Introduction
Peroxisomes are single membrane cytoplasmic organelles with diverse dynamic functions. The word 'peroxisome' was coined by Christian de Duve. Peroxisomes are involved in multiple metabolic functions in the body (Figurel). They do not have their own DNA like mitochondria. Membrane proteins, matrix proteins, and peroxins are peroxisomal proteins that are responsible for maintaining the structure of peroxisomes, for peroxisomal division/assembly, and for performing various cellular biochemical functions.
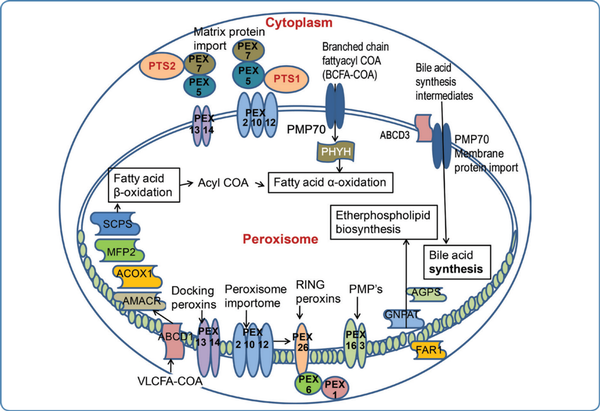
Figure 1
Functions of peroxisomes and the genes involved in peroxisomal disorders
The main functions of peroxisomes are a and (3 oxidation of fatty acids, synthesis of plasmalogen, cholesterol and bile acids, detoxification of glyoxylate and hydrogen peroxide, and catabolism of lysine.
Peroxisomal disorders are a broad spectrum of disorders with phenotypic and genotypic variability. Most of them present in the neonatal and pediatric age groups. They are classified into two subgroups. The first group is peroxisome biogenesis disorders (PBDs) which occur due to defective peroxisome assembly. The second group is peroxisomal dysfunction due to single peroxisome enzyme deficiencies. The most typical disorder under peroxisome biogenesis disorders is the 'classical' Zellweger syndrome, a severe form of the Zellweger spectrum disorders (ZSDs). The most common disorder of peroxisomal dysfunction is X-linked adrenoleukodystrophy (X-ALD).
Classification of peroxisomal disorders
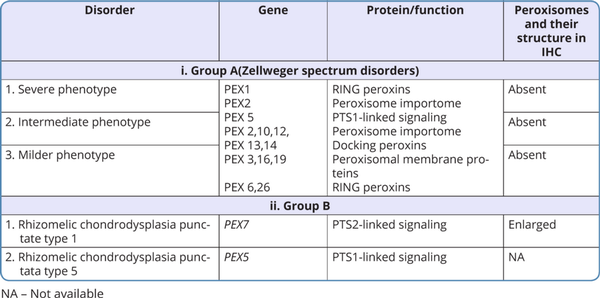
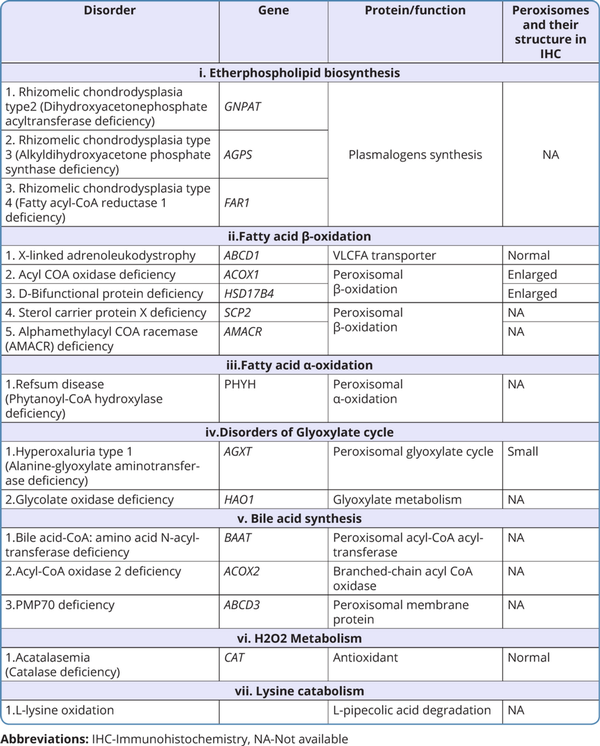
The classification of peroxisomal disorders is outlined in Tables 1 and 2 (Takashima et al., 2019; Wanders, 2017).
