughter ). Its level also increases during fermentation and depends on the yeast strain used (Sakuma et al.). It is derived partly from α-ketoglutarate catabolism and de novo synthesis of the amino acid threonine during the fermentation (Tressl et al. ).
Terpenoids
Terpenoid synthesis in hops
Terpenoids are the largest and most diverse group of flavor compounds. In particular, two of the plant species that produce terpenoids are Vitis vinifera (grapes) and Humulus lupulus (hops), important raw ingredients of wine and beer industries, respectively. They are synthesized de novo in the plastids from pyruvate and glyceraldehyde-3-phosphate in the methylerythritol 4-phosphate (MEP) pathway, or from acetyl coenzyme A (acetyl-coA) in the cytosol, the ER and the peroxisome through the mevalonate (MVA) pathway (Singh and Sharma ). The MEP pathway genes are highly expressed and have for each enzyme several paralogs in hop, compared to the MVA pathway genes (Nagel et al.; Wang et al.). It is therefore thought to be the major pathway responsible for provision of isoprenoid precursors for terpenoid synthesis. This pathway has seven sequential enzymatic steps, requiring carbon influx and energy charged molecules (ATP, CTP) (Fig. 7). Hydroxymethylbutenyl 4-diphosphate, the end product of the MEP pathway, is transformed into the isoprenoid precursors isopentenyl diphosphate (IPP, C5) and dimethylallyl diphosphate (DMAPP, C5) by IPP/DMAPP synthase and can be interconverted by IPP/DMAPP isomerase. Next, IPP and DMAPP are condensed by prenyltransferases to geranyl diphosphate (GPP, C10) and neryl diphosphate (C10), farnesyl diphosphate (FPP, C15) and geranylgeranyl diphosphate (GGPP, C20). These are the basic units for further processing by terpene synthase enzymes into monoterpenes (C10), sesquiterpenes (C15) and diterpenes (C20) using mono-, sesqui- and diterpene synthases (MTS, STS and DTS), respectively (Chen et al. ). The wealth of different terpenoids in plant products is due to terpene synthases in different plant species yielding multiple different products from the same substrate and the subsequent addition of functional groups (Tholl ; Nagegowda ; Chen et al. ). Notably, the functional groups can also come directly from the terpene synthase activity. For example, linalool synthase cleaves the oxygen from the phosphate of GPP, and finally yields β-linalool, which contains an alcohol group (Cseke, Dudareva and Pichersky ). Addition of functional groups (alcohols, ketones, aldehydes, etc.) can occur either by enzymatic reactions in the hop plant or by yeast in the brewing process. The latter is discussed in detail later.
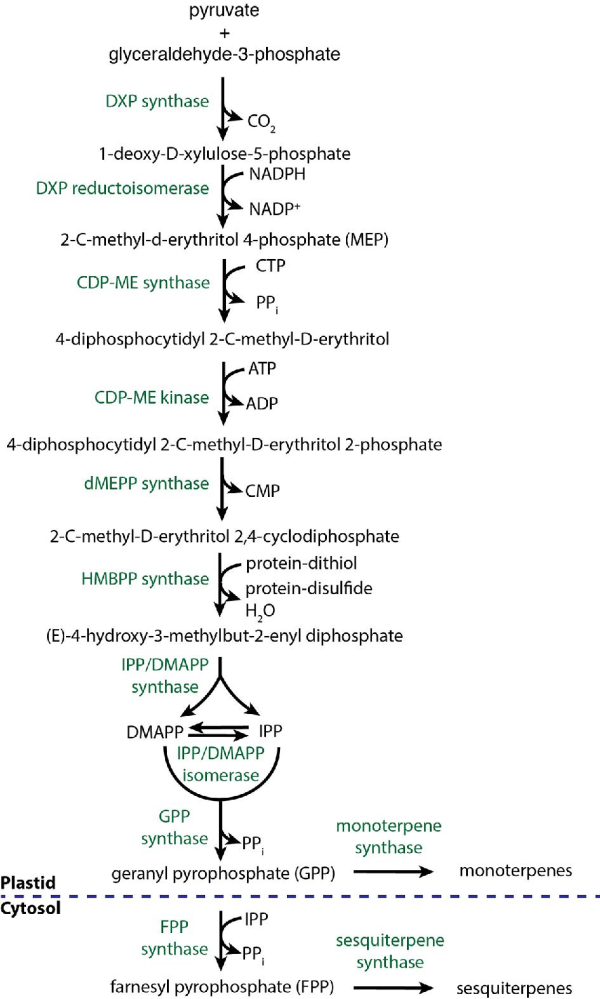
Figure 7
The MEP terpenoid biosynthesis pathway in hops. The pathway consists of seven enzymatic steps, including five synthases, two isomerases and a kinase, leading to IPP and DMAPP. Subsquent conversion to GPP and FPP, which are the direct precursors of mono- and sesquiterpenes, respectively, occurs through the GPP (plastidic) and FPP (cytosolic) synthases. DXP, 1-deoxy-D-xylulose-5-phosphate; CDP-ME, 4-diphosphocytidyl-2-C-methyl-D-erythritol; cMEPP, 2-C-methyl-D-erythritol 2,4-cyclodiphosphate; HMBPP, (E)-4-hydroxy-3-methyl-but-2-enyl pyrophosphate.
Hop terpenoid synthesis has been investigated by Wang and colleagues (Wang et al.; Wang and Dixon ). Using a cDNA library made from RNA of the glandular trichomes (hairs) and flowers of the hop plant, GPP synthase, two monoterpene synthase and two sesquiterpene synthase homologs were identified, expressed in Escherichia coli and enzymatically characterized. The GPP synthase consists of a small and a large subunit that form a heterodimer producing GPP (59.5%) and GGPP (40.5%) when incubated with the substrates IPP and DMAPP, and uniquely GGPP when IPP and GPP or GFP is supplied in vitro (Wang and Dixon ). The large subunit is the catalytic entity that on its own has a higher tendency to form GGPP (68.2%) than GPP (26.9%). Expression levels of the small subunit of GPP synthase are highest in glandular trichomes, and are directly correlated with the myrcene concentration, whereas the large subunit is also expressed in stems, leafs and flowers (Wang and Dixon ). The MTS enzyme, Mts2, catalyzes the formation of myrcene from GPP, while the STS enzyme, Sts1, synthesizes β-caryophyllene and α-humulene from FPP (Wang et al.). Among these, only myrcene is a powerful aroma-active terpenoid. The terpene synthases responsible for formation of some of the most aroma-active terpenoids have not been characterized yet. Interestingly, Wang and colleagues identified a putative β-linalool synthase predominantly expressed in flowers, but did not investigate it further. Comparative RNA-Seq analysis showed a higher expression of the MTS1 gene in the hop cone of the cultivated hop variety cv. Shinshu Wase compared to a wild hop variety (Natsume et al.). This indicates that a selection occurred in the cultivar for upregulated specialized flavor metabolism, leading to increased aroma production. Recently, nine terpenoid syntheases from glandular trichomes of Cannabis sativa, belonging to the same Cannabaceae family as hops, were functionally characterized and linked to the production of β-myrcene, (E)-β-ocimene, (–)-limonene, (+)-α-pinene, β-caryophyllene and α-humulene (Booth, Page and Bohlmann ). These terpenoid synthases, as well as the already characterized hop terpene synthases Mts1, Mts2, Sts1 and Sts2, belonged to the TPS-b and TPS-a subfamilies, which typically contain mono or sesquiterpenoid syntheases, respectively.
Some aroma-active terpenoids, termed norisoprenoids (e.g. β-ionone, ‘artificial raspberry’, ‘cedarwood’, ‘violet’), are formed from oxidative cleavage of carotenoids (C40, tetraterpenoids) by carotenoid cleavage dioxygenases (CCDs) (El Hadi et al.). Interestingly, some yeasts and fungi can also produce this compound from oxidation and cleavage of β-carotene (a major carotenoid compound) (Zorn et al., ; Romero-Guido et al.). There is also a high interest in norisoprenoids from the flavor and fragrance industry due to their pleasant aroma and low sensory thresholds. This has led to the construction of a transgenic S. cerevisiae strain that produced considerable amounts of β-ionone by introduction of genes for both the formation and oxidative degradation of β-carotene (López et al.). In wine production, carotenoids are typically absent in musts. The presence of β-ionone is likely due to the activity in the grapevine of the CCD enzyme VvCCD4b that is induced during ripening of grapes, when the level of carotenoids decreases steadily (Mendes-Pinto ; Lashbrooke et al.). A study on Pinot grapevine showed that the degradation of carotenoids during ripening is well correlated with the formation of some norisoprenoids, such as β-damascone (‘blackcurrant’, ‘raspberry’, ‘menthol’), but not linked to others such as β-ionone (Yuan and Qian ), suggesting that some of these compounds form during fermentation or during maturation.
Genomic regions (quantitative trait loci, QTLs) linked to the production of 17 mono- and sesquiterpenoids in hops showed mostly complex pleiotropic interactions between QTLs with linkage for multiple compounds and also some unique QTLs for specific compounds (humulene, δ/γ-cadinene and ρ-cymene) (McAdam et al.). This overlap between genomic regions for several terpenoids may be due to selection for regulatory elements responsible for high expression of terpenoid synthases or common genes in the biosynthesis pathway as described above. However, the large QTLs, typically identified in plant studies, do not allow to pinpoint the specific genes responsible without further targeted molecular analysis, such as genome editing facilitated by CRISPR/Cas (Ricroch, Clairand and Harwood ). This technique is currently being explored in the genetically related cannabis plant (Maxmen ), but still needs to be implemented for hops. Important work therefore remains to be performed regarding functional and genetic characterization of the remaining terpenoid synthases, their regulation and transformation by brewing yeast.
Another important aspect of terpenoid biosynthesis is the formation of flavorless glucosides of terpenoids that are being produced by glucosyltransferase activity in hops, and later being hydrolyzed during the brewing process into flavor-active terpenoids. The glycosylation of terpenoids occurs through transfer of the sugar molecule from the nucleoside diphosphate-activated group [e.g. uridine diphosphate (UDP)-glucose] to the functional group of the terpenoid (e.g. an alcohol or acid) (Tiwari, Sangwan and Sangwan ). Glycosylation is known to aid detoxification by solubilizing these hydrophobic compounds, which allows transport over the cell membrane (Zhao et al.). Screening for glycosylation activity by in vitro assays with glycosyltransferases from the plant model organism Arabidopsis thaliana identified 27 glycosyltransferases with diverse activities towards the aroma-active monoterpenoid alcohols geraniol, linalool, menthol, α-terpineol, β-citronellol and the sesquiterpenoid farnesol (Caputi, Lim and Bowles ). Recently, four hop terpenoid glycosyltransferases were shown to have high activity towards UPD-glucose together with linalool and geraniol, which was patented for purpose of reducing their activity, potentially leading to a higher level of free flavor-active terpenoids (Ono and Nobuo ). In grapevine, the two UDP-glucose monoterpenol glycosyltransferases, VvGT7 and VvGT14, were shown to be induced by ripening, which correlated with glycoside formation of a range of monoterpenoids, including geraniol and linalool (Li et al.). UDP-glucose:cinnamate glycosyltransferase expression in strawberry was also shown to be correlated with fruit ripening, specific for fruit-forming tissue, downregulated by auxin and induced by oxidative stress (Lunkenbein et al.). The molecular mechanisms of regulation of glycosyltransferases related to flavor-active terpenoids have not been investigated to the same level as conjugates with flavonoids and hormones (Gachon, Langlois-Meurinne and Saindrenan ; Tiwari, Sangwan and Sangwan ), and it is therefore not clear how the environmental signals are transmitted.
Hop terpenoids in the brewing process
The most abundant terpenoids found in the essential oil of hops, namely the sesquiterpenes: α-humulene, β-caryophyllene and β-farnesene and the monoterpenoid myrcene, do not directly shape the flavor profile of most beers. Instead, they undergo significant chemical and functional modifications during the brewing process (thermal reactions, oxidation, hydrolysis or isomerization) (Fig. 8) as well as biochemical transformations by yeast during fermentation (esterification, ester exchange and enzymatic cleavage). That said, these effects on the hop oil components have not yet been fully investigated. Generally, it is known that the extraction of terpenoids from hops depends on the time that the hop was boiled. The amount decreases with increasing boiling time due to evaporation (Kishimoto et al.). Therefore, late, whirlpool or dry hopping is advised to achieve richer flavor and aroma profile in final beers (Sharp, Steensels and Shellhammer ). The impact of boiling parameters (heat, light or oxygen) on the components of essential oils, in terms of their stability and possible reactions, has been reviewed elsewhere (Turek and Stintzing ). In general, it is known that oxygenated derivatives of the terpenoids (e.g. in the form of alcohols) have a much higher probability to remain in the final beer due to their higher relative solubility.
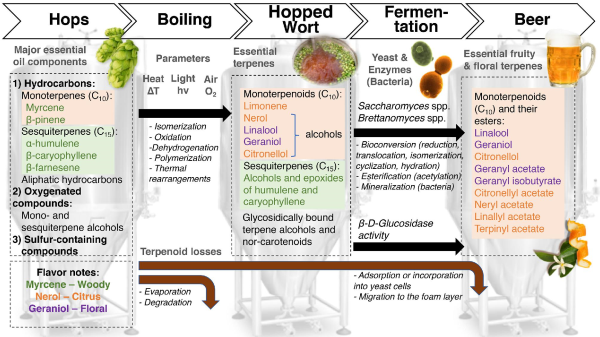
Figure 8
Overview of chemical, enzymatic and metabolic transformations of terpenoids throughout the brewing process, with a focus on ‘fruity’ and ‘floral’ notes development in the final beer.
The behavior and fate of each aroma-active terpenoid is unique and compound-specific. The loss of the above-mentioned most abundant hop-derived terpene hydrocarbons during fermentation of hopped wort was explained by their adsorption to the hydrophobic yeast cells, by binding with yeast cell wall components and by migration to the foam layer (Lam, Foster and Deinzer ; King and Dickinson ; Praet et al.). However, it was not originally attributed to bioconversion by yeast, because no yeast transformation products of myrcene, α-humulene and β-caryophyllene could be detected (King and Dickinson ). An early study reported that the hop-derived monoterpenoids linalool and geraniol survived the entire brewing process and that β-citronellol was formed during fermentation from a hop precursor (most likely geraniol). This suggested that yeast has an ability to transform certain terpenoids into other terpenoids enzymatically (Lam, Foster and Deinzer ). Many subsequent reports focused on the use of mono- and sesquiterpene alcohols as potential precursors for bioconversion reactions performed by yeast (King and Dickinson ; Takoi et al.,; Praet et al.). Regardless of the hop cultivar used, the same tendencies were observed during fermentation: slight decrease in linalool and α-terpinol, followed by an increase in β-citronellol and nerol and finally an increase in geraniol (Takoi et al. ). The proposed metabolic reactions for transformation of monoterpene alcohols by Torulaspora delbrueckii and Kluyveromyces lactis, as well as S. pastorianus lager and S. cerevisiae ale yeast, are outlined in Fig. 9. However, the enzymes responsible for these transformations have not been identified yet, also very little is known about the genes involved. In general, it was observed that yeasts were able to convert hop-derived monoterpene alcohols, specifically geraniol into linalool or β-citronellol, nerol into geraniol, α-terpinol or linalool and the latter into α-terpinol (King and Dickinson ). Monoterpene alcohols can be further metabolized by yeasts, through esterification or hydrolysis, particularly acetylation of monoterpenes by AATase enzymes (King and Dickinson ) or by cleavage of monoterpene esters by esterases or lipases (Lam, Foster and Deinzer ; Chatterjee, Chatterjee and Bhattacharyya ). Using gene overexpression and gene deletion analysis, it was demonstrated that the AATase enzyme, Atf1, is responsible for acetylation of the major monoterpenoids (geraniol, citronellol and nerol) in S. cerevisiae S288c (Steyer et al. 2013). The same effect was not observed for Atf2. Notably, the non-conventional yeast Williopsis saturnus was able to produce higher concentrations of acetate ester derivatives of monoterpenoids (geranyl- citronellyl-) and retain better the original terpenoid content compared to brewer's yeast (Liu and Quek ).
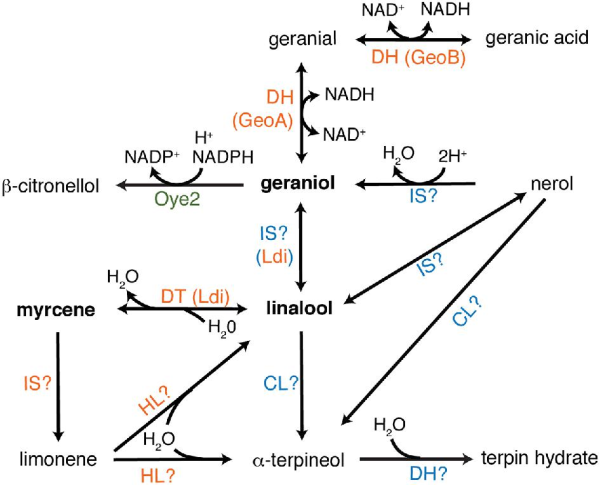
Figure 9
Overview of the biotransformation reactions of monoterpene alcohols and esters catalyzed by lager, ale and non-conventional yeasts and proposed transformation of myrcene from bacterial studies. The enzymatic reaction carried out by Oye2 has been experimentally verified in S. cerevisiae and is indicated in green, putative enzymatic activities that have been implied from metabolite profiling in fermentations with Saccharomyces yeast (King and Dickinson , ) are indicated in blue, and reactions in Castellaniella defragrans and Pseudomonas aeruginosa that have not yet been demonstrated in yeast (Brodkorb et al.; Esmaeili and Hashemi ) are indicated in orange. The monoterpenoids can also be acetylated by Atf1/2 via their functional group, which is not indicated in the figure. CL, cyclase; DH, dehydrogenase; DT, dehydratase; IS, isomerase; HL, hydroxylase.
Geraniol is a major precursor for yeast transformation, and its initial level correlates with the final content of the other monoterpene alcohols in beer (Takoi et al. ). During brewing, the rates of bioconversion reactions were dependent on yeasts’ growth phase and the timings of hop addition. Several researchers have reported that geraniol is mainly converted to β-citronellol during the first 2–4 days in model fermentations producing late-hopped beers (Takoi et al. ). This phenomenon was explained by Yuan et al. (), who revealed the involvement of NADPH dehydrogenase 2, encoded by OYE2 (old yellow enzyme 2), in geraniol to citronellol reduction (Fig. 9). Overexpression of S. cerevisiae OYE2 increased the reduction of geraniol to β-citronellol to 87% in comparison to the 50% obtained with the control S. cerevisiae strain (Steyer et al. 2013). Deletion of the OYE2 or ATF1 genes, both involved in endogenous conversion of geraniol to other terpenoids, improved geraniol production by 1.7-fold or 1.6-fold in batch fermentation, respectively (Zhao et al.). Apart from Oye2, Atf1 and Atf2, the remaining enzymes involved in the biotransformation reactions of monoterpene alcohols in S. cerevisiae have not yet been identified. However, monoterpene degradation pathways, including the transformation between linalool and geraniol, have been more intensively studied in bacteria (Fig. 9). The bifunctional linalool dehydratase-isomerase (encoded by the LDI gene) was isolated from the bacterium Castellaniella defragrans, which catalyzes the hydration of β-myrcene to linalool in the thermodynamically unfavorable direction (dehydratase reaction). It further catalyzes the isomerization to geraniol (geraniol isomerase). These constitute the initial steps in anaerobic β-myrcene biodegradation (Brodkorb et al.). Geraniol can be further converted to geranial and geranic acid by geraniol- and geranial dehydrogenases, respectively (Luddeke et al.). In C. defragrans, geraniol dehydrogenase (NAD+-dependent) is encoded by the GEOA gene, while geranial dehydrogenase (NAD+-dependent) by the GEOB gene. Myrcene was also transformed by Pseudomonas aeruginosa to α-terpineol with limonene as intermediate (Esmaeili and Hashemi ). It has been suggested that limonene hydroxylase enzyme is possibly involved in conversion of limonene into linalool, perillyl alcohol and α-terpineol (Yang, Park and Chang). Besides, other enzymes involved in monoterpene transformations of microbial origin have been reviewed elsewhere (Marmulla and Harder ).
Among microorganisms, yeasts usually employ the MVA pathway for terpene synthesis, whereas bacteria mainly use the MEP route, except for some species, which use either the MVA route or both. In S. cerevisiae, firstly IPP (C5) is synthetized from mevalonate by the enzymes mevalonate kinase (Erg12/Rar1), phosphomevalonate kinase (Erg8) and diphospho-mevalonate decarboxylase (Mvd1/Erg19). Then, IPP (C5) can be rearranged to DMAPP (C5) catalyzed by the IDI1-encoded enzyme IPP isomerase. Next, two reactions leading to the formation of GPP (C10) and then FPP (C15) are conducted by the bifunctional Erg20 enzyme, which has both GPP synthase and FPP synthase activities. Firstly, GPP synthase catalyzes condensation of IPP (C5) with DMAPP (C5) into GPP, followed by a second condensation of GPP with another IPP that is performed with FPP synthase to form the FPP molecule. GPP and FPP are key precursors for further mono- and sesquiterpenoid synthesis (Cordente et al.).
Normally, yeasts do not synthesize significant quantities of terpenoids de novo, due to lack of the MTS in the monoterpene synthesis pathway. In addition, they also lack a specific GPP synthase, and this metabolite only occurs as an intermediate in the synthesis of FPP, the precursor of several essential metabolites (Grabinska and Palamarczyk ). A relatively low level of the monoterpenoids, geraniol and linalool, could be synthesized de novo by Hanseniaspora uvarum and S. cerevisiae (1.2 and 4 μg/L, respectively) (Carrau et al.). However, new strategies in genetic modification helped to overcome these constraints. This included enginieering and optimization of the upstream isoprenoid pathway flux (prior to mevalonate synthesis) together with introduction of an appropriate plant-originated terpenoid synthase. For example, dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism (redistributed precursor GPP flux) contributed to the 3.4-fold improvement in geraniol production in S. cerevisiae with introduced geraniol synthase from valerian flowers (Valeriana officinalis) (Zhao et al.). Moreover, the combination of dynamic control of ERG20 expression with OYE2 deletion increased geraniol production up to 1.69 g/L (Zhao et al.). The enzyme hexaprenyl pyrophosphate synthetase, encoded by the COQ1 gene, which is known to catalyze the first step in ubiquinone (coenzyme Q) biosynthesis, is involved in monoterpene synthesis in S. cerevisiae. Overexpression of COQ1 together with a mutated FPP synthetase, Erg20K197E, resulted in significantly higher levels of linalool (above 750 μg/L), geraniol, α-terpineol and the sesquiterpenes, farnesol and nerolidol (Camesasca et al.). Recently, a methodology was developed to create brewer's yeasts capable of biosynthesizing monoterpenes to similar levels as those typically found in finished beer, and importantly, beer brewed with this yeast was characterized by a hoppy aroma in sensory analysis, without the addition of any external hops (Denby et al.). The successful strain contained a unique combination of strong promoters driving expression of the four modulated genes, which ensured variation in the ratio of linalool to geraniol: (1) a truncated form of yeast HMG acetyl-coA reductase lacking the regulatory domain, Hmg1M1_S511del; (2) the mutated FPP synthase with enhanded activity, Erg20K197E; (3) a truncated linalool synthase from mint (Mentha citrata), McLISM1_E67del without the plastidic targeting peptide; and (4) a geraniol synthase from basil (Ocimum basilicum) ObGES. In fermentation experiments, it was verified that the selected genes indeed control monoterpene production since the total production of monoterpenes was correlated with Hmg1M1_S511del and Erg20K197E abundance, linalool correlated with McLISM1_E67del abundance, but ObGES abundance was not promotional with geraniol synthesis. Although these engineered strains can improve fruity and floral notes in beer, genetically modified brewing yeasts often still lack acceptance by the consumer.