Genetics of peroxisomal disorders
Most of the peroxisomal disorders have an autosomal recessive pattern of inheritance, with few exceptions such as X-linked adrenoleukodystrophy. The phenotypic severity of the ZSDs is based on the type of pathogenic variants in PEX genes. Mutations in the PEX1 gene account for 60% and mutations in PEX6 account for 10-15% of cases of ZSDs. As ZSDs are a rare group of disorders, the exact genotype and phenotype correlations have not been established but loss-of-function (LoF) variants like truncating variants have been found to lead to a more severe phenotype than missense variants. Biallelic pathogenic variants in the PEX7 gene are known to cause severe rhizomelic chondrodysplasia punctata type 1, but some variants in PEX7 can cause the less severe peroxisome biogenesis disorder 9B with absence of chondrodysplasia and rhizomelia, which includes the adult-onset Refsum disease phenotype of cataracts, retinitis pigmentosa, hearing loss, ataxia, neuropathy and ichthyosis, and the more recently described phenotype of cataract with neurodevelopmental disability (Masih et al., 2021).
Some of the peroxisomal disorders are now being identified to be autosomal dominant. PEX6 is associated with autosomal recessive PBD 4A and 4B, but in a few patients with a heterozygous variant in the PEX6 gene, allelic expression imbalance leading to an overrepresentation of a mutant allele and ZSD phenotype has been reported (Falkenberg et al., 2017). Likewise, biallelic pathogenic variants in the FAR1 gene lead to the autosomal recessive peroxisomal fatty acyl-CoA reductase 1 disorder, which is characterized by severe psychomotor retardation during infancy followed by childhood spasticity, but a few patients with a heterozygous pathogenic variant in FAR1 have been reported to have cataracts and spastic paraparesis.
Peroxisome biogenesis disorders also exhibit mosaicism in a few patients. In type 1 mosaicism, normal peroxisomal activity is revealed in fibroblasts with abnormal biochemical profiles. In type 2 mosaicism, with the same genotype, there is a difference in peroxisome morphology in different tissues.
Clinical features of peroxisomal disorders
1. Peroxisome biogenesis disorders (PBDs)
These are autosomal recessive genetic disorders with an incidence of approximately 1:30,000 to 1:50,000 newborns. The Zellweger spectrum of disorders and rhizomelic chondrodysplasia punctata (RCDP) spectrum are included under this category.
i. Zellweger spectrum of disorders (ZSDs)
The phenotype of ZSDs usually ranges from severe form to intermediate and milder forms with the typical presentation. Patients with atypical presentation lacking the classical signs and symptoms of ZSDs have also been described. Dysmorphic features in severe ZSDs include the high forehead, large anterior fontanelle, hypoplastic supraorbital ridges, epicanthal folds, corneal clouding, cataract, and broad nasal bridge (Figure 2). Prognosis is guarded with early neonatal or infantile death. Most of the intermediate forms of ZSDs have late childhood deaths. Clues to the diagnosis of Zellweger spectrum disorders in different age groups with the differential diagnosis are depicted in Figure 3. The neonatal phenotype of X-linked adrenoleukodystrophy due to contiguous deletion of the ABCD1 gene which mimics peroxisome biogenesis disorders has also been described.