Release of terpenoids from glycosides
Due to the described transformations, terpenoids present in wort and beer undergo dynamic changes and interactions, usually leading to their decrease under traditional brewing conditions (Takoi et al. ; Liu and Quek ). However, in beer brewed with hop pellets accumulation of linalool and β- damascone throughout the fermentation of wort has been reported (Kishimoto et al.; Kollmannsberger, Biendl and Nitz ). The observed increase in monoterpenoid levels is due to release from glycosidically conjugated flavor-inactive precursors (glycosides) of terpenoids and norisoprenoids found in hopped wort, which undergo hydrolysis during fermentation with release of their aromatic counterparts (aglycones) (Fig. 10). Plant materials used in the food industry contain up to five times more flavor compounds bound to glucose than free, aroma-active flavor compounds (Vervoort et al.). The cleavage of glycosides originating from hops or fruits is most commonly observed in spontaneously fermented beers with fruit maceration, such as Lambic and Gueuze base beers with cherries (Kriek), or in sour beer varieties (Daenen and De Clerck ). Principally, two mechanisms of hydrolysis are responsible for their release from the flavor-inactive glucosides. In sour beers, at low pH (3.0–3.5), acidic hydrolysis by terpene glycosidases can induce rearrangement of monoterpene alcohols into other compounds (Maicas and Mateo ). First, acidic hydrolysis (pH 3.0) is mainly responsible for release of linalool and α-terpineol, α-ionol and α-damascone in sour cherry beers (Daenen et al.). The second mechanism, enzymatic hydrolysis, can be initiated by addition of exogenous enzymes or by enzymatic activity of yeast, although the enzymatic activity in conventional brewing yeast is generally very low. Commercially available enzymes used in winemaking for aroma enrichment but also in fruit and vegetable processing for hydrolysis of glycosides act sequentially in two steps, as shown in Fig. 10 (Daenen and De Clerck ; Wen et al.). First, disaccharide glycosides are cleaved by the action of α-L-rhamnosidase, α-L-arabinosidase and β-D-apiosidase liberating the corresponding terpene monoglucosides by cleavage at the (1→6) glycoside bond. In the second step, the aglycone-carbohydrate bond is cleaved by β-D-glucosidase releasing monoterpene alcohols such as linelool. For example, the enzyme preparation Rapidase AR2000, containing pectinase and glycosidases derived from Aspergillus niger, has been successfully applied to release aglycones from glycosides in hops, hop products and beer (Kollmannsberger, Biendl and Nitz ; Daenen and De Clerck ), in cherry juice (Wen et al.) and sour cherry Kriek beer (Daenen et al.; Daenen et al.; Daenen and De Clerck ; Vervoort et al.).
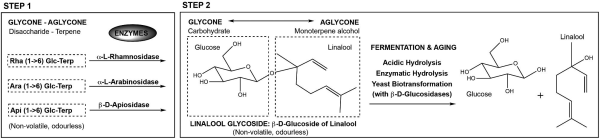
Figure 10
Sequential enzymatic hydrolysis of disaccharide flavor precursors, STEP 1—the disaccharides: α-L-rhamnopyranosyl-β-D-glucopyranosides (designated Rha-Glc), α-L-arabinofuranosyl-β-D-glucopyranosides (designated Ara-Glc) and β-D-apiofuranosyl-β-D-glucopyranosides (designated Api-Glc), are cleaved by corresponding enzymes (α-L-rhamnosidase, α-L-arabinosidase and β-D-apiosidase) from the terpene residues (designated Terp), STEP 2—liberation of free linalool from a linalyl glycoside containing β-D-glucose, via acidic or enzymatic hydrolysis [adapted from Maicas and Mateo (Maicas and Mateo )].
Yeasts with β-glucoside hydrolase activity can also release flavor-active compounds from hop-derived glucosides (Daenen et al.; Vervoort et al.; Sharp, Steensels and Shellhammer ). Daenen et al. () first reported that the Saccharomyces brewing yeasts that they tested did not show 1,4-β-D-glucosidase (BGL) activity, only a strain-dependent low-to-moderate activity of exo-1,3-β-glucanase (EXG). The major exoglucanase enzyme in S. cerevisiae, encoded by the EXG1 gene, has also been shown to have non-specific activity, which can act on glucose polymers and smaller glycosidically conjugated substrates (Larriba et al.; Suzuki et al.; Schmidt et al.). Thus, Saccharomyces strains characterized by high EXG activity demonstrated BGL-like activity against hop glycosides during fermentation (Daenen et al.), and their BGL-like activity varied widely among different strains (Sharp, Steensels and Shellhammer ). The Exg1 protein is proteolytically hydrolyzed by the Kex2 protease and subsequently released into the extracellular space via the secretion (Sec) pathway, through the endoplamatic reticulum, the golgi apparatus and the periplasmic space from where it diffuses into the extracellular medium (Cenamor et al.; Larriba et al.; Cappellaro, Mrsa and Tanner ). Release of active Exg1 into the extracellular medium is enhanced in a strain lacking the ß-glucan synthase Kre6, which has compromised cell integrity (Wang et al.). The EXG1 gene shows high expression under its native promoter during exponential growth, but is repressed in low hypoxic conditions that occur during fermentation (Lu-Chau et al.). Overexpression of the EXG1 gene in a Saccharomyces wine yeast strain under the constitutive ACT1 promoter showed 20- to 40-fold higher EXG activity and enabled release of significantly higher levels of terpenoids than with a control strain from glycoside-rich grapevine extract in Muscat wine fermentations (Gil et al.). Interestingly, the levels of yeast-derived esters and higher alcohols were also enhanced significantly in the wines, similarly to what was observed upon overexpression of the ß-glucosidases from A. nidulans and Saccharomycopsis fibuligera (Ganga et al.; Van Rensburg et al.). This is consistent with differences observed in the yeast-derived ester profile in beers hopped with four different hop varieties (Steyer et al.), indicating an uncharacterized interaction between hop compounds and regulation of yeast aroma pathways.
Some of the non-conventional Brettanomyces yeasts possessed active BGL with the highest activity found in Brettanomyces custersii LD72, isolated from Lambic beer (Daenen et al.). In refermentation experiments with addition of amygdalin (cyanogenic glycoside present in the seeds of the sour cherry), B. custersii LD72 demonstrated higher release of sour cherry glycosides compared with Saccharomyces ale strains with low and high BGL-like activity (Daenen et al.). The strain B. custersii LD72 was further tested in different refermentation conditions with whole cherries, cherry pulp, cherry juice and cherry stones. Similar patterns of released linalool, α-terpineol, geraniol and α-ionol were observed in all conditions. Out of 428 different yeasts that were screened for BGL activity, B. anomalus YV396 and B. bruxellensis YV397 showed exceptionally high activities (Vervoort et al.). Their genomes were sequenced, and the β-glucosidase-encoding genes were codon-optimized and expressed in E. coli. The β-D-glucosidase from B. anomalus was thoroughly characterized for its technological and industrial application for instance fruity cherry beer production. Isolating Brettanomyces β-D-glucosidase enzymes and adding them during the brewing process without the Brettanomyces yeast can enhance the fruitiness of beer without production of the characteristic off-flavors that typically develop with Brettanomyces yeasts during fermentation (Vervoort et al.).
CONCLUSIONS AND PERSPECTIVES
Flavor compound biosynthesis, especially ester biosynthesis, has now been investigated in considerable detail in yeast, while the corresponding pathways in plants, including barley grains, hops and grapes, are much less known. The latter is also true for the pathways responsible for biosynthesis of precursors present in fermentation substrates that are converted by the yeast into highly active flavor compounds. For some plant-derived precursors, the genes encoding the transporters as well as the enzymes responsible for their uptake and conversion into active flavor compounds in the yeast are still unknown. Major examples are the lactones, polyfunctional thiols and terpenoids. For example, only a fraction of the S-conjugated polyfunctional thiols can be converted into the free thiols by yeast and the concentration of the precursors is generally not directly correlated with the final amount of the released thiol during alcoholic fermentation. A major shortage in our knowledge is whether variability in the activity of the biosynthetic pathways for flavor compound production is responsible for the strong variation in flavor production between different yeast strains, especially for the brewing yeasts which have a dramatic effect on beer flavor. Recent QTL mapping research on flavor production in yeast has revealed multiple additional genes affecting flavor production (Steyer et al.; Den Abt et al.; Trindade de Carvalho et al.; Eder et al.; Holt et al. ). Many of the gene products concerned do not seem to be true intermediates of metabolic pathways involved in flavor biosynthesis and/or breakdown, but likely act indirectly through effects on substrate provision or other factors influencing the flux through these pathways. Higher alcohols and esters are among the most important flavor compounds in beer and are almost uniquely synthesized by the yeast. The ATF1 gene product clearly plays a vital role in this respect. We have gained much insight about the many molecular signaling pathways and transcription factors that affect expression of ATF1, which turned out to be highly complex. It remains unclear what the precise relationship is between this complex regulation and the ecological function of yeast flavor production in its natural habitats. Most research on yeast flavor production has been performed under aerobic or semi-anaerobic laboratory conditions. However, the largely anaerobic and high-pressure conditions in large-scale industrial beer fermentations are quite different and might certainly have a major effect on the profile of the flavor compounds produced. Hence, more research is needed on the regulation of flavor production under conditions more closely mimicking the industrial brewing conditions. Specially selected and maintained strains of S. cerevisiae have been the cornerstone of the industrial brewing process for many centuries. However, for production of certain specialty beers, evolving communities of yeast and other microorganisms have been essential for production of beer with the appropriate flavor. We highlight the capacity of non-conventional yeast species to produce novel flavor profiles, different from the characteristic S. cerevisiae profile, and examples where such alternative yeast species can be used successfully in beer brewing. Very little is known about the alternative pathways and/or alternative regulation of flavor production in non-conventional yeast species. It is likely that these yeast species still hold a large untapped potential for successful application in the production of beer and other alcoholic beverages.
ACKNOWLEDGEMENT
The authors would like to acknowledge Jens Eiken and Luc Didierjean from the Carlsberg research center for establishing the collaboration and for advice regarding the section on terpenoids in the brewing process. We also like to thank Nico Vangoethem for practical advice with the design of figures.
REFERENCES
- Aguilar PS, de Mendoza D. Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Mol Microbiol2006;62:1507–14.
- Aguilera J, Petit T, de Winde JH, et al Physiological and genome-wide transcriptional responses of to high carbon dioxide concentrations. FEMS Yeast Res2005a;5:579–93.
- Aguilera J, Van Dijken JP, De Winde JH, et al Carbonic anhydrase (Nce103p): an essential biosynthetic enzyme for growth of Saccharomyces cerevisiae at atmospheric carbon dioxide pressure. Biochem J2005b;391:311–6.
- Akada R, Matsuo K, Aritomi K, et al Construction of recombinant sake yeast containing a dominant FAS2 mutation without extraneous sequences by a two-step gene replacement protocol. J Biosci Bioeng1999;87:43–48.
- Akakabe Y, Sakamoto M, Ikeda Y, et al Identification and characterization of volatile components of the Japanese sour citrus fruit citrus nagato-yuzukichi Tanaka. Biosci Biotechnol Biochem2008;72:1965–8.
- Anfang N, Brajkovich M, Goddard MR. Co-fermentation with Pichia kluyverii increases varietal thiol concentrations in Sauvignon Blanc. Aust J Grape Wine R2009;15:1–8.
- Angelino SAGF. Beer. In Maarse H (ed.) Volatile Compounds in Foods and Beverages. New York, USA: Marcel Dekker, Inc., 1991;581–616.
- Aritomi K, Hirosawa I, Hoshida H, et al Self-cloning yeast strains containing novel FAS2 mutations produce a higher amount of ethyl caproate in Japanese sake. Biosci Biotechnol Biochem2004;68:206–14.
- Asano T, Inoue T, Kurose N, et al Improvement of isoamyl acetate productivity in sake yeast by isolating mutants resistant to econazole. J Biosci Bioeng1999;87:697–9.
- Ashida S, Ichkawa E, Suginami K, et al Isolation and application of mutants producing sufficient isoamyl acetate, a sake flavor component. Agr Biol Chem1987;51:2061–5.
- Avalos JL, Fink GR, Stephanopoulos G. Compartmentalization of metabolic pathways in yeast mitochondria improves the production of branched-chain alcohols. Nat Biotechnol2013;31:335–41.
- Baert JJ, De Clippeleer J, Hughes PS, et al On the origin of free and bound staling aldehydes in beer. J Agr Food Chem2012;60:11449–72.
- Bansil R, Turner BS. Mucin structure, aggregation, physiological functions and biomedical applications. Curr Opin Colloid In2006;11:164–70.
- Belda I, Ruiz J, Navascues E, et al Improvement of aromatic thiol release through the selection of yeasts with increased β-lyase activity. Int J Food Microbiol2016;225:1–8.