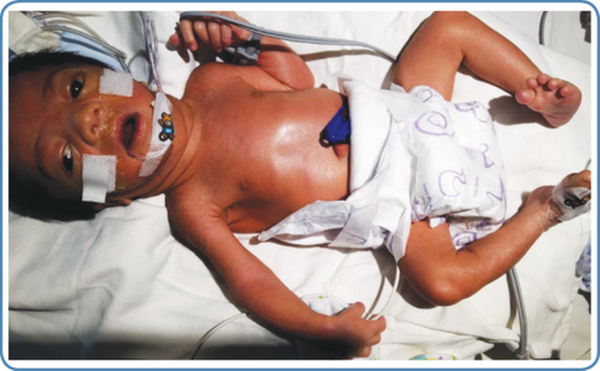
Figure 2
Dysmorphic features in a neonate with the severe Zellweger spectrum disorder
ii. Rhizomelic chondrodysplasia punctata spectrum (RCDP 1 and 5)
The phenotype of RCDP spectrum usually ranges from the severe form who present with midfacial hypoplasia, rhizomelic shortening at birth, to the intermediate form who present in childhood with joint contractures and spastic quadriparesis, and the milder forms who present with mild rhizomelic shortening. Severe phenotype can present either in the prenatal period or in the neonatal period.
2. Disorders of peroxisome function
i. Etherphospholipid biosynthesis disorders
RCDP types 2, 3, 4 are included under this category. The clinical features are similar to RCDP 1, 5 and they are differentiated by their genetic etiology. FARI-related disorder is referred to as RCDP4 by some authors, but this disorder lacks the classical skeletal features of RCDP.
ii. Disorders with impaired fatty acid p-oxidation
X-linked adrenoleukodystrophy (X-ALD): X-ALD is an X-linked recessive disorder. It does not have any clinical features at birth. It can manifest with three different phenotypes. Different forms of X-ALD, their specific clinical features, and their age of presentation are depicted in Figure 4. Some female carriers can present with adrenomyeloneuropathy.
Alpha methylacyl-CoA racemase (AMACR) deficiency, acyl-CoA oxidase 1 (ACOX1) deficiency, D-bifunctional protein (DBP) deficiency, and sterol carrier protein X deficiency (SCPx) are the other disorders included under impaired fatty acid p-oxidation (Arora et al;2020). SCPx deficiency has been described in one adult patient with dystonia, cerebellar signs, and motor neuropathy (Ferdinandusse et al., 2006).
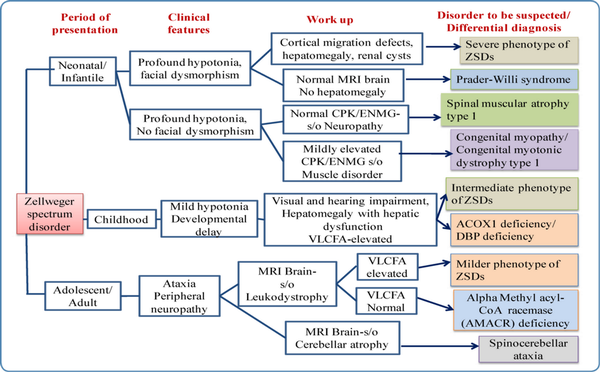
Figure 3
Clues to diagnosis of Zellweger spectrum disorders in different age groups with differential diagnosis
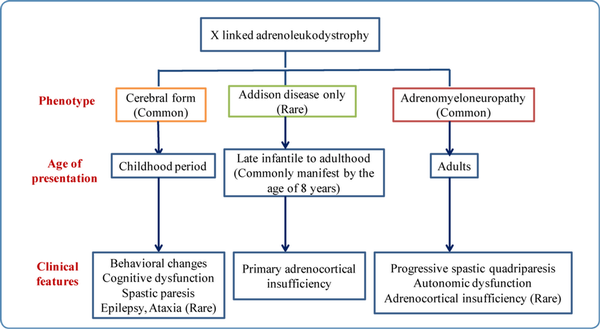
Figure 4
Different phenotypes, age of onset, and clinical features of X-linked adrenoleukodystrophy
iii. Disorders with impaired fatty acids a-oxidation
Ref sum Disease (RD): RD is caused due to phytanoyl-CoA hydroxylase deficiency which is the first enzyme involved in the a-oxidation of fatty acids. Patients with RD usually present in late childhood. Clinical features of this disorder are represented in Figure-5. The entire spectrum of clinical manifestations is not seen in all cases.