- Boeing H, Bechthold A, Bub A, et al Critical review: vegetables and fruit in the prevention of chronic diseases. Eur J Nutr2012;51:637–63.
- Bojanowski V, Hummel T. Retronasal perception of odors. Physiol Behav2012;107:484–7.
- Booth JK, Page JE, Bohlmann J. Terpene synthases from Cannabis sativa. PLoS One2017;12:e0173911.
- Boubekeur S, Bunoust O, Camougrand N, et al A mitochondrial pyruvate dehydrogenase bypass in the yeast Saccharomyces cerevisiae. J Biol Chem. 1999;274:21044–8.
- Boubekeur S, Camougrand N, Bunoust O, et al Participation of acetaldehyde dehydrogenases in ethanol and pyruvate metabolism of the yeast Saccharomyces cerevisiae. Eur J Biochem2001;268:5057–65.
- Braus GH. Aromatic amino acid biosynthesis in the yeast Saccharomyces cerevisiae: a model system for the regulation of a eukaryotic biosynthetic pathway. Microbiol Rev1991;55:349–70.
- Brodkorb D, Gottschall M, Marmulla R, et al Linalool dehydratase-isomerase, a bifunctional enzyme in the anaerobic degradation of monoterpenes. J Biol Chem2010;285:30436–42.
- Buck LB. The molecular architecture of odor and pheromone sensing in mammals. Cell2000;100:611–8.
- Caetano SM, Menezes R, Amaral C, et al Repression of the low affinity iron transporter gene FET4: a novel mechanism against cadmium toxicity orchestrated by Yap1 via Rox1. J Biol Chem2015;290:18584–95.
- Cai L, Sutter BM, Li B, et al Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell2011;42:426–37.
- Cai L, Tu BP. Acetyl-CoA drives the transcriptional growth program in yeast. Cell Cycle2011;10:3045–6.
- Calderbank J, Hammond JMR. Influence of higher alcohol availability on ester formation by yeast. J Am Soc Brew Chem1994;52:84–90.
- Camesasca L, Minteguiaga M, Farina L, et al Overproduction of isoprenoids by Saccharomyces cerevisiae in a synthetic grape juice medium in the absence of plant genes. Int J Food Microbiol2018;282:42–48.
- Cappellaro C, Mrsa V, Tanner W. New potential cell wall glucanases of Saccharomyces cerevisiae and their involvement in mating. J Bacteriol1998;180:5030–7.
- Caputi L, Lim EK, Bowles DJ. Discovery of new biocatalysts for the glycosylation of terpenoid scaffolds. Chem Eur J2008;14:6656–62.
- Carrau FM, Medina K, Boido E, et al De novo synthesis of monoterpenes by Saccharomyces cerevisiae wine yeasts. FEMS Microbiol Lett2005;243:107–15.
- Casalone E, Fia G, Barberio C, et al Genetic and biochemical characterization of Saccharomyces cerevisiae mutants resistant to trifluoroleucine. Res Microbiol1997;148:613–23.
- Cavalieri D, Casalone E, Bendoni B, et al Trifluoroleucine resistance and regulation of alpha-isopropyl malate synthase in Saccharomyces cerevisiae. Mol Gen Genet1999;261:152–60.
- Cenamor R, Molina M, Galdona J, et al Production and secretion of Saccharomyces cerevisiae -glucanases: differences between protoplast and periplasmic enzymes. Microbiology1987;133:619–28.
- Chatterjee T, Chatterjee BK, Bhattacharyya DK. Study of lipase-catalyzed hydrolysis of some monoterpene esters. Can J Microbiol2001;47:397–403.
- Chellappa R, Kandasamy P, Oh CS, et al The membrane proteins, Spt23p and Mga2p, play distinct roles in the activation of Saccharomyces cerevisiae OLE1 gene expression. Fatty acid-mediated regulation of Mga2p activity is independent of its proteolytic processing into a soluble transcription activator. J Biol Chem2001;276:43548–56.
- Chen F, Tholl D, Bohlmann J, et al The family of terpene synthases in plants: a mid-size family of genes for specialized metabolism that is highly diversified throughout the kingdom. Plant J2011a;66:212–29.
- Chen Y, Rai R, Zhou ZR, et al A conserved motif within RAP1 has diversified roles in telomere protection and regulation in different organisms. Nat Struct Mol Biol2011b;18:213–21.
- Christiaens JF, Franco LM, Cools TL, et al The fungal aroma gene ATF1 promotes dispersal of yeast cells through insect vectors. Cell Rep2014;9:425–32.
- Cibaka ML, Decourriere L, Lorenzo-Alonso CJ, et al 3-Sulfanyl-4-methylpentan-1-ol in dry-hopped beers: first evidence of glutathione s-conjugates in hop (Humulus lupulus L.). J Agric Food Chem2016;64:8572–82.
- Cibaka ML, Gros J, Nizet S, et al Quantitation of selected terpenoids and mercaptans in the dual-purpose hop varieties Amarillo, Citra, Hallertau Blanc, Mosaic, and Sorachi Ace. J Agr Food Chem2015;63:3022–30.
- Conrad M, Schothorst J, Kankipati HN, et al Nutrient sensing and signaling in the yeast Saccharomyces cerevisiae. FEMS Microbiol Rev2014;38:254–99.
- Cordente AG, Capone DL, Curtin CD. Unravelling glutathione conjugate catabolism in Saccharomyces cerevisiae: the role of glutathione/dipeptide transporters and vacuolar function in the release of volatile sulfur compounds 3-mercaptohexan-1-ol and 4-mercapto-4-methylpentan-2-one. Appl Microbiol Biot2015;99:9709–22.
- Cordente AG, Curtin CD, Varela C, et al Flavour-active wine yeasts. Appl Microbiol Biot2012;96:601–18.
- Cordente AG, Cordero-Bueso G, Pretorius IS, et al Novel wine yeast with mutations in YAP1 that produce less acetic acid during fermentation. FEMS Yeast Res2013;13:62–73.
- Cseke L, Dudareva N, Pichersky E. Structure and evolution of linalool synthase. Mol Biol Evol1998;15:1491–8.
- Cullen PJ, Sabbagh WJ, Graham E, et al A signaling mucin at the head of the Cdc42- and MAPK-dependent filamentous growth pathway in yeast. Genes Dev2004;18:1695–708.
- Dabbou S, Lussiana C, Maatallah S, et al Changes in biochemical compounds in flesh and peel from Prunus persica fruits grown in Tunisia during two maturation stages. Plant Physiol Biochem2016;100:1–11.
- Daenen L, Saison D, Sterckx F, et al Screening and evaluation of the glucoside hydrolase activity in Saccharomyces and Brettanomyces brewing yeasts. J Appl Microbiol2008;104:478–88.
- Daenen L, Sterckx F, Delvaux FR, et al Evaluation of the glycoside hydrolase activity of a Brettanomyces strain on glycosides from sour cherry (Prunus cerasus L.) used in the production of special fruit beers. FEMS Yeast Res2008;8:1103–14.
- Dastidar RG, Hooda J, Shah A, et al The nuclear localization of SWI/SNF proteins is subjected to oxygen regulation. Cell Biosci2012;2:30.
- De Keersmaecker J. The mystery of lambic beer. Sci Am1996;56–62.
- Dekoninck TML, Verbelen PJ, Delvaux F, et al The Importance of wort composition for yeast metabolism during accelerated brewery fermentations. J Am Soc Brew Chem2012;70:195–204.
- Den Abt T, Souffriau B, Foulquié-Moreno MR, et al Genomic saturation mutagenesis and polygenic analysis identify novel yeast genes affecting ethyl acetate production, a non-selectable polygenic trait. Microb Cell2016;3:159–75.
- Denby CM, Li RA, Vu VT, et al Industrial brewing yeast engineered for the production of primary flavor determinants in hopped beer. Nat Commun2018;9:965.
- Dickinson JR, Harrison SJ, Dickinson JA, et al An investigation of the metabolism of isoleucine to active Amyl alcohol in Saccharomyces cerevisiae. J Biol Chem2000;275:10937–42.
- Dickinson JR, Harrison SJ, Hewlins MJ. An investigation of the metabolism of valine to isobutyl alcohol in Saccharomyces cerevisiae. J Biol Chem1998;273:25751–6.
- Dickinson JR, Lanterman MM, Danner DJ, et al A 13C nuclear magnetic resonance investigation of the metabolism of leucine to isoamyl alcohol in Saccharomyces cerevisiae. J Biol Chem1997;272:26871–8.
- Dickinson JR, Salgado LE, Hewlins MJ. The catabolism of amino acids to long chain and complex alcohols in Saccharomyces cerevisiae. J Biol Chem2003;278:8028–34.
- Ding BJ, Lager I, Bansal S, et al The yeast ATF1 acetyltransferase efficiently acetylates insect pheromone alcohols: implications for the biological production of moth pheromones. Lipids2016;51:469–75.
- Doyle JA. Molecular and fossil evidence on the origin of angiosperms. Annu Rev Earth Pl Sc2012;40:301–26.
- Dubourdieu D, Tominaga T. Polyfunctional thiol compounds. In: Moreno-Arribas MV, Polo MC (eds). Wine Chemistry and Biochemistry. Sawston, Cambridge, UK: Springer Science+Business Media, 2009,275–93.
- Dufour M, Zimmer A, Thibon C, et al Enhancement of volatile thiol release of Saccharomyces cerevisiae strains using molecular breeding. Appl Microbiol Biot2013;97:5893–905.
- Dzialo MC, Park R, Steensels J, et al Physiology, ecology and industrial applications of aroma formation in yeast. FEMS Microbiol Rev2017;41:S95–S128.
- Eden A, Simchen G, Benvenisty N. Two yeast homologs of Eca39, a target for c-myc regulation, code for cytosolic and mitochondrial branched-chain amino acid aminotransferases. J Biol Chem1996;271:20242–5.
- Eden A, Van Nedervelde L, Drukker M, et al Involvement of branched-chain amino acid aminotransferases in the production of fusel alcohols during fermentation in yeast. Appl Microbiol Biot2001;55:296–300.
- Eder M, Sanchez I, Brice C, et al QTL mapping of volatile compound production in Saccharomyces cerevisiae during alcoholic fermentation. BMC Genomics2018;19:166.
- Ehrentraut S, Weber JM, Dybowski JN, et al Rpd3-dependent boundary formation at telomeres by removal of Sir2 substrate. Proc Natl Acad Sci USA2010;107:5522–7.
- El Hadi MA, Zhang FJ, Wu FF, et al Advances in fruit aroma volatile research. Molecules2013;18:8200–29.
- Endo M, Takesako K, Kato I, et al Fungicidal action of aureobasidin A, a cyclic depsipeptide antifungal antibiotic, against Saccharomyces cerevisiae. Antimicrob Agents Ch1997;41:672–6.
- Endrizzi A, Pagot Y, Clainche AL, et al Production of lactones and peroxisomal beta-oxidation in yeasts. Crit Rev Biotechnol1996;16:301–29.
- Engan S. Esters in beer. Brewers Digest1974;49:40–48.
- Engel KH, Heidlas J, Tressl R. The flavour of tropical fruits (banana, melon, pineapple). In Morton ID, Macleod AJ (eds) Food flavors Part C: The flavor of fruits. Amsterdam, The Netherlands: Elsevier Science Publishers, 1990;195–219.
- Engel KH, Tressl R. Identification of new sulfur-containing volatiles in yellow passionfruit (Passiflora edulis f. flavicarpa). J Agr Food Chem1991;39:2249–52.
- Esmaeili A, Hashemi E. Biotransformation of myrcene by Pseudomonas aeruginosa. Chem Cent J2011;5:26.
- Espinosa Vidal E, de Morais MA Jr, Francois JM, et al Biosynthesis of higher alcohol flavour compounds by the yeast Saccharomyces cerevisiae: impact of oxygen availability and responses to glucose pulse in minimal growth medium with leucine as sole nitrogen source. Yeast2015;32:47–56.
- Etschmann MM, Bluemke W, Sell D, et al Biotechnological production of 2-phenylethanol. Appl Microbiol Biot2002;59:1–8.
- Fleet G. Yeast interactions and wine flavour. Int J Food Microbiol2003;86:11–22.
- Frick O, Wittmann C. Characterization of the metabolic shift between oxidative and fermentative growth in Saccharomyces cerevisiae by comparative 13C flux analysis. Microb Cell Fact2005;4:30.
- Friis EM, Pedersen KR, Crane PR. Diversity in obscurity: fossil flowers and the early history of angiosperms. Philos T R Soc B2010;365:369–82.
- Fujii T, Kobayashi O, Yoshimoto H, et al Effect of aeration and unsaturated fatty acids on expression of the Saccharomyces cerevisiae alcohol acetyltransferase gene. Appl Environ Microb1997;63:910–5.
- Fujii T, Nagasawa N, Iwamatsu A, et al Molecular cloning, sequence analysis, and expression of the yeast alcohol acetyltransferase gene. Appl Environ Microb1994;60:2786–92.
- Fujii T, Yoshimoto H, Tamai Y. Acetate ester production by Saccharomyces cerevisiae lacking the ATF1 gene encoding the alcohol acetyltransferase. J Ferment Bioeng1996;81:538–42.
- Fujiwara D, Kobayashi O, Yoshimoto H, et al Molecular mechanism of the multiple regulation of the Saccharomyces cerevisiae ATF1 gene encoding alcohol acetyltransferase. Yeast1999;15:1183–97.
- Fujiwara D, Yoshimoto H, Sone H, et al Transcriptional co-regulation of Saccharomyces cerevisiae alcohol acetyltransferase gene,ATF1 and ?-9 fatty acid desaturase gene,OLE1 by unsaturated fatty acids. Yeast1998;14:711–21.
- Fukuda K, Kiyokawa Y, Yanagiuchi T, et al Purification and characterization of isoamyl acetate-hydrolyzing esterase encoded by the IAH1 gene of Saccharomyces cerevisiae from a recombinant Escherichia coli. Appl Microbiol Biot2000;53:596–600.
- Fukuda K, Kuwahata O, Kiyokawa Y, et al Molecular cloning and nucleotide sequence of the isoamyl acetate-hydrolyzing esterase gene (EST2) from Saccharomyces cerevisiae. J Ferment Bioeng1996;82:8–15.
- Fukuda K, Muromachi A, Watanabe M, et al Mutants producing high concentrations of the flavor components active amylalcohol and normal propanol in Saccharomyces cerevisiae. J Ferment Bioeng1993;75:288–92.
- Fukuda K, Watanabe M, Asano K. Altered regulation of aromatic amino acid biosynthesis in β-phenylethyl-alcohol-overproducing mutants of sake yeast Saccharomyces cerevisiae. Agric Biol Chem1990;54:3151–6.
- Fukuda K, Watanabe M, Asano K, et al Breeding of brewing yeast producing a large amount of β-phenylethyl alcohol and β-phenylethyl Acetate. Agr Biol Chem1990;54:269–71.
- Fukuda K, Yamamoto N, Kiyokawa Y, et al Balance of activities of alcohol acetyltransferase and esterase in Saccharomyces cerevisiae is important for production of isoamyl acetate. Appl Environ Microb1998;64:4076–8.
- Gachon CM, Langlois-Meurinne M, Saindrenan P. Plant secondary metabolism glycosyltransferases: the emerging functional analysis. Trends Plant Sci2005;10:542–9.
- Gancedo JM. Control of pseudohyphae formation in Saccharomyces cerevisiae. FEMS Microbiol Rev2001;25:107–23.
- Ganga MA, Piñaga F, Vallés S, et al Aroma improving in microvinification processes by the use of a recombinant wine yeast strain expressing the Aspergillus nidulans xlnA gene. Int J Food Microbiol1999;47:171–8.
- Garcia-Gonzalez L, Geeraerd AH, Spilimbergo S, et al High pressure carbon dioxide inactivation of microorganisms in foods: the past, the present and the future. Int J Food Microbiol2007;117:1–28.
- Gil JV, Manzanares P, Genoves S, et al Over-production of the major exoglucanase of leads to an increase in the aroma of wine. Int J Food Microbiol2005;103:57–68.
- Gmelin L. Ester oder sauerstoffsäure Ätherarten. In Handbuch der Organicshen Chemie. Vol. 4, Heidelberg, Germany: Universitäts-Buchhandlung von Karl Winter, 1848;178.
- Goldberg EM, Wang K, Goldberg J, et al Factors affecting the ortho- and retronasal perception of flavors: a review. Crit Rev Food Sci Nutr2016;58:913–23.
- Grabinska K, Palamarczyk G. Dolichol biosynthesis in the yeast Saccharomyces cerevisiae: an insight into the regulatory role of farnesyl diphosphate synthase. FEMS Yeast Res2002;2:259–65.
- Greger V, Schieberle P. Characterization of the key aroma compounds in apricots (Prunus armeniaca) by application of the molecular sensory science concept. J Agr Food Chem2007;55:5221–8.
- Gros J, Nizet S, Collin S. Occurrence of odorant polyfunctional thiols in the Super Alpha Tomahawk hop cultivar. Comparison with the thiol-rich Nelson Sauvin bitter variety. J Agr Food Chem2011;59:8853–65.
- Gros J, Tran TTH, Collin S. Enzymatic release of odourant polyfunctional thiols from cysteine conjugates in hop. J Inst Brew2013;119:221–7.
- Guichard E. Interactions between flavor compounds and food ingredients and their influence on flavor perception. Food Rev Int2002;18:49–70.
- Gulshan K, Thommandru B, Moye-Rowley WS. Proteolytic degradation of the Yap1 transcription factor is regulated by subcellular localization and the E3 ubiquitin ligase Not4. J Biol Chem2012;287:26796–805.
- Haffner T, Tressl R. Biosynthesis of (R)-γ-decanolactone in the yeast Sporobolomyces odorus. J Agr Food Chem1996;44:1218–23.
- Hartmann M, Schneider TR, Pfeil A, et al Evolution of feedback-inhibited / barrel isoenzymes by gene duplication and a single mutation. Proc Natl Acad Sci USA2003;100:862–7.
- Hazelwood LA, Daran JM, van Maris AJ, et al The Ehrlich pathway for fusel alcohol production: a century of research on Saccharomyces cerevisiae metabolism. Appl Environ Microb2008;74:2259–66.
- Helwi P, Guillaumie S, Thibon C, et al Vine nitrogen status and volatile thiols and their precursors from plot to transcriptome level. BMC Plant Biol2016;16:173.
- Hess KC, Liu J, Manfredi G, et al A mitochondrial CO2-adenylyl cyclase-cAMP signalosome controls yeast normoxic cytochrome c oxidase activity. FASEB J2014;28:4369–80.
- Hirooka K, Ogita A, Fujita K, et al Isolation of a copper-resistant sake yeast mutant with improved flavour compound production. J Inst Brew2010;116:261–4.
- Hirooka K, Yamamoto Y, Tsutsui N, et al Improved production of isoamyl acetate by a sake yeast mutant resistant to an isoprenoid analog and its dependence on alcohol acetyltransferase activity, but not on isoamyl alcohol production. J Biosci Bioeng2005;99:125–9.
- Holt S, Cordente AG, Williams SJ, et al Engineering Saccharomyces cerevisiae to release 3-Mercaptohexan-1-ol during fermentation through overexpression of an S. cerevisiae Gene, STR3, for improvement of wine aroma. Appl Environ Microb2011;77:3626–32.
- Holt S, Mukherjee V, Lievens B, et al Bioflavoring by non-conventional yeasts in sequential beer fermentations. Food Microbiol2018b;72:55–66.
- Holt S, Trindade de Carvalho B, Foulquié-Moreno MR, et al Polygenic analysis in absence of major effector ATF1 unveils novel components in yeast flavor ester biosynthesis. mBio2018a;9:e01279–01218.
- Hong DH, Forstner JF, Forstner GG. Protein kinase C-epsilon is the likely mediator of mucin exocytosis in human colonic cell lines. Am J Physiol Gastr L1997;272:G31–7.
- Hoppe T, Matuschewski K, Rape M, et al Activation of a membrane-bound transcription factor by regulated ubiquitin/proteasome-dependent processing. Cell2000;102:577–86.
- Howard D, Anderson RG. Cell-free synthesis of ethyl acetate by extracts from Saccharomyces cerevisiae. J Inst Brew1976;82:70–71.
- Imai S, Armstrong CM, Kaeberlein M, et al Transcriptional silencing and longevity protein Sir2 is an NAD-dependent histone deacetylase. Nature2000;403:795–800.
- Inokoshi J, Tomoda H, Hashimoto H, et al Cerulenin-resistant mutants of Saccharomyces cerevisiae with an altered fatty acid synthase gene. Mol Gen Genet1994;244:90–96.
- Inui T, Tsuchiya F, Ishimaru M, et al Different beers with different hops. Relevant compounds for their aroma characteristics. J Agr Food Chem2013;61:4758–64.
- Ishii A, Roudnitzky N, Beno N, et al Synergy and masking in odor mixtures: an electrophysiological study of orthonasal vs. retronasal perception. Chem Senses2008;33:553–61.
- Jarauta I, Ferreira V, Cacho JF. Synergic, additive and antagonistic effects between odorants with similar odour properties. Dev Food Sci2006;43:205–8.
- Jayanty S, Song J, Rubinstein NM, et al Temporal relationship between ester biosynthesis and ripening events in bananas. J Am Soc Hort Sci2002;127:998–1005.
- Jesch SA, Gaspar ML, Stefan CJ, et al Interruption of inositol sphingolipid synthesis triggers Stt4p-dependent protein kinase C signaling. J Biol Chem2010;285:41947–60.
- Jones RP, Greenfield PF. Effect of carbon dioxide on yeast growth and fermentation. Enzyme Microb Technol1982;4:210–23.
- Joo YC, Oh DK. Lipoxygenases: potential starting biocatalysts for the synthesis of signaling compounds. Biotechnol Adv2012;30:1524–32.
- Joo YJ, Kim JH, Kang UB, et al Gcn4p-mediated transcriptional repression of ribosomal protein genes under amino-acid starvation. EMBO J2011;30:859–72.
- Jordán MJ, Tandon K, Shaw PE, et al Aromatic profile of aqueous banana essence and banana fruit by gas chromatography−mass spectrometry (GC-MS) and gas chromatography−olfactometry (GC-O). J Agr Food Chem2001;49:4813–7.
- Jouhten P, Rintala E, Huuskonen A, et al Oxygen dependence of metabolic fluxes and energy generation of Saccharomyces cerevisiae CEN.PK113-1A. BMC Syst Biol2008;2:60.
- Jungbluth M, Mosch HU, Taxis C. Acetate regulation of spore formation is under the control of the Ras/cyclic AMP/protein kinase A pathway and carbon dioxide in Saccharomyces cerevisiae. Eukaryot Cell2012;11:1021–32.
- Kandasamy P, Vemula M, Oh CS, et al Regulation of unsaturated fatty acid biosynthesis in Saccharomyces: the endoplasmic reticulum membrane protein, Mga2p, a transcription activator of the OLE1 gene, regulates the stability of the OLE1 mRNA through exosome-mediated mechanisms. J Biol Chem2004;279:36586–92.
- Kastaniotis AJ, Zitomer RS. Rox1 mediated repression. Oxygen dependent repression in yeast. Adv Exp Med Biol2000;475:185–95.
- King A, Dickinson JR. Biotransformation of monoterpene alcohols by Saccharomyces cerevisiae, Torulaspora delbrueckii and Kluyveromyces lactis. Yeast2000;16:499–506.
- King AJ, Dickinson JR. Biotransformation of hop aroma terpenoids by ale and lager yeasts. FEMS Yeast Res2003;3:53–62.
- Kishimoto T, Kobayashi M, Yako N, et al Comparison of 4-mercapto-4-methylpentan-2-one contents in hop cultivars from different growing regions. J Agr Food Chem2008;56:1051–7.
- Kishimoto T, Wanikawa A, Kagami N, et al Analysis of hop-derived terpenoids in beer and evaluation of their behavior using the stir bar–sorptive extraction method with GC-MS. J Agr Food Chem2005;53:4701–7.
- Kishimoto T, Wanikawa A, Kono K, et al Comparison of the odor-active compounds in unhopped beer and beers hopped with different hop varieties. J Agr Food Chem2006;54:8855–61.
- Klengel T, Liang WJ, Chaloupka J, et al Fungal adenylyl cyclase integrates CO2 sensing with cAMP signaling and virulence. Curr Biol2005;15:2021–6.