iv. Disorders of the Glyoxylate cycle
Primary Hyperoxaluria type 1: Symptoms of this disorder manifest from infancy to adulthood but majority of them present in childhood or early adolescence. They present with recurrent nephrolithiasis due to deposition of calcium oxalate and nephrocalcinosis. Death in these cases is due to end-stage renal disease and renal failure.
v. Bile acid synthesis defects
Acyl-CoA oxidase 2 (ACOX2) deficiency, peroxisomal membrane protein 70 (ABCD3) deficiency, and bile acid-CoA: amino acid N-acyltransferase (BAAT) deficiency are included under this category. ACOX2 deficiency and ABCD3 deficiency present in childhood. Both these disorders have similar clinical features with predominant involvement of the liver (Figure 5). BAAT deficiency presents with itching and steatorrhea.
vi. H2O2 Metabolism
Acatalasemia/Hypocatalasemia: It is caused by either complete or partial loss of catalase activity in erythrocytes. This disorder is usually asymptomatic. In rare cases, it may be associated with oral ulcerations or gangrene, or diabetes mellitus.
The age of onset, clinical features, and features that should lead one to suspect the disorders of peroxisomal dysfunction are outlined in Figure 5.
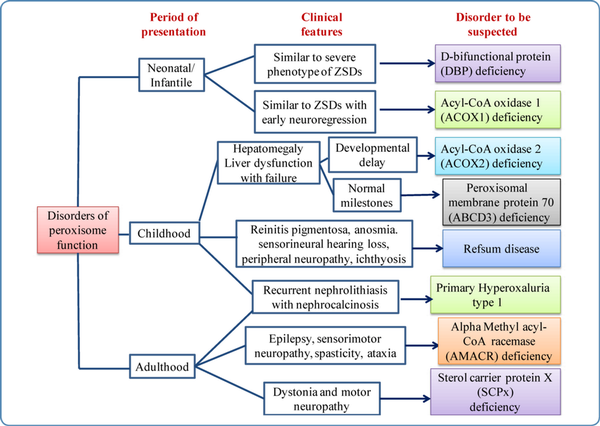
Figure 5
Flow chart showing clues to the diagnosis of disorders of peroxisomal function (single enzyme deficiency disorders)
Diagnosis of peroxisomal disorders
i. Biochemical workup
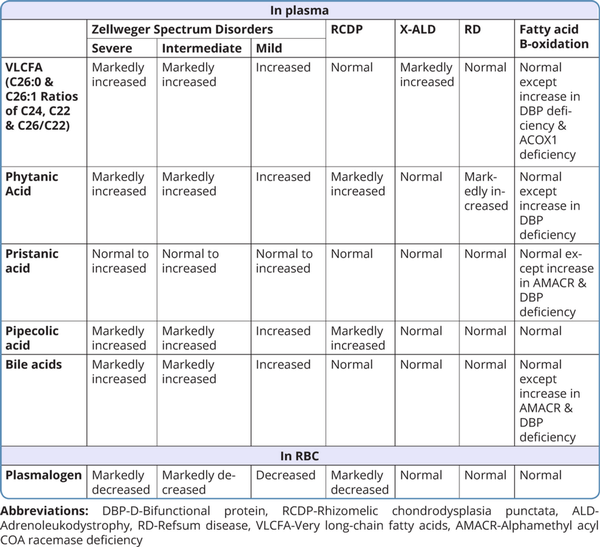
For most peroxisomal disorders, biochemical workup involves metabolite assay in plasma and/ or red blood cells(RBCs). Very long-chain fatty acids (VLCFA), docosahexaenoic acid, phytanic acid, and plasmalogen are the biochemical parameters measured by gas chromatography/ mass spectrometry (GCMS). VLCFAs are measured by analyzing the concentration of C26:0, the ratio of C24:0 to C22:0, and the ratio of C26:0 to C22:0. In ZSDs, VLCFA, phytanic acid, pristanic acid, docosahexaenoic acid, pipecolic acid, and bile acids are elevated in the plasma, and plasmalogens are decreased in the RBCs. An increase in phytanic acid with decreased pipecolic acid in plasma and decreased plasmalogen in RBC is suggestive of RCDP. In Refsum disease, phytanic acid levels are increased but plasmalogen and pipecolic acid levels are normal. In X-ALD, VLCFA is markedly elevated with normal levels of other biochemical substances (Wanders et al) 2018.