- Knatchbull FB, Slaughter JC. The effect of low CO2 pressures on the absorption of amino acids and production of flavour-active volatiles by yeast. J Inst Brew1987;93:420–4.
- Knight SJ, Klaere S, Morrison-Whittle P, et al Fungal diversity during fermentation correlates with thiol concentration in wine. Aust J Grape Wine Res2018;24:105–12.
- Kobayashi H, Takase H, Suzuki Y, et al Environmental stress enhances biosynthesis of flavor precursors, S-3-(hexan-1-ol)-glutathione and S-3-(hexan-1-ol)-L-cysteine, in grapevine through glutathione S-transferase activation. J Exp Bot2011;62:1325–36.
- Kobayashi N, Kaneda H, Kano Y, et al The production of linoleic and linolenic acid hydroperoxides during mashing. J Ferment Bioeng1993;76:371–5.
- Kohlhaw GB. Leucine biosynthesis in fungi: entering metabolism through the back door. Microbiol Mol Biol R2003;67:1–15.
- Kollmannsberger H, Biendl M, Nitz S. Occurrence of glycosidically bound flavour compounds in hops, hop products and beer. Brewing Sci2006;59:83–89.
- Koon N, Squire CJ, Baker EN. Crystal structure of LeuA from Mycobacterium tuberculosis, a key enzyme in leucine biosynthesis. Proc Natl Acad Sci USA2004;101:8295–300.
- Kovačevič M, Kač M. Determination and verification of hop varieties by analysis of essential oils. Food Chem2002;77:489–94.
- Krivoruchko A, Zhang Y, Siewers V, et al Microbial acetyl-CoA metabolism and metabolic engineering. Metab Eng2015;28:28–42.
- Kruis AJ, Levisson M, Mars AE, et al Ethyl acetate production by the elusive alcohol acetyltransferase from yeast. Metab Eng2017;41:92–101.
- Kuriyama K, Ashida S, Saito Y, et al Ethyl caproate synthesis and hydrolysis activity of Saké yeast. Hakkokokagu1986;64:175–80.
- Lam KC, Foster RTI, Deinzer ML. Aging of hops and their contribution to beer flavor. J Agr Food Chem1986;34:763–70.
- Langos D, Granvogl M, Schieberle P. Characterization of the key aroma compounds in two bavarian wheat beers by means of the sensomics approach. J Agr Food Chem2013;61:11303–11.
- Larriba G, Basco RD, Andaluz E, et al Yeast exoglucanases. Where redundancy implies necessity. Arch Med Res1993;24:293–9.
- Lashbrooke JG, Young PR, Dockrall SJ, et al Functional characterisation of three members of the Vitis vinifera L. carotenoid cleavage dioxygenase gene family. BMC Plant Biol2013;13:156.
- Lawless HT, Heymann H. Measurement of sensory thresholds. In Sensory Evaluation of Food: Principles and Practices. Boston, MA, Springer, 1999;173–207.
- Lee HW, Ahn DH, Crawley SC, et al Phorbol 12-myristate 13-acetate up-regulates the transcription of MUC2 intestinal mucin via Ras, ERK, and NF-kappa B. J Biol Chem2002;277:32624–31.
- Lee K, Hahn JS. Interplay of Aro80 and GATA activators in regulation of genes for catabolism of aromatic amino acids in Saccharomyces cerevisiae. Mol Microbiol2013;88:1120–34.
- Lee S, Gaspar ML, Aregullin MA, et al Activation of protein kinase C-mitogen-activated protein kinase signaling in response to inositol starvation triggers Sir2p-dependent telomeric silencing in yeast. J Biol Chem2013;288:27861–71.
- Li SS, Cheng C, Li Z, et al Yeast species associated with wine grapes in China. Int J Food Microbiol2010;138:85–90.
- Li XY, Wen YQ, Meng N, et al Monoterpenyl glycosyltransferases differentially contribute to production of monoterpenyl glycosides in two aromatic Vitis vinifera varieties. Front Plant Sci2017;8:1226.
- Lieb JD, Liu X, Botstein D, et al Erratum: Promoter-specific binding of Rap1 revealed by genome-wide maps of protein–DNA association. Nat Genet2001;28:327–34.
- Lilly M, Bauer FF, Lambrechts MG, et al The effect of increased yeast alcohol acetyltransferase and esterase activity on the flavour profiles of wine and distillates. Yeast2006a;23:641–59.
- Lilly M, Bauer FF, Styger G, et al The effect of increased branched-chain amino acid transaminase activity in yeast on the production of higher alcohols and on the flavour profiles of wine and distillates. FEMS Yeast Res2006b;6:726–43.
- Lindemann B. Receptors and transduction in taste. Nature2001;413:219–25.
- Liu SQ, Quek AY. Evaluation of beer fermentation with a novel yeast Williopsis saturnus. Food Technol Biotech2016;54:403–12.
- López J, Essus K, Kim I-k, et al Production of β-ionone by combined expression of carotenogenic and plant CCD1 genes in Saccharomyces cerevisiae. Microb Cell Fact2015;14:84.
- Loscos N, Hernandez-Orte P, Cacho J, et al Release and formation of varietal aroma compounds during alcoholic fermentation from nonfloral grape odorless flavor precursors fractions. J Agr Food Chem2007;55:6674–84.
- Lu-Chau TA, Guillan A, Nunez MJ, et al Anaerobic and aerobic continuous cultures of Saccharomyces cerevisiae : comparison of plasmid stability and EXG1 gene expression. Bioprocess Biosyst Eng2004;26:159–63.
- Luddeke F, Wulfing A, Timke M, et al Geraniol and geranial dehydrogenases induced in anaerobic monoterpene degradation by Castellaniella defragrans. Appl Environ Microb2012;78:2128–36.
- Lunkenbein S, Bellido M, Aharoni A, et al Cinnamate metabolism in ripening fruit. Characterization of a UDP-glucose:cinnamate glucosyltransferase from strawberry. Plant Physiol2006;140:1047–58.
- Lytra G, Tempere S, Le Floch A, et al Study of sensory interactions among red wine fruity esters in a model solution. J Agr Food Chem2013;61:8504–13.
- McAdam EL, Freeman JS, Whittock SP, et al Quantitative trait loci in hop (Humulus lupulus L.) reveal complex genetic architecture underlying variation in sex, yield and cone chemistry. BMC Genomics2013;14:360.
- McIntyre M, McNeil B. Morphogenetic and biochemical effects of dissolved carbon dioxide on filamentous fungi in submerged cultivation. Appl Microbiol Biot1998;50:291–8.
- Maicas S, Mateo JJ. Hydrolysis of terpenyl glycosides in grape juice and other fruit juices: a review. Appl Microbiol Biot2005;67:322–35.
- Malcorps P, Cheval JM, Jamil S, et al A new model for the regulation of ester synthesis by alcohol acetyltransferase in Saccharomyces cerevisiae during fermentation. J Am Soc Brew Chem1991;49:47–53.
- Malcorps P, Dufour J. Short-chain and medium-chain aliphatic-ester synthesis in Saccharomyces cerevisiae. Eur J Biochem1992;210:1015–22.
- Marmulla R, Harder J. Microbial monoterpene transformations-a review. Front Microbiol2014;5:346.
- Marriott J. Bananas–physiology and biochemistry of storage and ripening for optimum quality. Crit Rev Food Sci Nutr1980;13:41–88.
- Maxmen A. Coming soon to a lab near you? Genetically modified cannabis. Nature2018;559:162-.
- Mayr D, Märk T, Lindinger W, et al Breath-by-breath analysis of banana aroma by proton transfer reaction mass spectrometry. Int J Mass Spectrom2003;223–224:743–56.
- Meilgaard MC. Flavour chemistry of beer. part I: Flavour interaction between principal volatiles. MBAA TQ1975;12:107–17.
- Meilgaard MC. Prediction of flavor differences between beers from their chemical composition. J Agr Food Chem1982;30:1009–17.
- Meilgaard MC. Effects on flavour of innovations in brewery equipment and processing: a review. J Inst Brew2001;107:271–86.
- Meilgaard MC, Dalgliesh CE, Clapperton JF. Beer flavour terminology. J Inst Brew1979;85:38–42.
- Mendes-Pinto MM. Carotenoid breakdown products the—norisoprenoids—in wine aroma. Arch Biochem Biophys2009;483:236–45.
- Merkel O, Fido M, Mayr JA, et al Characterization and function in vivo of two novel phospholipases B/lysophospholipases from Saccharomyces cerevisiae. J Biol Chem1999;274:28121–7.
- Morano KA, Grant CM, Moye-Rowley WS. The response to heat shock and oxidative stress in Saccharomyces cerevisiae. Genetics2012;190:1157–95.
- Mosedale JR, Puech JL. Wood maturation of distilled beverages. Trends Food Sci Technol1998;9:95–101.
- Moshonas MG, Shaw PE. Analysis of flavor constituents from lemon and lime essence. J Agr Food Chem1972;20:1029–30.
- Muller CJ, Kepner RE, Webb AD. Lactones in wines–a review. Am J Enol Vitic1973;24:5–6.
- Nagegowda DA. Plant volatile terpenoid metabolism: biosynthetic genes, transcriptional regulation and subcellular compartmentation. FEBS Lett2010;584:2965–73.
- Nagel J, Culley LK, Lu Y, et al EST analysis of hop glandular trichomes identifies an O-methyltransferase that catalyzes the biosynthesis of xanthohumol. Plant Cell2008;20:186–200.
- Nakagawa Y, Sakumoto N, Kaneko Y, et al Mga2p is a putative sensor for low temperature and oxygen to induce OLE1 transcription in Saccharomyces cerevisiae. Biochem Bioph Res Co2002;291:707–13.
- Natsume S, Takagi H, Shiraishi A, et al The draft genome of hop (Humulus lupulus), an essence for brewing. Plant Cell Physiol2015;56:428–41.
- Njoroge SM, Ukeda H, Kusunose H, et al Volatile components of japanese yuzu and lemon oils. Flavour Fragr J1994;9:159–66.
- Nordström K. Formationof ethyl acetate in fermentation with brewer's yeast. J Inst Brew1961;67:173–81.
- Nordström K. Formationof ethyl acetate in fermentation with brewer's yeast III. Participation of coenzyme A. J Inst Brew1962;68:398–407.
- Nout MJR, Bartelt RJ. Attraction of a flying nitidulid (Carpophilus humeralis) to volatiles produced by yeasts grown on sweet corn and a corn-based medium. J Chem Ecol1998;24:1217–39.
- Oba T, Nomiyama S, Hirakawa H, et al Asp578 in Leu4p is one of the key residues for leucine feedback inhibition release in sake yeast. Biosci Biotechnol Biochem2005;69:1270–3.
- Ono E, Nobuo T. Monoterpene Glycosyltransferase Originating From Hop and Method for Using Same. Vol. PCT/JP2013/053166. Suntory Holdings Limited, 2013.
- Oshita K., Kubota M., Uchida M., Ono M. Clarification of the relationship between fusel alcohol formation and amino acid assimilation by brewing yeast using 13C-labeled amino acid, in Proc. Eur. Brew. Conv. Congr.City, Brussels, IRL Press, Oxford, 1995;25:387–402.
- Otsuka K, Zenibayashi Y, Itoh M. Presence and significance of two diastereomers of beta-methyl-gamma-octalactone in aged distilled liquors. Agr Biol Chem1974;38:485–90.
- Panter RA, Mudd JB. Carnitine levels in some higher plants. FEBS Lett1969;5:169–70.
- Parish-Virtue K, Herbst-Johnstone M, Bouda F, et al The impact of postharvest ultra-violet light irradiation on the thiol content of Sauvignon blanc grapes. Food Chem2019;271:747–52.
- Parker DK. Beer: Production, sensory characteristics and sensory analysis. In Piggott J (ed.) Alcoholic Beverages: Sensory Evaluation and Consumer Research. Woodhead Publishing, 2012;133–58.
- Peacock VE, Deinzer ML. Chemistry of hop aroma in beer. J Am Soc Brew Chem1981;39:136–41.
- Pino JA, Mesa J. Contribution of volatile compounds to mango (Mangifera indica L.) aroma. Flavour Fragr J2006;21:207–13.
- Pires EJ, Teixeira JA, Branyik T, et al Yeast: the soul of beer's aroma—a review of flavour-active esters and higher alcohols produced by the brewing yeast. Appl Microbiol Biot2014;98:1937–49.