Table 3 shows the list of various biochemical substances measured in blood and their levels in different peroxisomal disorders.
X-linked adrenoleukodystrophy is included in neonatal screening programs in several countries (Turk et al., 2020). Recently changes in phospholipid metabolites are found to be reliable biomarkers to indicate neuroinflammation in mice models and X-ALD patients. Further studies need to be done in a large cohort of X-ALD patients to use these metabolites as early biomarkers for neuroinflammation (Kettwig et al., 2021).
In primary hyperoxaluria type 1, the diagnosis is made by the presence of high urinary oxalate excretion and for glycolate oxidase deficiency the diagnosis is by documenting high urinary glycolate levels.
ii. Radiological features
Skeletal abnormalities
Chondrodysplasia punctata at the knee and/ or ankle joints and along the vertebrae in early childhood and rhizomelic shortening are noted in skeletal radiographs in cases with RCDP and Zellweger syndrome (Figure 6). In addition, vertebral cleftsare seen on the skeletal survey in RCDP.
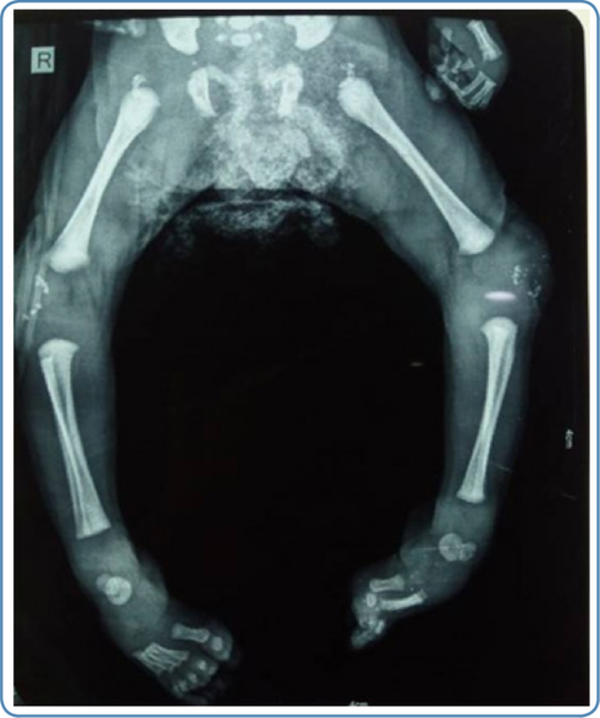
Figure 6
Skeletal radiograph showing chondrodysplasia punctata at the knee joint in a child with rhizomelic chondrodysplasia punctata
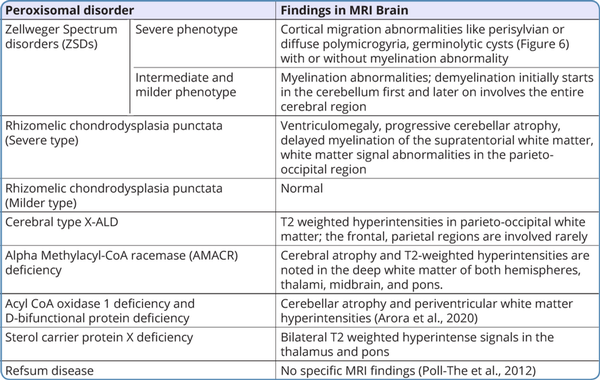
Neuroimaging findings
In peroxisomal disorders, specific findings in magnetic resonance imaging (MRI) of the brain are seen only in Zellweger syndrome (Figure 7) and X-ALD. In other peroxisomal disorders, MRI brain findings may provide clues to the diagnosis. MRI brain findings in peroxisomal disorders are listed in Table 4. Typical findings of the MRI brain may not be found in the initial stages of the disease in peroxisomal disorders as they evolve gradually during the disease course. In such a scenario biochemical workup and genetic evaluation would help in the diagnosis.