- Platt JM, Ryvkin P, Wanat JJ, et al Rap1 relocalization contributes to the chromatin-mediated gene expression profile and pace of cell senescence. Genes Dev2013;27:1406–20.
- Pohlers S, Martin R, Kruger T, et al Lipid signaling via Pkh1/2 regulates fungal CO2 sensing through the kinase Sch9. MBio2017;8;e02211–16.
- Praet T, Van Opstaele F, Jaskula-Goiris B, et al Biotransformations of hop-derived aroma compounds by Saccharomyces cerevisiae upon fermentation. Cerevisia2012;36:125–32.
- Pronk JT, Steensma HY, Van Dijken JP. Pyruvate metabolism in Saccharomyces cerevisiae. Yeast1996;12:1607–33.
- Quilter MG, Hurley JC, Lynch FJ, et al The production of isoamyl acetate from amyl alcohol by Saccharomyces cerevisiae. J Inst Brew2003;109:34–40.
- Rape M, Hoppe T, Gorr I, et al Mobilization of processed, membrane-tethered Spt23 transcription factor by Cdc48 Ufd1/Npl4, a ubiquitin-selective chaperone. Cell2001;107:667–77.
- Rapparini F, Predieri S. Pear fruit volatiles. In: Janick J (ed.) Horticultural Reviews, Vol. 28. Oxford, UK: John Wiley, Sons, Inc., 2002;237–324.
- Raspor P, Milek DM, Polanc J, et al Yeasts isolated from three varieties of grapes cultivated in different locations of the Dolenjska vine-growing region, Slovenia. Int J Food Microbiol2006;109:97–102.
- Renger RS, Hateren SHv, Luyben KCAM. The formation of esters and higher alcohols during brewery fermentation; the effect of carbon dioxide pressure. J Inst Brew1992;98:509–13.
- Rettberg N, Biendl M, Garbe L-A. Hop aroma and hoppy beer flavor: chemical backgrounds and analytical tools—a review. J Am Soc Brew Chem2018;76:1–20.
- Richard L, Guillouet SE, Uribelarrea JL. Quantification of the transient and long-term response of Saccharomyces cerevisiae to carbon dioxide stresses of various intensities. Process Biochem2014;49:1808–18.
- Ricroch A, Clairand P, Harwood W. Use of CRISPR systems in plant genome editing: toward new opportunities in agriculture. Emerg Top Life Sci2017;1:169–82.
- Riemenschneider W, Bolt HM. Esters, organic. Ullmann's Encyclopedia of Industrial Chemistry. Wiley-VCH Verlag GmbH & Co. KGaA, 2000.
- Roberts MT, Dufour J-P, Lewis AC. Application of comprehensive multidimensional gas chromatography combined with time-of-flight mass spectrometry (GC×GC-TOFMS) for high resolution analysis of hop essential oil. J Sep Sci2004;27:473–8.
- Rojas V, Gil JV, Piñaga F, et al Studies on acetate ester production by non-Saccharomyces wine yeasts. Int J Food Microbiol2001;70:283–9.
- Romagnoli G, Knijnenburg TA, Liti G, et al Deletion of the Saccharomyces cerevisiae ARO8 gene, encoding an aromatic amino acid transaminase, enhances phenylethanol production from glucose. Yeast2015;32:29–45.
- Romero-Guido C, Belo I, Ta TM, et al Biochemistry of lactone formation in yeast and fungi and its utilisation for the production of flavour and fragrance compounds. Appl Microbiol Biot2011;89:535–47.
- Roncoroni M, Santiago M, Hooks DO, et al The yeast IRC7 gene encodes a β-lyase responsible for production of the varietal thiol 4-mercapto-4-methylpentan-2-one in wine. Food Microbiol2011;28:926–35.
- Rossouw D, Naes T, Bauer FF. Linking gene regulation and the exo-metabolome: a comparative transcriptomics approach to identify genes that impact on the production of volatile aroma compounds in yeast. BMC Genomics2008;9:530.
- Sabate J, Cano J, Esteve-Zarzoso B, et al Isolation and identification of yeasts associated with vineyard and winery by RFLP analysis of ribosomal genes and mitochondrial DNA. Microbiol Res2002;157:267–74.
- Saerens SM, Delvaux FR, Verstrepen KJ, et al Production and biological function of volatile esters in Saccharomyces cerevisiae. Microb Biotechnol2010;3:165–77.
- Saerens SM, Verbelen PJ, Vanbeneden N, et al Monitoring the influence of high-gravity brewing and fermentation temperature on flavour formation by analysis of gene expression levels in brewing yeast. Appl Microbiol Biot2008;80:1039–51.
- Saerens SM, Verstrepen KJ, Van Laere SD, et al The Saccharomyces cerevisiae EHT1 and EEB1 genes encode novel enzymes with medium-chain fatty acid ethyl ester synthesis and hydrolysis capacity. J Biol Chem2006;281:4446–56.
- Sahara H, Kotaka A, Kondo A, et al Using promoter replacement and selection for loss of heterozygosity to generate an industrially applicable sake yeast strain that homozygously overproduces isoamyl acetate. J Biosci Bioeng2009;108:359–64.
- Sakai M, Hirata H, Sayama H, et al Production of 2-phenylethanol in roses as the dominant floral scent compound from L-phenylalanine by two key enzymes, a PLP-dependent decarboxylase and a phenylacetaldehyde reductase. Biosci Biotechnol Biochem2007;71:2408–19.
- Sakuma S, Kobayashi K, Tayama T, et al Formation of sweet flavor compounds during fermentation. J Am Soc Brew Chem1996;54:37–40.
- Salles C, Chagnon MC, Feron G, et al In-mouth mechanisms leading to flavor release and perception. Crit Rev Food Sci Nutr2010;51:67–90.
- Samel A, Rudner A, Ehrenhofer-Murray AE. Variants of the Sir4 coiled-coil domain improve binding to Sir3 for heterochromatin formation in Saccharomyces cerevisiae. G32017;7:1117–26.
- San-Juan F, Ferreira V, Cacho J, et al Quality and aromatic sensory descriptors (mainly fresh and dry fruit character) of Spanish red wines can be predicted from their aroma-active chemical composition. J Agr Food Chem2011;59:7916–24.
- Santiago M, Gardner RC. Yeast genes required for conversion of grape precursors to varietal thiols in wine. FEMS Yeast Res2015;15:fov034.
- Satyanarayana T, Umbarger HE, Lindegren G. Biosynthesis of branched-chain amino acids in yeast: regulation of leucine biosynthesis in prototrophic and leucine auxotrophic strains. J Bacteriol1968;96:2018–24.
- Schieberle P. Primary odorants of pale lager beer. Eur Food Res Technol1991;193:558–65.
- Schmidt S, Rainieri S, Witte S, et al Identification of a Saccharomyces cerevisiae glucosidase that hydrolyzes flavonoid glucosides. Appl Environ Microb2011;77:1751–7.
- Schwab W, Schaart JG, Rosati C. Molecular biology research. In Folta KM, Gardiner SE (eds.) Fragaria. Genetics and Genomics of Rosaceae, Vol. 6. New York, NY, USA: Springer, 2009;457–86.
- Semchyshyn HM, Abrat OB, Miedzobrodzki J, et al Acetate but not propionate induces oxidative stress in bakers' yeast Saccharomyces cerevisiae. Redox Rep2011;16:15–23.
- Seymour GB. Banana. In Seymour GB, Taylor JE, Tucker GA (eds.) Biochemistry of Fruit Ripening. Dordrecht, Springer Netherlands, 1993;83–106.
- Sharp DC, Steensels J, Shellhammer TH. The effect of hopping regime, cultivar and β-glucosidase activity on monoterpene alcohol concentrations in wort and beer. J Inst Brew2017;123:185–91.
- Sharpe FR, Laws DRJ. The essential oil of hops a review. J Inst Brew1981;87:96–107.
- Shen HY, De Schrijver S, Moonjai N, et al Effects of CO2 on the formation of flavour volatiles during fermentation with immobilised brewer's yeast. Appl Microbiol Biot2004;64:636–43.
- Shiba Y, Sasaki Y, Kanno Y. 12-O-tetradecanoylphorbol-13-acetate disrupts actin filaments and focal contacts and enhances binding of fibronectin-coated latex beads to 3t3-l1 cells. Exp Cell Res1988;178:233–41.
- Singh B, Sharma RA. Plant terpenes: defense responses, phylogenetic analysis, regulation and clinical applications. 3 Biotech2015;5:129–51.
- Slaughter JC. The naturally occurring furanones: formation and function from pheromone to food. Biol Rev1999;74:259–76.
- Slaughter JC, Flint PWN, Kular KS. The effect of CO2 on the adsorption of amino acids from malt extract by Saccharomyces cerevisiae. FEMS Microbiol Lett1987;40:239–43.
- Smith RL, Johnson AD. Turning genes off by Ssn6–Tup1: a conserved system of transcriptional repression in eukaryotes. Trends Biochem Sci2000;25:325–30.
- Staschke KA, Dey S, Zaborske JM, et al Integration of general amino acid control and target of rapamycin (TOR) regulatory pathways in nitrogen assimilation in yeast. J Biol Chem2010;285:16893–911.
- Steinhaus M, Schieberle P. Comparison of the most odor-active compounds in fresh and dried hop cones (Humulus lupulus L. Variety Spalter Select) based on GC−olfactometry and odor dilution techniques. J Agr Food Chem2000;48:1776–83.
- Steyer D, Ambroset C, Brion C, et al QTL mapping of the production of wine aroma compounds by yeast. BMC Genomics2012;13:573.
- Steyer D, Clayeux C, Laugel B. Characterization of the terpenoids composition of beers made with the French hop varieties: Strisselspalt, Aramis, Triskel and Bouclier. Brewing Sci2013;66:192–7.
- Steyer D, Erny C, Claudel P, et al Genetic analysis of geraniol metabolism during fermentation. Food Microbiol2013;33:228–34.
- Steyer D, Tristram P, Clayeux C, et al Yeast strains and hop varieties synergy on beer volatile compounds. Brewing Sci2017;70:131–41.
- Strejc J, Siříšťová L, Karabín M, et al Production of alcohol-free beer with elevated amounts of flavouring compounds using lager yeast mutants. J Inst Brew2013;119:149–55.
- Stribny J, Querol A, Perez-Torrado R. Differences in enzymatic properties of the Saccharomyces kudriavzevii and Saccharomyces uvarum alcohol acetyltransferases and their impact on aroma-active compounds production. Front Microbiol2016;7:897.
- Strijbis K, Distel B. Intracellular acetyl unit transport in fungal carbon metabolism. Eukaryot Cell2010;9:1809–15.
- Styger G, Jacobson D, Bauer FF. Identifying genes that impact on aroma profiles produced by Saccharomyces cerevisiae and the production of higher alcohols. Appl Microbiol Biot2011;91:713–30.
- Styger G, Jacobson D, Prior BA, et al Genetic analysis of the metabolic pathways responsible for aroma metabolite production by Saccharomyces cerevisiae. Appl Microbiol Biot2013;97:4429–42.
- Subileau M, Schneider R, Salmon JM, et al New insights on 3-mercaptohexanol (3MH) biogenesis in Sauvignon Blanc wines: Cys-3MH and (E)-hexen-2-al are not the major precursors. J Agr Food Chem2008a;56:9230–5.
- Subileau M, Schneider R, Salmon JM, et al Nitrogen catabolite repression modulates the production of aromatic thiols characteristic of Sauvignon Blanc at the level of precursor transport. FEMS Yeast Res2008b;8:771–80.
- Suzuki K, Yabe T, Maruyama Y, et al Characterization of recombinant yeast exo-beta-1,3-glucanase (Exg1p) expressed in Escherichia coli cells. Biosci Biotechnol Biochem2001;65:1310–4.
- Swiegers JH, Capone DL, Pardon KH, et al Engineering volatile thiol release in Saccharomyces cerevisiae for improved wine aroma. Yeast2007;24:561–74.
- Tabachnick J, Joslyn MA. Formation of esters of yeast. I. The production of ethyl acetate by standing surface cultures of Hansenula anomala. J Bacteriol1953;65:1–9.
- Takahashi T, Ohara Y, Sawatari M, et al Isolation and characterization of sake yeast mutants with enhanced isoamyl acetate productivity. J Biosci Bioeng2017;123:71–77.