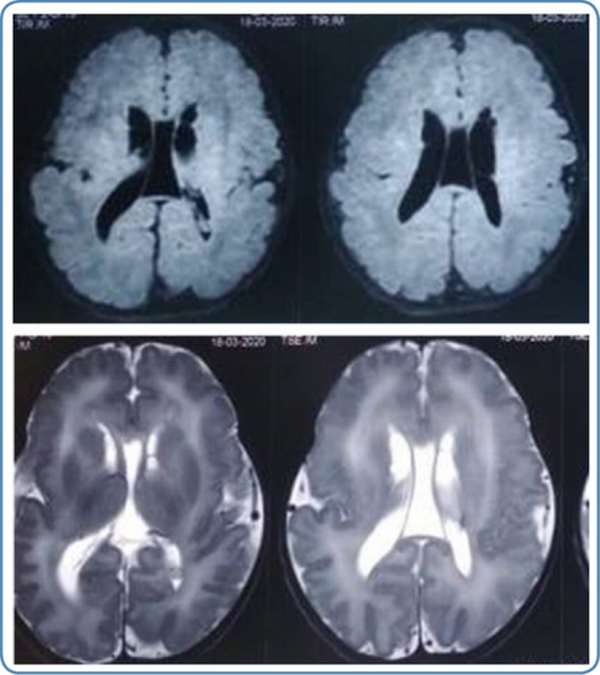
Figure 7
MRI brain findings in Zellweger syndrome 6A.T2 weighted MRI brain axial view showing germinolytic cysts 6B. T1 weighted MRI brain axial view showing diffuse polymicrogyria
Genetic evaluation
Peroxisomal disorders are rare inherited metabolic disorders. The diagnosis of a specific peroxisomal disorder can be made based on clinical features, biochemical workup, skeletal survey, MRI brain, and molecular genetic testing by whole/ clinical-exome sequencing. Biochemical analysis is required to corroborate the genetic diagnosis in some cases especially in those with variants of uncertain significance. The genomics-first approach would be helpful in cases with milder and atypical phenotypes.
A diagnostic flowchart that can be used for peroxisomal disorders is given in Figure 8.
Management
Surveillance of most of the peroxisomal disorders is by regular monitoring of liver functions, renal and adrenal functions, and annual hearing and ophthalmologic evaluation. In addition, MRI brain is recommended in suspected cases of ZSDs, X-ALD, and also in ACOX1, AMACR, and DBP deficiency.
There is no complete cure available for peroxisomal disorders at present. Supportive therapies like adequate nutrition, physiotherapy, and occupational therapy are to be given. Symptomatic management includes antiepileptic therapy for seizures, and gastrostomy tube feeding for those with feeding difficulty.
Dietary restriction of phytanic acid helps to some extent in patients with Refsum disease. Pyridoxine supplementation has been tried in hyperoxaluria type 1 but has limited success. Good hydration, lithotripsy, and surgical intervention would be helpful to some extent in hyperoxaluriatype 1 but there is a high chance of recurrence of renal stones. In cases with adrenocortical insufficiency, corticosteroid replacement therapy is recommended. In X-linked ALD, Lorenzo's oil has been tried but has limited success as it does not prevent the progression of neurological symptoms. If done in the early stages, allogeneic hematopoietic stem cell transplantation (HSCT) is shown to either prevent progression or reverse demyelination. Lenti-D gene therapy tried for patients in the early stages of cerebral type of X-ALD showed beneficial results in phase III clinical trial (Eichler et al., 2017). In 2018, Lenti-D(tm) was heralded as a breakthrough therapy by the United States Food and Drug Administration (US FDA) for treating the cerebral type of X-ALD, as it is found to provide significant improvement when compared to other available therapies.
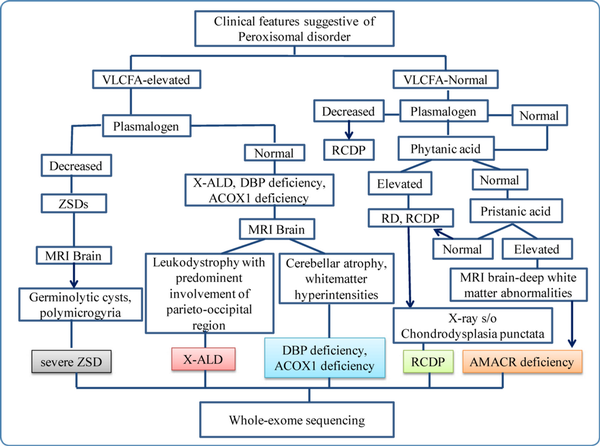
Figure 8
Diagnostic flow chart for peroxisomal disorders
Conclusion
Though most of the peroxisomal disorders can be recognized based on specific clinical clues and diagnostic workup, the milder and atypical phenotypes need a structured clinical and diagnostic approach to establish the exact diagnosis. There is no complete cure for these disorders except for supportive treatment and symptomatic management by a multidisciplinary team. Exact molecular diagnosis, therefore, helps in appropriate genetic counseling and definitive prenatal testing, and also helps the couples to make informed reproductive choices.
References
- 1. Arora V, et al. Eyes See what the Mind Knows: Clues to Pattern Recognition in Single Enzyme Deficiency-Related Peroxisomal Disorders. Mol Syndromol 2020; 11: 309–314.
- 2. Eichler F, et al. Hematopoietic stem-cell gene therapy for cerebral ad renoleukodystrophy. N Engl J Med 2017; 377: 1630–1638.
- 3. Falkenberg KD, et al. Allelic Expression Imbalance Promoting a Mutant PEX6 Allele Causes Zellweger Spectrum Disorder. Am J Hum Genet 2017; 101: 965–976.
- 4. Ferdinandusse S, et al. Mutations in the gene encoding peroxisomal sterol carrier protein X (SCPx) cause leukoencephalopathy with dystonia and motor neuropathy. Am J Hum Genet 2006; 78: 1046–1052.
- 5. Kettwig M, et al. Targeted metabolomics revealed changes in phospholipids during the development of neuroinflammation in Abcd1 (tm1 Kds) mice and X-linked adrenoleukodystrophy patients. J Inherit Metab Dis. 2021.44:1174–1185.
- 6. Masih S, et al. Twins with PEX7 related intellectual disability and cataract: Highlighting phenotypes of peroxisome biogenesis disorder 9B. Am J Med Genet 2021; 185: 1504–1508.
- 7. Poll-The BT, et al. Clinical diagnosis, biochemical findings and MRI spectrum of peroxisomal disorders. Biochim Biophys Acta 2012; 1822: 1421–1429.
- 8. Takashima S, et al. Expanding the concept of peroxisomal diseases and efficient diagnostic system in Japan. J Hum Genet 2019; 64:145–152.
- 9. Turk BR, et al. X-linked adrenoleukodystrophy: Pathology, pathophysiology, diagnostic testing, newborn screening, and therapies. Int J Dev Neurosci 2020; 80: 52–72.
- 10. Wanders RJA. Peroxisomal disorders: Improved laboratory diagnosis, new defects, and the complicated route to treatment. Mol Cell Probes 2018;40:60–69.