- Takoi K. Screening of geraniol-rich flavor hop and interesting behavior of beta-citronellol during fermentation under various hop-addition timings. J Am Soc Brew Chem2014;72:22–29.
- Takoi K, Degueil M, Shinkaruk S, et al Identification and characteristics of new volatile thiols derived from the hop (Humulus luplus L.) cultivar Nelson Sauvin (dagger). J Agr Food Chem2009;57:2493–502.
- Takoi K, Itoga Y, Koie K, et al The contribution of geraniol metabolism to the citrus flavour of beer: synergy of geraniol and beta-citronellol under coexistence with excess linalool. J Inst Brew2010a;116:251–60.
- Takoi K, Koie K, Itoga Y, et al Biotransformation of hop-derived monoterpene alcohols by lager yeast and their contribution to the flavor of hopped beer. J Agr Food Chem2010b;58:5050–8.
- Thibon C, Cluzet S, Merillon JM, et al 3-Sulfanylhexanol precursor biogenesis in grapevine cells: the stimulating effect of Botrytis cinerea. J Agr Food Chem2011;59:1344–51.
- Thibon C, Marullo P, Claisse O, et al Nitrogen catabolic repression controls the release of volatile thiols by Saccharomyces cerevisiae during wine fermentation. FEMS Yeast Res2008;8:1076–86.
- Tholl D. Terpene synthases and the regulation, diversity and biological roles of terpene metabolism. Curr Opin Plant Biol2006;9:297–304.
- Tiwari P, Sangwan RS, Sangwan NS. Plant secondary metabolism linked glycosyltransferases: an update on expanding knowledge and scopes. Biotechnol Adv2016;34:714–39.
- Tiwari R, Koffel R, Schneiter R. An acetylation/deacetylation cycle controls the export of sterols and steroids from S. cerevisiae. EMBO J2007;26:5109–19.
- Tominaga T, Dubourdieu D. Identification of cysteinylated aroma precursors of certain volatile thiols in passion fruit juice. J Agr Food Chem2000;48:2874–6.
- Tomiyama K, Aoki H, Oikawa T, et al Characteristic volatile components of Japanese sour citrus fruits: Yuzu, Sudachi and Kabosu. Flavour Fragr J2012;27:341–55.
- Tornow J, Zeng X, Gao W, et al GCR1, a transcriptional activator in Saccharomyces cerevisiae, complexes with RAP1 and can function without its DNA binding domain.. EMBO J1993;12:2431–7.
- Touhara K, Vosshall LB. Sensing odorants and pheromones with chemosensory receptors. Annu Rev Physiol2009;71:307–32.
- Tressl R, Apetz M, Arrieta R, et al Formation of lactones and terpenoids by microorganisms. In Charalambous G (ed.) Flavor of Foods and Beverages: Chemistry and Technology. Elsevier, 1978a;45–168.
- Tressl R, Friese L, Fendesack F, et al Gas chromatographic-mass spectrometric investigation of hop aroma constituents in beer. J Agr Food Chem1978b;26:1422–6.
- Tressl R, Kossa T, Renner R. Gaschromatographisch-massenspektrometrische untersuchungen flüchtiger inhaltsstoffe von hopfen, würze und bier und deren genese. II. Phenole und sauerstoffhaltige heterocyclen. Mschr Brauerei1975; 28:109–18.
- Trindade de Carvalho B, Holt S, Souffriau B, et al Identification of novel alleles conferring superior production of rose flavor phenylethyl acetate using polygenic analysis in yeast. mBio2017;8:e01173–01117.
- Turek C, Stintzing FC. Stability of essential oils: a review. Compr Rev Food Sci F2013;12:40–53.
- Tzin V, Malitsky S, Ben Zvi MM, et al Expression of a bacterial feedback-insensitive 3-deoxy-D-arabino-heptulosonate 7-phosphate synthase of the shikimate pathway in Arabidopsis elucidates potential metabolic bottlenecks between primary and secondary metabolism. New Phytol2012;194:430–9.
- Vadali RV, Bennett GN, San KY. Applicability of CoA/acetyl-CoA manipulation system to enhance isoamyl acetate production in Escherichia coli. Metab Eng2004;6:294–9.
- Vadali RV, Horton CE, Rudolph FB, et al Production of isoamyl acetate in ackA-pta and/or ldh mutants of Escherichia coli with overexpression of yeast ATF2/or ldh mutants of Escherichia coli with overexpression of yeast ATF2. Appl Microbiol Biot2004;63:698–704.
- Valsecchi F, Ramos-Espiritu LS, Buck J, et al cAMP and mitochondria. Physiology2013;28:199–209.
- Van Rensburg P, Stidwell T, Lambrechts MG, et al Development and assessment of a recombinant Saccharomyces cerevisiae wine yeast producing two aroma-enhancing β-glucosidases encoded by the Saccharomycopsis fibuligera BGL1 and BGL2 genes. Ann Microbiol2005;55:33–42.
- van Rijswijck IMH, Wolkers-Rooijackers JCM, Abee T, et al Performance of non-conventional yeasts in co-culture with brewers' yeast for steering ethanol and aroma production. Microb Biotechnol2017;10:1591–602.
- van Rossum HM, Kozak BU, Pronk JT, et al Engineering cytosolic acetyl-coenzyme A supply in Saccharomyces cerevisiae: pathway stoichiometry, free-energy conservation and redox-cofactor balancing. Metab Eng2016;36:99–115.
- Vandamme J, Castermans D, Thevelein JM. Molecular mechanisms of feedback inhibition of protein kinase A on intracellular cAMP accumulation. Cell Signal2012;24:1610–8.
- Vermeulen C, Lejeune I, Tran TT, et al Occurrence of polyfunctional thiols in fresh lager beers. J Agr Food Chem2006;54:5061–8.
- Verstrepen K, Derdelinckx G, Dufour J, et al The alcohol acetyl transferase gene is a target of the cAMP/PKA and FGM nutrient-signalling pathways. FEMS Yeast Res2003a;4:285–96.
- Verstrepen KJ, Derdelinckx G, Dufour J, et al Flavor-active esters: adding fruitiness to beer. J Biosci Bioeng2003b;96:110–8.
- Verstrepen KJ, Moonjai N, Bauer FF, et al Genetic regulation of ester synthesis in yeast: new facts, insights and implications for the brewer. In: Smart K. (ed):Brewing Yeast Fermentation Performance, 2nd edn, Blackwell Science, UK: Oxford. 2003c;234–48.
- Verstrepen KJ, Van Laere SD, Vanderhaegen BM, et al Expression levels of the yeast alcohol acetyltransferase genes ATF1, Lg-ATF1, and ATF2 control the formation of a broad range of volatile esters. Appl Environ Microb2003d;69:5228–37.
- Verstrepen KJ, Van Laere SD, Vercammen J, et al The Saccharomyces cerevisiae alcohol acetyl transferase Atf1p is localized in lipid particles. Yeast2004;21:367–77.
- Vervoort Y, Herrera-Malaver B, Mertens S, et al Characterization of the recombinant Brettanomyces anomalus beta-glucosidase and its potential for bioflavouring. J Appl Microbiol2016;121:721–33.
- Vrieling AM. Agitated fermentation in high fermentors. Eur Brew Conv Monograph1978;5:135–45.
- Vuralhan Z, Luttik MA, Tai SL, et al Physiological characterization of the ARO10-dependent, broad-substrate-specificity 2-oxo acid decarboxylase activity of Saccharomyces cerevisiae. Appl Environ Microb2005;71:3276–84.
- Vuralhan Z, Morais MA, Tai SL, et al Identification and characterization of phenylpyruvate decarboxylase genes in Saccharomyces cerevisiae. Appl Environ Microb2003;69:4534–41.
- Wache Y, Aguedo M, Nicaud JM, et al Catabolism of hydroxyacids and biotechnological production of lactones by Yarrowia lipolytica. Appl Microbiol Biot2003;61:393–404.
- Wang G, Dixon RA. Heterodimeric geranyl(geranyl)diphosphate synthase from hop (Humulus lupulus) and the evolution of monoterpene biosynthesis. Proc Natl Acad Sci USA2009;106:9914–9.
- Wang G, Tian L, Aziz N, et al Terpene biosynthesis in glandular trichomes of hop. Plant Physiol2008;148:1254–66.
- Wang R, Lin PY, Huang ST, et al Hyperproduction of beta-glucanase exg1 promotes the bioconversion of mogrosides in Saccharomyces cerevisiae mutants defective in mannoprotein deposition. J Agr Food Chem2015;63:10271–9.
- Wanikawa A, Hosoi K, Kato T. Conversion of unsaturated fatty acids to precursors of gamma-lactones by lactic acid bacteria during the production of malt whisky. J Am Soc Brew Chem2000;58:51–56.
- Wanikawa A, Hosoi K, Takise I, et al Detection or γ-lactones in malt whisky. J Inst Brew2000;106:39–44.
- Watanabe M, Nagai H, Kondo K. Properties of sake yeast mutants resistant to isoamyl monochloroacetate. J Ferment Bioeng1995;80:291–3.
- Watanabe M, Tanaka N, Mishima H, et al Isolation of sake yeast mutants resistant to isoamyl monofluoroacetate to improve isoamyl acetate productivity. J Ferment Bioeng1993;76:229–31.
- Weinert BT, Iesmantavicius V, Moustafa T, et al Acetylation dynamics and stoichiometry in Saccharomyces cerevisiae. Mol Syst Biol2014;10:716.
- Wen YQ, He F, Zhu BQ, et al Free and glycosidically bound aroma compounds in cherry (Prunus avium L.). Food Chem2014;152:29–36.
- Wurz REM, Kepner RE, Webb AD. The Biosynthesis of certain gamma-lactones from glutamic acid by film yeast activity on the surface of flor sherry. Am J Enol Viticult1988;39:234–8.
- Yang JE, Park YJ, Chang HC. Cloning of four genes involved in limonene hydroxylation from Enterobacter cowanii 6 L. J Microbiol Biotechnol2007;17:1169–76.
- Yu J, Huang S, Dong J, et al The influence of LOX-less barley malt on the flavour stability of wort and beer. J Inst Brew2014;120:93–98.
- Yuan F, Qian MC. Development of C13-norisoprenoids, carotenoids and other volatile compounds in Vitis vinifera L. Cv. Pinot noir grapes. Food Chem2016;192:633–41.
- Yuan T, Chen Q, Zhao P, et al. Identification of enzymes responsible for the reduction of geraniol to citronellol. Nat Prod Bioprospect2011;1:108–11.
- Zhang H, Cao M, Jiang X, et al De-novo synthesis of 2-phenylethanol by Enterobacter sp. CGMCC 5087. BMC Biotechnol2014;14:30.
- Zhang M, Galdieri L, Vancura A. The yeast AMPK homolog SNF1 regulates acetyl coenzyme A homeostasis and histone acetylation. Mol Cell Biol2013;33:4701–17.
- Zhang S, Skalsky Y, Garfinkel DJ. MGA2 or SPT23 is required for transcription of the delta9 fatty acid desaturase gene, OLE1, and nuclear membrane integrity in Saccharomyces cerevisiae. Genetics1999;151:473–83.
- Zhao J, Huhman D, Shadle G, et al MATE2 mediates vacuolar sequestration of flavonoid glycosides and glycoside malonates in medicago truncatula. Plant Cell2011;23:1536–55.
- Zhao J, Li C, Zhang Y, et al Dynamic control of ERG20 expression combined with minimized endogenous downstream metabolism contributes to the improvement of geraniol production in Saccharomyces cerevisiae. Microb Cell Fact2017;16:17.
- Zhong W, Murphy DJ, Georgopapadakou NH. Inhibition of yeast inositol phosphorylceramide synthase by aureobasidin A measured by a fluorometric assay. FEBS Lett1999;463:241–4.
- Zorn H, Langhoff S, Scheibner M, et al A Peroxidase from lepista irina cleaves beta,beta-carotene to flavor compounds. Biol Chem2003a;384:1049–56.
- Zorn H, Langhoff S, Scheibner M, et al Cleavage of beta,beta-carotene to flavor compounds by fungi. Appl Microbiol Biotechnol2003b;62:331–6.
- Zott K, Thibon C, Bely M, et al The grape must non-Saccharomyces microbial community: impact on volatile thiol release. Int J Food Microbiol2011;151:210–5.